Abstract
Background
Lokomat therapy for gait rehabilitation has become increasingly popular. Most evidence suggests that Lokomat therapy is equally effective as but not superior to standard therapy approaches. One reason might be that the Lokomat parameters to personalize therapy, such as gait speed, body weight support and Guidance Force, are not optimally used. However, there is little evidence available about the influence of Lokomat parameters on the effectiveness of the therapy. Nevertheless, an appropriate reporting of the applied therapy parameters is key to the successful clinical transfer of study results. The aim of this scoping review was therefore to evaluate how the currently available clinical studies report Lokomat parameter settings and map the current literature on Lokomat therapy parameters.
Methods and results
A systematic literature search was performed in three databases: Pubmed, Scopus and Embase. All primary research articles performing therapy with the Lokomat in neurologic populations in English or German were included. The quality of reporting of all clinical studies was assessed with a framework developed for this particular purpose. We identified 208 studies investigating Lokomat therapy in patients with neurologic diseases. The reporting quality was generally poor. Less than a third of the studies indicate which parameter settings have been applied. The usability of the reporting for a clinical transfer of promising results is therefore limited.
Conclusion
Although the currently available evidence on Lokomat parameters suggests that therapy parameters might have an influence on the effectiveness, there is currently not enough evidence available to provide detailed recommendations. Nevertheless, clinicians should pay close attention to the reported therapy parameters when translating research findings to their own clinical practice. To this end, we propose that the quality of reporting should be improved and we provide a reporting framework for authors as a quality control before submitting a Lokomat-related article.
Similar content being viewed by others
Background
Over the last two decades, robot-assisted gait therapy (RAGT) has emerged as a frequently used technique in gait rehabilitation for patients with central neurologic gait disorders. Advantages, such as the possibility to achieve a high number of repetitions and reduced physical demands on the therapist, make RAGT an attractive option to clinicians. One of the most widely used robots is the Lokomat (Hocoma AG, Volketswil, Switzerland) which has been installed over 1000 times according to the company’s website [1]. Originally developed for people with spinal cord injuries [2], it has also been used for rehabilitation in numerous other conditions including stroke, Parkinson’s disease, multiple sclerosis and cerebral palsy [3,4,5,6]. Over the years, a significant number of clinical studies have been conducted to support the effectiveness of Lokomat therapy with scientific evidence. Most evidence was collected and analyzed in systematic reviews that found Lokomat therapy to be effective, but generally not superior to other forms of therapy like overground walking or manual treadmill therapy [3,4,5,6,7]. Looking more closely at the included studies, some showed an advantage over traditional training methods [8,9,10,11,12,13,14,15,16,17], but others failed to demonstrate this and urge therapists to remain cautious [18,19,20,21,22,23,24,25,26,27]. This heterogeneity of the results cannot easily be explained; for example, studies investigating people with the same diagnosis such as subacute stroke can be found distributed across that spectrum [14, 23].
The literature suggests that there might be various reasons for these mixed results. Several studies have investigated the influence of the patient population. For example, there is some evidence that more severely impaired patients with stroke might improve more than less affected patients [28]. Similar results have been found for children with cerebral palsy [29], although again this is controversial [30]. With respect to diagnosis, children with an acquired brain injury seem to benefit more than their peers with cerebral palsy [31]. Other attempts to find correlates between responsiveness and diagnostic factors in stroke [32] and spinal cord injury [33] have not been very successful.
Another important contribution to the mixed results might be differences in the therapy process itself. Modern rehabilitation research has established that goal-oriented therapy [34], a large amount of practice [35], and an active participation of the patient [36] are important contributors to a successful rehabilitation process. This suggests that the actual therapy content is important. The term RAGT describes the modality by which the therapy is administered, but it does not define the therapy content. In case of the Lokomat, therapy content is influenced by a range of parameters with which the therapy can be optimally adapted to the individual patient. The three most commonly adapted parameters available to all Lokomat users are the regulation of gait speed, the amount of unloading via a harness, the so-called body weight support, and a scaling factor for the forcefield that keeps the legs on the desired spatiotemporal trajectory, the so-called Guidance Force [2]. Additional features include virtual reality environments to increase patient motivation and activity via “gamification” of the therapy [37], the FreeD module [38] to facilitate a physiological weight shift, and a newer control mode named Path Control to allow temporal variability [39]. The availability of these additional features depends on the version of the Lokomat. There is some, albeit limited, evidence from clinical trials that the role of therapy parameters might be important [40, 41]. Kuo et al., recently could show in a retrospective study that the trajectory of therapy parameters during RAGT over time correlates with the improvement of walking function [42].
It can be concluded that the therapy content is a key aspect when interpreting the effectiveness of RAGT. Nevertheless, in the existing literature, it has not yet been sufficiently investigated how the therapy content, in terms of therapy parameters, influences therapy success. A potential reason for the limited evidence might be that, due to the heterogeneous patient groups trained, the ideal Lokomat parameters vary between patients. Several studies have highlighted the importance of the therapist in individualizing therapy [38, 43,44,45]. However, since there are currently no guidelines, each clinic or even therapist has developed their own opinion and preferences on how to adjust therapy parameters. Nevertheless, an appropriate reporting of the applied therapy parameters is key to the successful clinical transfer of study results.
The aim of this scoping review was therefore to evaluate how the currently available clinical studies report the applied Lokomat parameter settings and map the current literature on Lokomat therapy parameters. This should inform Lokomat practitioners about existing strategies and inform future research on tailoring RAGT.
Methods
The protocol of this review was developed following the PRISMA extension for scoping reviews [46]. A completed checklist can be found in Additional file 1. The protocol of the scoping review was not published in advance. Three literature databases were searched for articles that investigated Lokomat therapy: PubMed, Embase and Scopus. Results were compared with the Hocoma literature database to identify potentially missed articles [47]. Search terms were designed to include at least one keyword from each of the following three categories: (1) study population (“patient”, “cerebral palsy”, etc.), (2) device (“Lokomat”, “robot” or “electromechanical”) and (3) activity (“walking”, “gait” or “Locomotion”). The exact search terms can be found in Additional file 2. The search results were retrieved on January 19th, 2021 and updated on February 22nd, 2022.
