Abstract
Background
Electromyography (EMG)-based audiovisual biofeedback systems, developed and tested in research settings to train neuromuscular control in patient populations such as cerebral palsy (CP), have inherent implementation obstacles that may limit their translation to clinical practice. The purpose of this study was to design and validate an alternative, plantar pressure-based biofeedback system for improving ankle plantar flexor recruitment during walking in individuals with CP.
Methods
Eight individuals with CP (11–18 years old) were recruited to test both an EMG-based and a plantar pressure-based biofeedback system while walking. Ankle plantar flexor muscle recruitment, co-contraction at the ankle, and lower limb kinematics were compared between the two systems and relative to baseline walking.
Results
Relative to baseline walking, both biofeedback systems yielded significant increases in mean soleus (43–58%, p < 0.05), and mean (68–70%, p < 0.05) and peak (71–82%, p < 0.05) medial gastrocnemius activation, with no differences between the two systems and strong relationships for all primary outcome variables (R = 0.89–0.94). Ankle co-contraction significantly increased relative to baseline only with the EMG-based system (52%, p = 0.03).
Conclusion
These findings support future research on functional training with this simple, low-cost biofeedback modality.
Similar content being viewed by others
Background
Effective recruitment of the ankle plantar flexor muscles is necessary to modulate the forward and vertical progression of the center of mass for an efficient exchange of potential and kinetic energy during bipedal walking [1, 2]. Individuals with cerebral palsy (CP) [3], stroke [4, 5], and the elderly [6], often lack the neuromuscular control to effectively utilize their plantar flexors during walking. For individuals with CP, the most prevalent pediatric-onset movement disorder, there is broad clinical agreement that plantar flexor dysfunction often contributes to gait impairment [7], creating a barrier to an active lifestyle and predisposing this population to a host of secondary effects associated with inactivity [8], including an eventual loss of independent ambulation [9]. For this reason, interventions designed to improve neuromuscular control of the ankle plantar flexors could have a significant impact on long-term mobility for individuals with CP, or any other patient populations that experience reduced motor control at the ankle.
Several audiovisual biofeedback systems (e.g., step-length feedback) have been developed for individuals with CP with the goal of modulating upper or lower limb position, force, or motor control [10, 11]. To date, most audiovisual biofeedback studies aimed at increasing lower-limb muscle control in CP have utilized an electromyography (EMG)-based system, whereby a user’s muscle activity is displayed to them in real-time [12,13,14]. While EMG-based audiovisual biofeedback provides direct feedback of the intervention’s target (i.e., increased muscle activity), there are significant limitations to the EMG biofeedback modality that prevents widespread adoption in clinical or home settings, including motion artifact noise during walking; skin-electrode interface reliability challenges, like hair and sweating; the necessity and complexity of proper anatomical placement of the sensors, particularly when placing sensors on small limbs; and the cost of an EMG system. This may explain why, despite a demonstrated benefit of plantar flexor EMG-based biofeedback for improving ankle function and gait symmetry in CP nearly three decades ago, this gait training tool has failed widespread adoption in clinical practice. Practical biofeedback modalities capable of increasing plantar flexor recruitment during gait training would likely have widespread appeal.
We theorize that a potential alternative to a plantar flexor EMG-based biofeedback system could be an underfoot plantar pressure-based system that would measure and provide feedback on the change in forefoot pressure generated from plantar flexor muscle recruitment. Pressure sensors are inexpensive and could be quickly and easily accommodated by most footwear, and have been used previously to modulate muscle activity at the ankle during walking for individuals with chronic ankle instability [15]. If effective, plantar pressure-based biofeedback may expand access to neuromuscular gait training by offering a practical solution for in-clinic and at-home use. Before a plantar pressure-based system like this could be clinically translated, however, it should be validated by comparing changes in muscle activity with those observed from an EMG-based system during walking.
The primary aim of this study was to clinically validate the use of a plantar pressure-based audiovisual biofeedback system to increase ankle plantar flexor engagement during walking by comparing changes in muscle activation levels to an EMG-based audiovisual biofeedback system in CP. We hypothesized that both biofeedback modalities would result in a significant increase in plantar flexor activity while walking, with no difference and strong relationships between the two systems, validating the use of the plantar pressure-based system as an alternative to an EMG-based system.
Methods
We developed a simple plantar pressure-based biofeedback system comprised of a force sensitive resistor (FSR) placed under the ball of the fore-foot that responded to plantar flexor muscle activity indirectly through push-off with the ground (Fig. 1A). We compared our plantar pressure-based system to EMG-based biofeedback, which we considered the “gold standard”, given that it provides direct feedback of the metric that a user is attempting to modulate (i.e., muscle activation), and has been tested in previous studies [13, 16]. To isolate the effects of each biofeedback modality while controlling for the components attached to the body, we used the same wearable setup (i.e., plantar pressure and EMG systems) during all walking conditions (i.e., baseline, plantar pressure-based biofeedback, and EMG-based biofeedback).
Plantar pressure biofeedback system
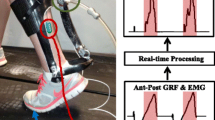
Plantar pressure was measured using an FSR (FlexiForce A502, Tekscan) secured to a thin carbon fiber foot plate. The FSR had a relatively large (51 × 51 mm) sensing area that was centered under the typical location for the head of the first metatarsal (the “pad” or “ball” of the foot). The FSR location remained the same for each participant, as an aspect of the present study was to evaluate if an FSR placed in this area could serve as a suitable metric for providing audiovisual biofeedback of ankle plantar flexion for different foot types. The FSR was wired to a custom printed circuit board with a microcontroller and Bluetooth transceiver, utilizing electronics from a modified version of our wearable robotic system presented in [17]. In this study, the Bluetooth transceiver communicated with a laptop running a real-time MATLAB graphical user interface (GUI). To demonstrate the broader applicability of our approach, we also created a stand-alone system with a smartphone application (available for iOS and Android from Biomotum, Inc.). The stand-alone system, weighing < 50 g, had the components condensed into a portable casing that can be attached to a user’s footwear (Fig. 1B).
A 5-s calibration procedure was completed at the beginning of each walking trial. The instantaneous plantar pressure data were then normalized by the average peak stance phase pressure during the calibration period and transmitted at 100 Hz to a moving bar graph on the MATLAB GUI. A “target” horizontal line was also included on the bar graph, which was initially set to 10% above the average peak plantar pressure from the calibration period. The target was then adjusted based on each user’s performance. Specifically, if a user reached their target greater than 75% of steps within a minute, the line was increased by 10%. If a user reached their target less than 50% of steps within a minute, the line was decreased by 10%. If a user reached their target between 50 and 75% of steps within a minute, the line was held constant. If a participant reached the target line, the bar graph changed from red to green, and a “ding” sound was emitted from the laptop speakers. Once the bar dropped back below the target line, the bars changed back to red (Fig. 1C). Biofeedback was provided for each participant’s more impaired limb.
A Plantar pressure-based setup, with a force sensitive resistor (FSR) placed under the pad of the foot to measure changes in plantar pressure with contraction of the ankle plantar flexors; B standalone plantar pressure-based biofeedback system; C experimental protocol for validating the plantar pressure-based audiovisual biofeedback system against an EMG-based system (measuring and displaying soleus muscle activity), where both systems used an identical display of a real-time moving bar graph and horizontal target line with an audible “ding” when this target line was passed
EMG biofeedback system
We used a commercially available system for EMG-based biofeedback (Desktop DTS, Noraxon), which displayed real-time soleus muscle activity after filtering with a 1000 ms root mean square envelope. The filtered muscle activity was presented in an identical display as the plantar pressure-based system; the same auditory feedback was also provided. To match the plantar pressure-based system, a “target” horizontal line was also set at 10% above a user’s baseline peak soleus activity, with the same performance-based adjustments to this target as the plantar pressure system (Fig. 1C). Feedback was provided on each participant’s more impaired limb.
Participants
This protocol was approved by the Northern Arizona University Institutional Review Board (#986744) and completed at the Northern Arizona University–Phoenix Biomedical Campus (Phoenix, AZ). The protocol utilized participants recruited for a clinical trial, which was prospectively registered at ClinicalTrials.gov (NCT04119063). Prior to starting the study, informed written consent was provided by each participant if 18 years or older, or the parent/legal guardian in the case of minors (minors also provided verbal assent).