Titles, abstracts and full texts were screened independently by the two review authors to identify all original articles that met the following eligibility criteria: (1) The study population involved children, adolescents, or adults with a diagnosed neurological mobility impairment, (2) the study investigated Lokomat therapy. Reasons for exclusion were: (1) wrong devices (not Lokomat), (2) wrong study population (e.g. healthy subjects, orthopedic patients), (3) studies that focused only on assessments or technology, (4) wrong publication types (including reviews, editorials or letters) and (5) languages other than English or German. Conflicts were resolved by discussion until mutual agreement. The open source program Rayyan was used for the screening process [48].
The studies were divided into two groups: (1) Clinical studies that investigated the effectiveness of the therapy in multiple patients over multiple therapy sessions, (2) other studies that investigated Lokomat therapy, e.g. short-term effects within one therapy session. We assessed the quality of reporting for all clinical studies and used the articles from the group other studies as an additional source to provide an overview of the current evidence on the Lokomat parameters. Information about the therapy parameters, any rationale for their administration and any findings about these parameters were extracted from all included studies. In the scope of this study, therapy parameters included body weight support, Guidance Force, Path Control and gait speed. Although it is important to distinguish between Guidance Force and Path Control in the clinical context, in this text we group both parameters under the term robotic assistance.
Reporting quality assessment framework
To assess the reporting quality of Lokomat therapy parameters, we developed a framework to evaluate the three most common parameters gait speed, body weight support and robotic assistance for all clinical studies. Four different categories were included to cover different aspects important for clinical transfer and the usability for a potential meta-analysis:
-
1.
Reference Referencing a specific parameter illustrates that the therapists/scientists were aware of this parameter and readers can assume that certain considerations were made regarding this parameter.
-
2.
Strategy This category refers to the mentioning of a strategy that guides the adjustment of a specific parameter. The reporting of the strategy is important as strategies might vary between studies. For example, the transfer of study results obtained by a therapy with a constant gait speed may not be comparable to results obtained by a therapy in a similar group of patients but with a progressively increasing gait speed. Mentioning a strategy therefore belongs to the minimum information needed by the reader to place study results within the context of their own work.
-
3.
Limits The third category refers to setting boundaries for the therapy parameters. Often therapists do not use the full range of available therapy parameters. For example, therapists might never use a body weight support above 50%, nor reduce it below a minimum of 10%. A progressive reduction within these limits might mean something different than a progressive reduction from 100% body weight support to 50% body weight support. Therefore, this information complements the general strategy and helps to translate a therapy approach from a clinical study with promising results into the clinical setting.
-
4.
Actual settings The intended strategy referred to in the first three categories might differ from the actually applied settings of parameters during therapy. For example, if authors intend to substantially decrease Guidance Force, but were not able to decrease it below 90%, this is a relevant information to judge whether the presented approach was successful. Moreover, knowledge about the actual settings is the main prerequisite for meta-analyses.
The complete framework with scoring rules can be found in Table 1. The points obtained for each individual category were added to a sum score per clinical study for each of the three parameters. The maximum number of points per parameter was 8. In addition, medians and interquartile ranges per parameter were calculated across all clinical studies to quantify the overall quality.
Levels of reporting quality
To provide guidance for authors of future Lokomat publications, we introduced a traffic light system for the reporting of Lokomat parameters. Studies with 0–2 points were categorized to have a poor reporting quality. These studies do not allow a transfer of a therapy approach to clinical practice. Studies with 3–6 points were categorized to have a limited reporting quality. These studies allow some transfer of strategies but are not eligible for meta-analyses. Finally, studies with the highest scores, 7–8, were categorized to have a sufficient reporting quality and are expected to be eligible for full clinical transfer and suitable for meta-analyses.
Results
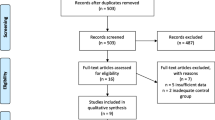
The complete overview of the screening and selection procedure according to the PRISMA guidelines [49] is shown in Fig. 1.
The reporting of the gait speed in clinical studies was rated with a median of 3 and an interquartile range (IQR) of 2, the reporting of the body weight support with 3 (IQR: 3) and the reporting of the robotic assistance with 1 (IQR: 3.5). All individual sum scores by study and parameter can be found in Additional file 3.
Within the first category, Reference, 83% of the clinical studies mentioned the gait speed and thus received 1 point, followed by 79% that mentioned the body weight support and 51% that mentioned the robotic assistance (Fig. 2A). In terms of the subdivisions of robotic assistance, a single clinical study mentioned the application of Path Control Guidance Force.
The dark portion in A illustrates the number of studies that mentioned the given Lokomat parameter. This corresponds to all studies that received one point in category Reference within the reporting framework. In B, the dark portion refers to all studies that reported the actual Lokomat parameter setting in some form. This corresponds to at least one or more points in category Actual Settings within the framework
Within the second category, Strategy, 69% reported a strategy for the gait speed and body weight support and thus received 1 point. Forty-one percent reported a strategy for the parameter robotic assistance. In detail, most of the studies progressively increased gait speed and decreased bodyweight support (Fig. 3A). Six percent of the clinical studies reported a fixed gait speed, around 4.2% of the studies reported a fixed body weight support. A progressive reduction in Guidance Force was less common, but was still reported in 32% of the studies whereas 6.3% reported a fixed Guidance Force.
Around 50% of the clinical studies reported in some form, which parameter they prioritized to personalize or progress the therapy. Body weight support and gait speed were cited as the first or most important parameter in 15% and 14%, respectively, of the studies (Fig. 3B). About 18% reported simultaneous adaptions or combinations of more than one parameter and only one single study reported Guidance Force as the most important factor. Approximately half of the studies did not report any kind of prioritization of parameters.
For the third category, Limits, 38% of all clinical studies reported one limit for the gait speed and thus received 1 point, and 16% of all clinical studies reported both lower and upper limits and received the maximum of 2 points. For the body weight support, 30% of the clinical studies reported one limit and 30% both limits. Finally, for the Guidance Force, 28% reported one limit and 7% both limits.
Less than one-third of all clinical studies reported information about the Actual settings used (Fig. 2B). Most commonly, an actual gait speed was reported in 28% of the cases, followed by body weight support (18%), and Guidance Force (17%). These fractions correspond to all studies that received at least 1 point in the category actual settings.
Discussion
The present scoping review is, to the best of our knowledge, the first article summarizing the reporting of and evidence on the choice of Lokomat parameters. The results clearly illustrate that therapy parameters play a minor role in most of the currently published clinical studies. While there are numerous systematic reviews summarizing the clinical evidence for RAGT for different pathologies, the majority has not paid much attention to the actual therapy content of RAGT. Figure 2 illustrates that many authors appear to consider the parameters in some form, but only a small fraction provides detailed information about their actual settings in therapy. The more detailed the reporting according to our framework needs to be, the lower the proportion of studies that meets the requirements for points within these categories. This might seem logical, as a more detailed reporting requires more effort by the authors, but this decrease in reporting quality might also indicate a discrepancy between the importance therapists attribute to the parameters and the attention that they receive in the literature. While the high number of studies that consider at least some form of adjustment indicates that some importance is attributed to Lokomat parameters, the low quality of the reporting illustrates that the parameters are largely neglected in the scientific debate.
Despite the poor reporting quality within our framework, some information on the therapy parameters is available and we found that various strategies for their adjustment exist. While these different strategies might potentially be due to specific rehabilitation goals or different patient populations, it is currently not possible to underline most of these strategies with evidence and further clarifying studies are necessary. This also applies to differences and similarities across diagnoses.
The evidence currently available for therapy parameters comes predominantly from research-oriented studies that investigated reactions of patient groups during a single therapy session. Strategies and key findings on Lokomat parameters are summarized in the following sections.
Gait speed
While most of the studies tend to increase the gait speed as the patients improve [10, 14, 20, 21, 24, 50,51,52,53,54,55,56,57], others even reduce the treadmill speed [41, 58,59,60,61]. Little research has been conducted on the influence of gait speed. However, research suggests that an increase in gait speed also increases the heart rate and thus the intensity of the therapy [62]. Along the same lines, research on patients with stroke and cerebral palsy suggests that an increase in gait speed increases the muscle activation [63, 64]. Nevertheless, there is some evidence that supports a reduction in walking speed with the purpose of activating supraspinal centers [41, 58, 60, 61].
Body weight support
Variable strategies do also exist for the body weight support. While in the majority of the studies, the support never exceeded 50% of the body weight [8,9,10, 14, 15, 18, 19, 21, 22, 24,25,26,27, 32, 41, 50,51,52, 54, 56, 58, 65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95], other studies never decreased the support below 50% [96,97,98,99,100,101,102]. Current evidence on the body weight support suggests that a decrease in the body weight support can increase the metabolic costs [103] and can elicit higher heart rates [62]. This is related to the fact that a reduction in body weight support elicits a higher muscle activation [63, 64, 104, 105]. However, the muscle synergies were found to be robust across different levels of body weight support [105]. Besides these direct effects, there is some evidence that the body weight support interacts with the effects of other therapy parameters. For example, a high body weight support attenuated the effects of gait speed and Guidance Force, suggesting that load bearing is crucial for an adequate patient activity [63].
Robotic assistance
While some therapists are in favor of minimizing the interactions between the robot and the patients [106] and aim for a low Guidance Force, others proposed to perform resistance training with the Lokomat [57]. The current evidence stems mostly from research oriented studies which generally take a look at short term effects but not the effectiveness of the therapy. König et al. found that a reduction of the Guidance Force did not significantly increase heart rate [62]. However, a reduction of the Guidance Force can increase the muscle activation [63, 64, 104]. Cherni et al. found a non-trivial influence of Guidance Force on muscle activations, partially attenuated previous results [105]. A pilot study could also show that during learning a trajectory tracking task in the Lokomat with a low Guidance Force, muscle activation could be increased and tracking errors could be reduced [106]. For Path Control, research suggests that its application normalizes the muscle activation patterns [38, 107].
Although these findings emphasize that therapy parameters alter the physiologic responses during the therapy and might be important to consider, they do not allow conclusions to be drawn about the effectiveness of specific adjustments of therapy parameters on therapy success. Further research is needed to advance the field and enable therapists to choose the best possible therapy parameter setting for their patients. The simplest form to gather additional evidence would be clinical studies where single parameters are being manipulated as it has been done in a very limited number of studies. For example, Park et al. [40], albeit using a different device, were able to show that reducing robotic assistance could be beneficial for the rehabilitation outcome. Similarly, Rodrigues et al. could show that the gait speed could influence the success of the training program [41]. However, simple manipulations of a single parameter might not be sufficient as complex interactions exist between the different therapy parameters [63]. Designing clinical studies that investigate combined effects of parameters would require an tremendous effort that hardly any clinic can afford.
A possible alternative approach would be to synthesize results of various studies to evaluate the contributions of therapy parameters on the effectiveness of RAGT. Such a synthesis is currently already being impeded by three main factors: (1) There are many different pathologies involved, (2) many different outcome measures used and (3) different strategies applied as mentioned above. In addition, the finding that only 4–7% of the studies did report parameter settings in a way that they could be used for such a meta-analysis (Fig. 4), makes the clinical transfer of such results very difficult. Even more so, since these 4–7% include mostly studies that applied a fixed parameter setting which clinically rarely makes sense. Even though many studies reported an increase or a decrease in one or more parameters as the therapeutic strategy, a synthesis of the study results without knowing the actual magnitude of such a change would make little sense. For example, a decrease in guidance force from 100 to 80% could have a very different influence on the outcome compared to a decrease from 100 to 30%. We present the available information per study to interested readers in Additional file 3.