Participant inclusion criteria was as follows: confirmed diagnosis of CP (hemi- or diplegic distributions), Gross Motor Function Classification System (GMFCS) level I–III, the ability to walk on a treadmill with support for at least ten minutes, and 10–21 years of age. Exclusion criteria included orthopedic surgery within the past 6 months, botulinum toxin injections to the triceps surae muscles within the past 6 months, and any other conditions that would preclude safe participation. Eight participants were recruited (Table 1).
Protocol
To begin, height, weight, and lower limb anthropometrics were measured for each participant. Next, participants were outfitted with reflective markers on their lower limbs in accordance with Vicon’s lower body Plug-In Gait model (Vicon, 100 Hz; Denver, CO, USA), and wireless surface EMG sensors (Noraxon, 1000 Hz; Scottsdale, AZ, USA) were placed on the more affected limb’s soleus, medial gastrocnemius, and tibialis anterior muscles according to SENIAM recommendations [18]. For participants who were diplegic, the more affected limb was determined by asking the participant or participant’s guardian which side was weaker and/or less coordinated. All participants and/or participant guardians were able to clearly identify a more affected side.
Participants walked under the following three conditions at a self-selected speed on the treadmill for two minutes and 30 s while lower body kinematics and muscle activity were measured: (1) baseline (i.e., no audiovisual biofeedback), (2) unilateral plantar pressure-based audiovisual biofeedback, and (3) unilateral EMG-based audiovisual biofeedback. All participants started with the baseline condition, followed by the plantar pressure and EMG biofeedback conditions in random order. P1, a younger participant, was re-tested on a separate day because of an apparent behavior non-compliance issue.
Data analysis
Twenty gait cycles of each condition were analyzed. Three-dimensional marker data was used to calculate ankle and knee joint angles for both biofeedback conditions versus baseline using Vicon’s Plug-In Gait Model and inverse kinematics. Angle data was low-pass filtered (4th order Butterworth, 4 Hz low-pass cutoff) and an average curve for both ankle angle and knee angle was generated. EMG data was bandpass filtered (4th order Butterworth, 20–400 Hz band-pass cutoff), rectified, and low-pass filtered (4th order Butterworth, 4 Hz low-pass cutoff [19]), and then time normalized to a gait cycle (heel strike to heel strike). The twenty recorded gait cycles were averaged together for a single activation curve for each muscle, and normalized to peak baseline activation of that respective muscle.
Peak stance phase ankle plantar flexion and knee extension angles were calculated for each condition. We chose to assess lower limb kinematics to determine if either system resulted in compensatory strategies that would negatively impact gait. In addition, mean and peak propulsive phase (51–100% of stance phase [20]) soleus and medial gastrocnemius activation was calculated for each condition. Finally, stance-phase co-contraction at the ankle between the soleus and tibialis anterior was calculated for each condition using a co-contraction index (CCI) [21]:
where i represents the individual time points of stance phase (0–100%, or 101 total data points), LEMG represents the normalized magnitude of the less active muscle at time point i, and MEMG represents the normalized magnitude of the more active muscle at time point i. CCI values for the audiovisual biofeedback conditions were then normalized to baseline values.
Statistical analysis
We validated plantar pressure-based biofeedback through three main statistical comparisons: (1) by assessing the change in muscle activity relative to baseline; (2) by comparing the change in muscle activity relative EMG biofeedback; and (3) similar to other validation studies [22, 23], by assessing the relationship to EMG-based biofeedback.
The primary outcome measures for our a-priori hypotheses included mean and peak propulsive phase soleus and medial gastrocnemius muscle activity. Secondary outcome measures included ankle CCI, and peak ankle plantar flexion and knee extension angle. To assess our primary objective of validating plantar pressure vs. EMG biofeedback, we performed one-way repeated measures Analysis of Variance (RM ANOVA) to determine the effect of biofeedback condition (i.e., baseline, plantar pressure biofeedback, and EMG biofeedback) on these outcome measures. If a significant effect of walking condition was found, we ran two-tailed pairwise comparisons with Holm–Bonferroni correction for multiple comparisons. In addition, we assessed the relationship between the plantar pressure-based and EMG-based primary outcome measures by calculating a Pearson product-moment correlation coefficient (R), where 0.3 was considered a weak relationship, 0.5 a moderate relationship, and 0.7 a strong relationship [24]. To ensure that outlier values did not significantly influence this relationship, we ran an outlier analysis where any values 1.5 times the interquartile range below the first quartile or above the third quartile were removed the correlation calculation [25]. P7’s medial gastrocnemius data was not available for the primary objective due to a dropped signal from the EMG sensors. All reported p-values are the adjusted values from the multiple comparisons correction, and significance level was set at p < 0.05.
Results
All participants successfully completed the protocol, walking with both audiovisual biofeedback systems and understanding their objective of reaching their target line on each step.
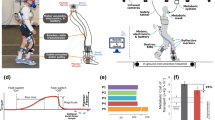
There was a significant effect of walking condition on mean soleus activity (p < 0.01; Fig. 2A). Pairwise comparisons indicated a significant increase in mean soleus activity for both the plantar pressure-based (mean ± SD) (43 ± 33%, p < 0.05) and EMG-based systems (58 ± 42%, p < 0.05) relative to mean baseline soleus activity, with no difference between the two systems (p = 0.09). There was no significant effect of walking condition on peak soleus activity (p = 0.08; Fig. 2B). Strong relationships were observed between the two systems for both mean (R = 0.89, p < 0.01, Fig. 3A) and peak (R = 0.92, p < 0.01, Fig. 3B) soleus activation. See Additional file 1 for individual soleus activation curves for these conditions.
Average A mean and B peak propulsive phase soleus activation relative to baseline activity for the two audiovisual biofeedback systems, and C representative soleus activation curves (average of 20 gait cycles) across the three walking conditions: baseline (blue), plantar pressure-based biofeedback (orange), and EMG-based biofeedback (yellow); Error bars represent standard error of the mean, brackets indicate pairwise comparisons between plantar pressure-based and EMG-based systems, upward arrows represent pairwise comparisons between baseline walking and the respective audiovisual biofeedback system; *p < 0.05
A significant effect of walking condition was observed for both mean (p < 0.01; Fig. 4A) and peak (p < 0.01; Fig. 4B) medial gastrocnemius activity. Pairwise comparisons indicated a significant increase in mean medial gastrocnemius activation for both the plantar pressure-based (68 ± 50%; p < 0.05) and EMG-based (77 ± 44%; p < 0.05) systems relative to baseline, with no difference between systems (p = 0.33). Additionally, both systems had a significant increase in peak medial gastrocnemius activation relative to baseline (plantar pressure-based: 82 ± 51%, p < 0.05; EMG-based: 71 ± 35%, p < 0.01), but no difference was observed between systems (p = 0.36). There were strong relationships between the two systems for both mean (R = 0.94, p < 0.01, Fig. 3C) and peak (R = 0.90, p < 0.01, Fig. 3D) medial gastrocnemius activation. See Additional file 2 for individual medial gastrocnemius activation curves for these conditions.
Average A mean and B peak propulsive phase medial gastrocnemius activation relative to baseline activity for the two audiovisual biofeedback systems, and C representative medial gastrocnemius activation curves (average of 20 gait cycles) across the three walking conditions: baseline (blue), plantar pressure-based biofeedback (orange), and EMG-based biofeedback (yellow); Error bars represent standard error of the mean, brackets indicate pairwise comparisons between plantar pressure-based and EMG-based systems, upward arrows represent pairwise comparisons between baseline walking and the respective audiovisual biofeedback system; *p < 0.05
A significant effect of walking condition on ankle CCI was found (p < 0.01; Fig. 5); pairwise comparisons indicated a significant increase for the EMG-based system relative to baseline walking (52 ± 41%, p = 0.03), but no difference between the plantar pressure-based system and baseline walking (p = 0.07) or between the two systems (p = 0.07). No significant effects of walking condition were observed for the lower limb kinematic measures (peak knee extension angle: p = 0.6, peak ankle plantar flexion angle: p = 0.5; see Additional file 3 for individual joint angle curves).