The figure illustrates the distribution of the reporting quality by parameter. All clinical studies were scored for the three categories Gait speed, Bodyweight support and Robotic Assistance with the scheme in Table 1. Scores from 0 to 2 correspond to a poor reporting not eligible to allow a comparison of studies, scores from 3 to 6 to a limited reporting that provide some insights in the therapy approach and allow to transfer the strategy, and scores from 7 to 8 to a sufficient reporting to include the results in a meta-analysis and judge whether the strategy matches the actually performed therapy
Despite the current shortcomings, we believe that meta-analyses including the therapy parameters would have a high potential for further knowledge gain in the future and therefore, the reporting of the therapy parameters in clinical studies should to be improved.
In addition, as illustrated by Fig. 4, depending on the parameter, 39–64% of the studies do not allow any transfer of the therapy strategy employed into the clinical setting. Therapists, who want to know whether or not to use Lokomat therapy for a particular patient, cannot adopt the strategy from studies with promising results, nor can they assess the trustworthiness of the results and compare the choice of the therapy parameters with their own clinical experience. The results of these studies are therefore difficult to translate and as such, their usefulness for therapists is very limited. Despite the poor overall reporting quality on therapy parameters, we would like to encourage RAGT practitioners to take a close look at the therapy content when interpreting clinical study results. Furthermore, an improved reporting would enable an evidence-based translation into Lokomat therapy practice.
The reporting quality assessment framework developed in this review could serve researchers to assess the quality of their reporting before submission of a Lokomat-related paper and could help establishing a minimal information standard similar to the ones of other research areas such as biochemistry [108]. To encourage adoption of such reporting standards by as many scientists as possible, it should be easy to implement and not require much additional effort. We suggest that clinical studies investigating RAGT should at least describe the actual training settings of the three parameters gait speed, body weight support and robotic assistance. Authors should consider reporting at least an average value and a standard deviation for each category per training and subject. This would be equivalent to 7 or more points within the framework presented above. With this fairly simple approach the quality of reporting could be improved. It would allow therapists and researchers to better compare and synthesize studies, as well as facilitate the transfer of promising study findings into clinical practice.
In conclusion, there is an underreporting of therapy parameters in the literature, although there is evidence to suggest that the therapy parameter settings are important. To enable further advances in the field, particularly with regards to the effectiveness of certain parameter settings for individual patient groups, a common minimal reporting standard is proposed. We invite researchers, who are about to publish Lokomat-related research, to make use of the developed reporting framework to check and improve the reporting quality of their work. Furthermore, current evidence suggests that therapy parameters might be important and RAGT practitioners should pay attention to the reported therapy content when translating research findings to their own clinical practice.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- RAGT:
-
Robot-assisted gait therapy
References
Hocoma. www.hocoma.com/about-us.
Colombo G, Wirz M, Dietz V. Driven gait orthosis for improvement of locomotor training in paraplegic patients. Spinal Cord. 2001;39(5):252–5.
Mehrholz J, Thomas S, Kugler J, Pohl M, Elsner B. Electromechanical-assisted training for walking after stroke. Cochrane Database Syst Rev. 2020. https://doi.org/10.1002/14651858.CD006185.pub5.
Alwardat M, Etoom M, Al Dajah S, Schirinzi T, Di Lazzaro G, Sinibaldi Salimei P, et al. Effectiveness of robot-assisted gait training on motor impairments in people with Parkinson’s disease: a systematic review and meta-analysis. Int J Rehabil Res. 2018;41(4):287–96.
Yeh S-W, Lin L-F, Tam K-W, Tsai C-P, Hong C-H, Kuan Y-C. Efficacy of robot-assisted gait training in multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. 2020;41:102034.
Carvalho I, Pinto SM, Chagas DdV, Praxedes dos Santos JL, de Sousa Oliveira T, Batista LA. Robotic gait training for individuals with cerebral palsy: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98(11):2332–44.
Nam Y-G, Lee JW, Park JW, Lee HJ, Nam KY, Park JH, et al. Effects of electromechanical exoskeleton-assisted gait training on walking ability of stroke patients: a randomized controlled trial. Arch Phys Med Rehabil. 2019;100(1):26–31.
Bang D-H, Shin W-S. Effects of robot-assisted gait training on spatiotemporal gait parameters and balance in patients with chronic stroke: a randomized controlled pilot trial. NeuroRehabilitation. 2016;38(4):343–9.
Bergmann J, Krewer C, Jahn K, Müller F. Robot-assisted gait training to reduce pusher behavior. Neurology. 2018;91(14):e1319–27.
Dundar U, Toktas H, Solak O, Ulasli AM, Eroglu S. A comparative study of conventional physiotherapy versus robotic training combined with physiotherapy in patients with stroke. Top Stroke Rehabil. 2014;21(6):453–61.
Uçar DE, Paker N, Buǧdayci D. Lokomat: a therapeutic chance for patients with chronic hemiplegia. NeuroRehabilitation. 2014;34(3):447–53.
Furnari A, Calabrò RS, De Cola MC, Bartolo M, Castelli A, Mapelli A, et al. Robotic-assisted gait training in Parkinson’s disease: a three-month follow-up randomized clinical trial. Int J Neurosci. 2017;127(11):996–1004.
Klobucká S, Klobucký R, Kollár B. Effect of robot-assisted gait training on motor functions in adolescent and young adult patients with bilateral spastic cerebral palsy: a randomized controlled trial. NeuroRehabilitation. 2020;47(4):495–508.
Schwartz I, Sajin A, Fisher I, Neeb M, Shochina M, Katz-Leurer M, et al. The effectiveness of locomotor therapy using robotic-assisted gait training in subacute stroke patients: a randomized controlled trial. PM&R. 2009;1(6):516–23.
Schwartz I, Sajina A, Neeb M, Fisher I, Katz-Luerer M, Meiner Z. Locomotor training using a robotic device in patients with subacute spinal cord injury. Spinal Cord. 2011;49(10):1062–7.
Wallard L, Dietrich G, Kerlirzin Y, Bredin J. Effects of robotic gait rehabilitation on biomechanical parameters in the chronic hemiplegic patients. Neurophysiol Clin Neurophysiol. 2015;45(3):215–9.
Wallard L, Dietrich G, Kerlirzin Y, Bredin J. Effect of robotic-assisted gait rehabilitation on dynamic equilibrium control in the gait of children with cerebral palsy. Gait Posture. 2018;60:55–60.
Belas dos Santos M, Barros de Oliveira C, dos Santos A, Garabello Pires C, Dylewski V, Arida RM. A comparative study of conventional physiotherapy versus robot-assisted gait training associated to physiotherapy in individuals with ataxia after stroke. Behav Neurol. 2018;2018:1–6.
Carda S, Invernizzi M, Baricich A, Comi C, Croquelois A, Cisari C. Robotic gait training is not superior to conventional treadmill training in Parkinson disease. Neurorehabil Neural Repair. 2012;26(9):1027–34.
Clerici I, Ferrazzoli D, Maestri R, Bossio F, Zivi I, Canesi M, et al. Rehabilitation in progressive supranuclear palsy: effectiveness of two multidisciplinary treatments. PLoS ONE. 2017;12(2):e0170927.
Field-Fote EC, Roach KE. Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys Ther. 2011;91(1):48–60.
Fundarò C, Maestri R, Ferriero G, Chimento P, Taveggia G, Casale R. Self-selected speed gait training in Parkinson’s disease: robot-assisted gait training with virtual reality versus gait training on the ground. Eur J Phys Rehabil Med. 2019;55(4):456–62.
Hidler J, Nichols D, Pelliccio M, Brady K, Campbell DD, Kahn JH, et al. Multicenter randomized clinical trial evaluating the effectiveness of the Lokomat in subacute stroke. Neurorehabil Neural Repair. 2009;23(1):5–13.
Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke. Stroke. 2008;39(6):1786–92.
Labruyère R, van Hedel HJA. Strength training versus robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study in patients depending on walking assistance. J Neuroeng Rehabil. 2014;11(1):4.
Mayr A, Quirbach E, Picelli A, Kofler M, Smania N, Saltuari L. Early robot-assisted gait retraining in non-ambulatory patients with stroke: a single blind randomized controlled trial. Eur J Phys Rehabil Med. 2019;54(6):819–26.
Straudi S, Manfredini F, Lamberti N, Martinuzzi C, Maietti E, Basaglia N. Robot-assisted gait training is not superior to intensive overground walking in multiple sclerosis with severe disability (the RAGTIME study): a randomized controlled trial. Mult Scler J. 2020;26(6):716–24.
Morone G, Bragoni M, Iosa M, De Angelis D, Venturiero V, Coiro P, et al. Who may benefit from robotic-assisted gait training? Neurorehabil Neural Repair. 2011;25(7):636–44.
van Hedel HJA, Meyer-Heim A, Rüsch-Bohtz C. Robot-assisted gait training might be beneficial for more severely affected children with cerebral palsy. Dev Neurorehabil. 2016;19(6):410–5.
Schroeder AS, Von Kries R, Riedel C, Homburg M, Auffermann H, Blaschek A, et al. Patient-specific determinants of responsiveness to robot-enhanced treadmill therapy in children and adolescents with cerebral palsy. Dev Med Child Neurol. 2014;56(12):1172–9.
Beretta E, Storm FA, Strazzer S, Frascarelli F, Petrarca M, Colazza A, et al. Effect of robot-assisted gait training in a large population of children with motor impairment due to cerebral palsy or acquired brain injury. Arch Phys Med Rehabil. 2020;101(1):106–12.
Dierick F, Dehas M, Isambert J-L, Injeyan S, Bouché A-F, Bleyenheuft Y, et al. Hemorrhagic versus ischemic stroke: who can best benefit from blended conventional physiotherapy with robotic-assisted gait therapy? PLoS ONE. 2017;12(6):e0178636.
Alcobendas-Maestro M, Esclarín-Ruz A, Casado-López R. Lokomat training, cervical versus thoracic spinal cord injuries: comparative study. In: Biosystems and biorobotics. 2013. p. 229–31.
Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation: a 35-year odyssey. Am Psychol. 2002;57(9):705–17.
Fahey M, Brazg G, Henderson CE, Plawecki A, Lucas E, Reisman DS, et al. The value of high intensity locomotor training applied to patients with acute-onset neurologic injury. Arch Phys Med Rehabil. 2021. https://doi.org/10.1016/j.apmr.2020.09.399.
Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126(4):866–72.
Mirelman A, Patritti BL, Bonato P, Deutsch JE. Effects of robot-virtual reality compared with robot alone training on gait kinetics of individuals post stroke. In: 2007 virtual rehabilitation. IEEE; 2007. p. 65–9.
Aurich-Schuler T, Grob F, van Hedel HJA, Labruyère R. Can Lokomat therapy with children and adolescents be improved? An adaptive clinical pilot trial comparing Guidance force, Path control, and FreeD. J Neuroeng Rehabil. 2017;14(1):76.
Duschau-Wicke A, von Zitzewitz J, Caprez A, Lunenburger L, Riener R. Path control: a method for patient-cooperative robot-aided gait rehabilitation. IEEE Trans Neural Syst Rehabil Eng. 2010;18(1):38–48.