Average co-contraction index (CCI) between the soleus and tibialis anterior during the stance phase of gait for the two audiovisual biofeedback systems alone; Error bars represent standard error of the mean, brackets indicate pairwise comparisons between plantar pressure-based and EMG-based systems, and upward arrows represent pairwise comparisons between baseline walking and the respective audiovisual biofeedback system
Discussion
We achieved our primary goal of validating a plantar pressure biofeedback system for increasing ankle plantar flexor muscle activity relative to EMG biofeedback, and demonstrated several expected and one surprising potential benefit of our novel system compared to the “gold-standard.” The findings from this study partially supported our hypothesis that both plantar pressure-based and EMG-based audiovisual biofeedback systems would increase ankle plantar flexor muscle activity while walking, with significant increases in mean soleus muscle activity and mean and peak medial gastrocnemius muscle activity relative to baseline for both systems, and trends toward increases in peak soleus activity. Importantly, there was no statistical difference in activation between the two biofeedback modalities, and strong relationships for all primary outcome variables between the two systems. Our finding of consistent lower limb gait kinematics across both biofeedback conditions, and relative to baseline walking, indicated that participants did not adopt any compensatory movement strategies in response to feedback that could negatively affect their gait.
While an ankle plantar flexor EMG-based audiovisual biofeedback system provides direct feedback of a muscle’s activation level, a plantar pressure-based system provides indirect feedback of a muscle’s activation level, focusing instead on the functional output of the muscle (i.e., plantar flexor force). With the EMG-based system, a user could raise the bar past their target level by contracting their soleus muscle without necessarily increasing plantar flexor force if the antagonist dorsiflexor muscle (i.e., tibialis anterior) was contracting at the same time. Our finding of increased co-contraction at the ankle with the EMG-based system relative to baseline indicates that this strategy was indeed adopted by our study participants. With the plantar pressure-based system, on the other hand, increasing co-contraction at the ankle would counter the desired output of increase plantar flexor force, likely explaining why we did not observe increases with this system relative to baseline. With the goal of using a biofeedback system to train improved neuromuscular control at the ankle for enhanced walking ability, the use of a plantar pressure-based system may result in a more functional outcome. This is corroborated by the observation reported in the literature that external focus of attention (i.e., increased force against an FSR) is superior to internal focus of attention (i.e., increased activation of the soleus) for functional motor learning [26]. To our knowledge, this is only the second study to evaluate the ability of an audiovisual biofeedback system to influence ankle plantar flexor muscle activity while walking in CP. The previous study focused on spatiotemporal and kinematic outcomes, and did not report the neuromuscular response (i.e., relative triceps surae activation) while walking with feedback [13]. The findings from the present study, therefore, are particularly enlightening, indicating that providing feedback in this manner to children and young adults with CP results in significantly increased neuromuscular recruitment while walking after only a few minutes of acclimation.
We observed statistically significant increases in mean and peak propulsive phase medial gastrocnemius activation with both biofeedback systems. The medial gastrocnemius serves a unique role relative to the soleus during walking, contributing more to forward propulsion [20]. It has been observed that individuals with CP have deficits in neuromuscular control of the medial gastrocnemii during the push-off phase of gait, leading to reduced ankle power and a slow and inefficient gait pattern [27]. Our finding of increased recruitment of this essential plantar flexor muscle, specifically during the propulsive phase of gait, supports the potential of this audiovisual biofeedback system to positively impact walking performance in this population.
There are instances where an EMG-based biofeedback system may be more advantageous than a plantar pressure-based system, such as barefoot walking or for a user whose orthotics are not compatible with a pressure-sensitive insole. This study, however, was motivated by the desire to test a more clinically accessible form of audiovisual biofeedback to improve neuromuscular control at the ankle while walking. From our anecdotal observations on the amount of time required to set up both systems, it became clear that a plantar pressure-based system was a less resource- and time-intensive process. For example, one can consider the complexity of placing the sensor for the two systems: for the plantar pressure-based system, it simply requires placing the sensor on the medial forefoot of the shoe insole. For the EMG-based system, one must utilize anatomical landmarks to locate an appropriate measurement spot of a muscle belly, which can be challenging for young children with low muscle volume; carefully prepare the skin interface by shaving hair and swabbing with alcohol; and carefully securing the sensor components to the skin to mitigate disturbances in the signal from movement artifacts. In addition, there are notable differences in the cost required for both systems, with materials required for a plantar pressure-based system coming in at a fraction of the price of the materials required for an EMG-based system. These practical differences, in conjunction with the findings from this study that a plantar pressure-based system is able to produce comparable or even more-favorable neuromuscular control at the ankle, support the future investigation of plantar pressure-based systems in clinical settings.
There are notable limitations of this study. First, we tested a relatively small sample of individuals with CP, which is an inherently heterogenous condition. Still, we observed relatively consistent increases in muscle activity across our participants with both audiovisual biofeedback systems. In addition, the primary aim of this study was to validate the plantar pressure-based system against an EMG-based system for a clinical population, and not necessarily to demonstrate any kind of training effect that may require greater statistical power. Second, this clinical validation was limited to individuals with CP. The primary outcome of improved plantar flexor recruitment, however, could be valuable to several patient populations with deficits in neuromuscular control at the ankle. Third, our validation was limited to treadmill walking, which was necessary to isolate the comparison between biofeedback systems and provide continuous visual feedback to the participants. Future work will explore the translation of the plantar pressure-based system to an overground walking context with audio-only biofeedback. Finally, this study did not include qualitative assessments of how the user experience compared between the two biofeedback systems, which should be explored in future applications of this plantar pressure-based system to confirm clinical viability.
Conclusion
In conclusion, we demonstrated that a simple plantar pressure-based biofeedback system is capable of increasing functional recruitment of the ankle plantar flexor muscles in children and young adults with CP. We observed comparable or even more-favorable neuromuscular control at the ankle when using this system relative to direct EMG biofeedback. We also confirmed that these neuromuscular responses were not a result of compensatory walking patterns, with consistent lower limb kinematics compared to baseline walking. Future studies and clinical interventions should evaluate if functional training with this simple, low-cost system can result in lasting improvements in walking ability in CP and other patient populations.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CP:
-
Cerebral palsy
- EMG:
-
Electromyography
- FSR:
-
Force sensitive resistor
- GUI:
-
Graphical user interface
- GMFCS:
-
Gross Motor Function Classification System
- CCI:
-
Co-contraction index
References
Sutherland DH, Cooper L, Daniel D. The role of the ankle plantar flexors in normal walking. J Bone Joint Surg Am. 1980;62:354–63.
Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol Regul Integr Comp Physiol. 1977;2:R243–61.
Dietz V, Berger W. Cerebral palsy and muscle transformation. Dev Med Child Neurol. 2008;37:180–4.
Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech. 1999;14:125–35.
Awad LN, Lewek MD, Kesar TM, Franz JR, Bowden MG. These legs were made for propulsion: advancing the diagnosis and treatment of post-stroke propulsion deficits. J Neuroeng Rehabil. 2020;17:139.
Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998;79:317–22.
Johnson D, Damiano D, Abel M. The evolution of gait in childhood and adolescent cerebral palsy. J Pediatr Orthop. 1997;17:392–6.
Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–40.
Day SM, Wu YW, Strauss DJ, Shavelle RM, Reynolds RJ. Change in ambulatory ability of adolescents and young adults with cerebral palsy. Dev Med Child Neurol. 2007;49:647–53.
MacIntosh A, Lam E, Vigneron V, Vignais N, Biddiss E. Biofeedback interventions for individuals with cerebral palsy: a systematic review. Disabil Rehabil. 2019;41:2369–91.
Fang Y, Lerner ZF. Feasibility of augmenting ankle exoskeleton walking performance with step length biofeedback in individuals with cerebral palsy. IEEE Trans Neural Syst Rehabil Eng. 2021;29:442–9.
Toner LV, Cook K, Elder GC. Improved ankle function in children with cerebral palsy after computer-assisted motor learning. Dev Med Child Neurol. 1998;40:829–35.
Colborne GR, Wright FV, Naumann S. Feedback of triceps surae EMG in gait of children with cerebral palsy: a controlled study. Arch Phys Med Rehabil. 1994;75:40–5.