Park IJ, Park J-H, Seong HY, You JSH, Kim SJ, Min JH, et al. Comparative effects of different assistance force during robot-assisted gait training on locomotor functions in patients with subacute stroke. Am J Phys Med Rehabil. 2019;98(1):58–64.
Rodrigues TA, Goroso DG, Westgate PM, Carrico C, Batistella LR, Sawaki L. Slow versus fast robot-assisted locomotor training after severe stroke. Am J Phys Med Rehabil. 2017;96(10):S165–70.
Kuo C-Y, Liu C-W, Lai C-H, Kang J-H, Tseng S-H, Su EC-Y. Prediction of robotic neurorehabilitation functional ambulatory outcome in patients with neurological disorders. J Neuroeng Rehabil. 2021;18(1):174.
Aurich Schuler T, Müller R, Van Hedel HJ. Leg surface electromyography patterns in children with neuro-orthopedic disorders walking on a treadmill unassisted and assisted by a robot with and without encouragement. J Neuroeng Rehabil. 2013;10(1):78.
Esquenazi A, Maier IC, Schuler TA, Beer SM, Borggraefe I, Campen K, et al. Clinical application of robotics and technology in the restoration of walking. In: Reinkensmeyer DJ, Dietz V, editors., et al., Neurorehabilitation technology. Cham: Springer International Publishing; 2016. p. 223–48.
Warken B, Graser J, Ulrich T, Borggraefe I, Heinen F, Meyer-Heim A, et al. Practical recommendations for robot-assisted treadmill therapy (Lokomat) in children with cerebral palsy: indications, goal setting, and clinical implementation within the WHO-ICF framework. Neuropediatrics. 2015;46(04):248–60.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Hocoma Research Database. https://knowledge.hocoma.com/research/#publications.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;n71.
Mıdık M. Effects of robot-assisted gait training on lower extremity strength, functional independence, and walking function in men with incomplete traumatic spinal cord injury. Turk J Phys Med Rehabil. 2020;66(1):54–9.
Ruiz J, Labas MP, Triche EW, Lo AC. Combination of robot-assisted and conventional body-weight–supported treadmill training improves gait in persons with multiple sclerosis. J Neurol Phys Ther. 2013;37(4):187–93.
Schwartz I, Sajin A, Moreh E, Fisher I, Neeb M, Forest A, et al. Robot-assisted gait training in multiple sclerosis patients: a randomized trial. Mult Scler J. 2012;18(6):881–90.
Aras B. Comparison of the effectiveness of partial body weight-supported treadmill exercises, robotic-assisted treadmill exercises, and anti-gravity treadmill exercises in spastic cerebral palsy. Turk J Phys Med Rehabil. 2019;65(4):361–70.
Bae Y-H, Lee SM, Ko M. Comparison of the effects on dynamic balance and aerobic capacity between objective and subjective methods of high-intensity robot-assisted gait training in chronic stroke patients: a randomized controlled trial. Top Stroke Rehabil. 2017;24(4):309–13.
Cherni Y, Ballaz L, Lemaire J, Begon M. Effects of robot-assisted gait training on walking abilities of children with cerebral palsy. Neurophysiol Clin. 2019;49(6):421.
Husemann B, Müller F, Krewer C, Heller S, Koenig E. Effects of locomotion training with assistance of a robot-driven gait orthosis in hemiparetic patients after stroke: a randomized controlled pilot study. Stroke. 2007;38:349–54.
Lam T, Pauhl K, Ferguson A, Malik R, Krassioukov A, Eng J. A new training paradigm using robot-applied resistance to enhance skilled walking in people with spinal cord injury. Physiotherapy. 2015;101:e813–4.
Danzl MM, Chelette KC, Lee K, Lykins D, Sawaki L. Brain stimulation paired with novel locomotor training with robotic gait orthosis in chronic stroke: a feasibility study. NeuroRehabilitation. 2013;33(1):67–76.
Gorman P, Scott W, York H, Theyagaraj M, Price-Miller N, McQuaid J, et al. Robotic treadmill training improves peak exercise capacity in chronic incomplete spinal cord injury: a pilot controlled clinical trial. Top Spinal Cord Inj Rehabil. 2011;16:40–1.
Prideaux N, van den Berg M, Drummond C, Barr C. Augmented performance feedback during robotic gait therapy results in moderate intensity cardiovascular exercise in subacute stroke. J Stroke Cerebrovasc Dis. 2020;29(6):104758.
Raithatha R, Carrico C, Powell ES, Westgate PM, Chelette KC II, Lee K, et al. Non-invasive brain stimulation and robot-assisted gait training after incomplete spinal cord injury: a randomized pilot study. NeuroRehabilitation. 2016;38(1):15–25.
Koenig A, Caruso A, Bolliger M, Somaini L, Omlin X, Morari M, et al. Model-based heart rate control during robot-assisted gait training. In: 2011 IEEE international conference on robotics and automation. IEEE; 2011. p. 4151–6.
van Kammen K, Reinders-Messelink HA, Elsinghorst AL, Wesselink CF, Meeuwisse-de Vries B, van der Woude LHV, et al. Amplitude and stride-to-stride variability of muscle activity during Lokomat guided walking and treadmill walking in children with cerebral palsy. Eur J Paediatr Neurol. 2020;29:108–17.
van Kammen K, Boonstra AM, van der Woude LHV, Visscher C, Reinders-Messelink HA, den Otter R. Lokomat guided gait in hemiparetic stroke patients: the effects of training parameters on muscle activity and temporal symmetry. Disabil Rehabil. 2020;42(21):2977–85.
Beretta E, Molteni E, Biffi E, Morganti R, Avantaggiato P, Strazzer S. Robotically-driven orthoses exert proximal-to-distal differential recovery on the lower limbs in children with hemiplegia, early after acquired brain injury. Eur J Paediatr Neurol. 2018;22(4):652–61.