Dursun E, Dursun N, Alican D. Effects of biofeedback treatment on gait in children with cerebral palsy. Disabil Rehabil. 2004;26:116–20.
Donovan L, Feger MA, Hart JM, Saliba S, Park J, Hertel J. Effects of an auditory biofeedback device on plantar pressure in patients with chronic ankle instability. Gait Posture. 2016;44:29–36.
Intiso D, Santilli V, Grasso MG, Rossi R, Caruso I. Rehabilitation of walking with electromyographic biofeedback in foot-drop after stroke. Stroke. 1994;25:1189–92.
Orekhov G, Fang Y, Cuddeback CF, Lerner ZF. Usability and performance validation of an ultra-lightweight and versatile untethered robotic ankle exoskeleton. J Neuroeng Rehabil. 2021;18:163.
Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, et al. European recommendations for Surface ElectroMyoGraphy results of the SENIAM project. Roessingh Research and Development; 1999.
Ranganathan R, Krishnan C. Extracting synergies in gait: using EMG variability to evaluate control strategies. J Neurophysiol. 2012;108:1537–44.
Gottschall JS, Kram R. Energy cost and muscular activity required for propulsion during walking. J Appl Physiol. 2003;94:1766–72.
Knarr BA, Zeni JA, Higginson JS. Comparison of electromyography and joint moment as indicators of co-contraction. J Electromyogr Kinesiol. 2012;22:607–11.
Hillman SJ, Hazlewood ME, Schwartz MH, van der Linden ML, Robb JE. Correlation of the Edinburgh gait score with the Gillette gait index, the Gillette functional assessment questionnaire, and dimensionless speed. J Pediatr Orthop. 2007;27:7–11.
Read HS, Hazlewood ME, Hillman SJ, Prescott RJ, Robb JE. Edinburgh visual gait score for use in cerebral palsy. J Pediatr Orthop. 2003;23:296–301.
Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24:69–71.
Tukey JW. Exploratory data analysis. Reading: Addison-Wesley; 1977.
Wulf G, Prinz W. Directing attention to movement effects enhances learning: a review. Psychon Bull Rev. 2001;8:648–60.
Barber L, Carty C, Modenese L, Walsh J, Boyd R, Lichtwark G. Medial gastrocnemius and soleus muscle-tendon unit, fascicle, and tendon interaction during walking in children with cerebral palsy. Dev Med Child Neurol. 2017;59:843–51.
Acknowledgements
The authors would like to thank Greg Orekhov, Leah Liebelt, and Sam Maxwell for their assistance with device manufacturing, as well as Cassidy Michaels and Alyssa Spomer for their assistance with collecting and processing kinematic data. The authors would also like to thank the participants and their families for their involvement in this study.
Funding
This work was supported in part by the National Science Foundation under Award Number 2045966, and in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number F30HD103318. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
BCC designed the study, led data collection and analysis, generated the figures, and wrote the manuscript. YF led portions of data analysis, interpretation of results, and assisted in writing the manuscript. ZFL was the principal investigator, conceived, designed, and led the study, interpreted the data, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Northern Arizona University Institutional Review Board (#986744). Informed written consent was provided by a parent or legal guardian for each participant after the nature and possible consequences of the study was explained; participants provided verbal assent.
Consent for publication
All participants 18 and over read and signed photo and video release forms. For all minors, we obtained assent and written consent from a parent.
Competing interests
ZFL is a co-founder with shareholder interest of a university start-up company that may leverage the findings from this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Individual soleus activation curves for baseline, plantar pressure biofeedback, and EMG biofeedback walking conditions.
Additional file 2.
Individual medial gastrocnemius activation curves for baseline, plantar pressure biofeedback, and EMG biofeedback walking conditions.
Additional file 3.
Individual knee and ankle joint angles for baseline, plantar pressure biofeedback, and EMG biofeedback walking conditions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Conner, B.C., Fang, Y. & Lerner, Z.F. Under pressure: design and validation of a pressure-sensitive insole for ankle plantar flexion biofeedback during neuromuscular gait training. J NeuroEngineering Rehabil 19, 135 (2022). https://doi.org/10.1186/s12984-022-01119-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-022-01119-y