Beretta E, Romei M, Molteni E, Avantaggiato P, Strazzer S. Combined robotic-aided gait training and physical therapy improve functional abilities and hip kinematics during gait in children and adolescents with acquired brain injury. Brain Inj. 2015;29(7–8):955–62.
Molteni E, Beretta E, Altomonte D, Formica F, Strazzer S. Combined robotic-aided gait training and 3D gait analysis provide objective treatment and assessment of gait in children and adolescents with Acquired Hemiplegia. In: 2015 37th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC). United States: IEEE; 2015. p. 4566–9.
Bertolucci F, Di Martino S, Orsucci D, Ienco EC, Siciliano G, Rossi B, et al. Robotic gait training improves motor skills and quality of life in hereditary spastic paraplegia. NeuroRehabilitation. 2015;36(1):93–9.
Chang WH, Kim MS, Huh JP, Lee PKW, Kim Y-H. Effects of robot-assisted gait training on cardiopulmonary fitness in subacute stroke patients. Neurorehabil Neural Repair. 2012;26(4):318–24.
Cherni Y, Ballaz L, Lemaire J, Dal Maso F, Begon M. Effect of low dose robotic-gait training on walking capacity in children and adolescents with cerebral palsy. Neurophysiol Clin. 2020;50(6):507–19.
Cheung EYY, Yu KKK, Kwan RLC, Ng CKM, Chau RMW, Cheing GLY. Effect of EMG-biofeedback robotic-assisted body weight supported treadmill training on walking ability and cardiopulmonary function on people with subacute spinal cord injuries—a randomized controlled trial. BMC Neurol. 2019;19(1):140.
Chin LF, Lim WS, Kong KH. Evaluation of robotic-assisted locomotor training outcomes at a rehabilitation centre in Singapore. Singapore Med J. 2010;51(9):709–15.
Chisari C, Bertolucci F, Monaco V, Venturi M, Simonella C, Micera S, et al. Robot-assisted gait training improves motor performances and modifies Motor Unit firing in poststroke patients. Eur J Phys Rehabil Med. 2015;51(1):59–69.
Cho DY, Park S-W, Lee MJ, Park DS, Kim EJ. Effects of robot-assisted gait training on the balance and gait of chronic stroke patients: focus on dependent ambulators. J Phys Ther Sci. 2015;27(10):3053–7.
Esquenazi A, Lee S, Wikoff A, Packel A, Toczylowski T, Feeley J. A comparison of locomotor therapy interventions: partial-body weight−supported treadmill, Lokomat, and G-EO training in people with traumatic brain injury. PM&R. 2017;9(9):839–46.
Esquenazi A, Lee S, Packel AT, Braitman L. A randomized comparative study of manually assisted versus robotic-assisted body weight supported treadmill training in persons with a traumatic brain injury. PM&R. 2013;5(4):280–90.
Fundarò C, Giardini A, Maestri R, Traversoni S, Bartolo M, Casale R. Motor and psychosocial impact of robot-assisted gait training in a real-world rehabilitation setting: a pilot study. PLoS ONE. 2018;13(2):e0191894.
Hwang S, Kim H-R, Han Z-A, Lee B-S, Kim S, Shin H, et al. Improved gait speed after robot-assisted gait training in patients with motor incomplete spinal cord injury: a preliminary study. Ann Rehabil Med. 2017;41(1):34.
Kim HY, Shin J-H, Yang SP, Shin MA, Lee SH. Robot-assisted gait training for balance and lower extremity function in patients with infratentorial stroke: a single-blinded randomized controlled trial. J Neuroeng Rehabil. 2019;16(1):99.
Lewek MD, Cruz TH, Moore JL, Roth HR, Dhaher YY, Hornby TG. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89(8):829–39.
Lo AC, Chang VC, Gianfrancesco MA, Friedman JH, Patterson TS, Benedicto DF. Reduction of freezing of gait in Parkinson’s disease by repetitive robot-assisted treadmill training: a pilot study. J Neuroeng Rehabil. 2010;7(1):51.
Lo AC, Triche EW. Improving gait in multiple sclerosis using robot-assisted, body weight supported treadmill training. Neurorehabil Neural Repair. 2008;22(6):661–71.
Mayr A, Kofler M, Quirbach E, Matzak H, Fröhlich K, Saltuari L. Prospective, blinded, randomized crossover study of gait rehabilitation in stroke patients using the Lokomat gait orthosis. Neurorehabil Neural Repair. 2007;21(4):307–14.
Mazzoleni S, Boldrini E, Laschi C, Carrozza MC, Stampacchia G, Rossi B. Changes on EMG activation in healthy subjects and incomplete SCI patients following a robot-assisted locomotor training. In: 2011 IEEE international conference on rehabilitation robotics. IEEE; 2011. p. 1–6.
Meyer-Heim A, Borggraefe I, Ammann-Reiffer C, Berweck S, Sennhauser FH, Colombo G, et al. Feasibility of robotic-assisted locomotor training in children with central gait impairment. Dev Med Child Neurol. 2007;49(12):900–6.
Mustafaoglu R, Erhan B, Yeldan I, Gunduz B, Tarakci E. Does robot-assisted gait training improve mobility, activities of daily living and quality of life in stroke? A single-blinded, randomized controlled trial. Acta Neurol Belg. 2020;120(2):335–44.
Nardo A, Anasetti F, Servello D, Porta M. Quantitative gait analysis in patients with Parkinson treated with deep brain stimulation: the effects of a robotic gait training. NeuroRehabilitation. 2014;35(4):779–88.
Piira A, Lannem A, Sørensen M, Glott T, Knutsen R, Jørgensen L, et al. Robot-assisted locomotor training did not improve walking function in patients with chronic incomplete spinal cord injury: a randomized clinical trial. J Rehabil Med. 2019;51(5):385–9.
Taveggia G, Borboni A, Mulé C, Villafañe JH, Negrini S. Conflicting results of robot-assisted versus usual gait training during postacute rehabilitation of stroke patients. Int J Rehabil Res. 2016;39(1):29–35.
Erdoğan Uçar D, Paker N, Buğdaycı D. Lokomat: a therapeutic chance for patients with chronic hemiplegia. NeuroRehabilitation. 2014;34(3):447–53.
van Silfhout L, Váňa Z, Pĕtioký J, Edwards MJR, Bartels RHMA, van de Meent H, et al. Highest ambulatory speed using Lokomat gait training for individuals with a motor-complete spinal cord injury: a clinical pilot study. Acta Neurochir (Wien). 2020;162(4):951–6.
Vaney C, Gattlen B, Lugon-Moulin V, Meichtry A, Hausammann R, Foinant D, et al. Robotic-assisted step training (Lokomat) not superior to equal intensity of over-ground rehabilitation in patients with multiple sclerosis. Neurorehabil Neural Repair. 2012;26(3):212–21.
Yildirim MA, Öneş K, Gökşenoğlu G. Early term effects of robotic assisted gait training on ambulation and functional capacity in patients with spinal cord injury. TURK J Med Sci. 2019;49(3):838–43.
Yun N, Joo MC, Kim S-C, Kim M-S. Robot-assisted gait training effectively improved lateropulsion in subacute stroke patients: a single-blinded randomized controlled trial. Eur J Phys Rehabil Med. 2019;54(6):827–36.
Zarkovic D, Sorfova M, Tufano JJ, Kutilek P, Viteckova S, Groleger-Srsen K, et al. Effect of robot-assisted gait training on selective voluntary motor control in ambulatory children with cerebral palsy. Indian Pediatr. 2020;57(10):964–6.
Calabrò RS, Naro A, Leo A, Bramanti P. Usefulness of robotic gait training plus neuromodulation in chronic spinal cord injury: a case report. J Spinal Cord Med. 2017;40(1):118–21.
Gorman PH, Scott W, York H, Theyagaraj M, Price-Miller N, McQuaid J, et al. Robotically assisted treadmill exercise training for improving peak fitness in chronic motor incomplete spinal cord injury: a randomized controlled trial. J Spinal Cord Med. 2016;39(1):32–44.
Krewer C, Rieß K, Bergmann J, Müller F, Jahn K, Koenig E. Immediate effectiveness of single-session therapeutic interventions in pusher behaviour. Gait Posture. 2013;37(2):246–50.
Łyp M, Stanisławska I, Witek B, Olszewska-Żaczek E, Czarny-Działak M, Kaczor R. Robot-assisted body-weight-supported treadmill training in gait impairment in multiple sclerosis patients: a pilot study. In: Advances in experimental medicine and biology. United States; 2018. p. 111–5.
Peri E, Turconi AC, Biffi E, Maghini C, Panzeri D, Morganti R, et al. Effects of dose and duration of Robot-Assisted Gait Training on walking ability of children affected by cerebral palsy. Technol Health Care. 2017;25(4):671–81.
Romei M, Montinaro A, Piccinini L, Maghini C, Germiniasi C, Bo I, et al. Efficacy of robotic-assisted gait training compared with intensive task-oriented physiotherapy for children with Cerebral Palsy. In: 2012 4th IEEE RAS & EMBS international conference on biomedical robotics and biomechatronics (BioRob). IEEE; 2012. p. 1890–4.
Tamburella F, Moreno JC, Herrera Valenzuela DS, Pisotta I, Iosa M, Cincotti F, et al. Influences of the biofeedback content on robotic post-stroke gait rehabilitation: electromyographic vs joint torque biofeedback. J Neuroeng Rehabil. 2019;16(1):95.
Krewer C, Müller F, Husemann B, Heller S, Quintern J, Koenig E. The influence of different Lokomat walking conditions on the energy expenditure of hemiparetic patients and healthy subjects. Gait Posture. 2007;26(3):372–7.
Lin J, Hu G, Ran J, Chen L, Zhang X, Zhang Y. Effects of bodyweight support and guidance force on muscle activation during Locomat walking in people with stroke: a cross-sectional study. J Neuroeng Rehabil. 2020;17(1):5.
Cherni Y, Hajizadeh M, Dal Maso F, Turpin NA. Effects of body weight support and guidance force settings on muscle synergy during Lokomat walking. Eur J Appl Physiol. 2021;121(11):2967–80.
Krishnan C, Kotsapouikis D, Dhaher YY, Rymer WZ. Reducing robotic guidance during robot-assisted gait training improves gait function: a case report on a stroke survivor. Arch Phys Med Rehabil. 2013;94(6):1202–6.
Schück A, Labruyère R, Vallery H, Riener R, Duschau-Wicke A. Feasibility and effects of patient-cooperative robot-aided gait training applied in a 4-week pilot trial. J Neuroeng Rehabil. 2012;9(1):31.
Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)—toward standards for microarray data. Nat Genet. 2001;29(4):365–71.
Acknowledgements
Not applicable.
Funding
Open access funding provided by Swiss Federal Institute of Technology Zurich. This review was supported by the Stiftung Cerebral, the Walter Muggli Fund of the ACCENTUS Foundation and the J&K Wonderland Foundation. The funders did not have any role in the design of the study, the data analysis and writing the manuscript.
Author information
Authors and Affiliations
Contributions
FVD and RL developed the search strategy and the assessment framework for this review. They screened the search hits for eligibility, and extracted as well as synthesized and rated the relevant data. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests and there are no financial competing interests to declare in relation to this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA Checklist for Scoping Reviews.
Additional file 2.
Search terms.
Additional file 3.
Information about Settings and Scores per study. Scores from 0 to 2 are marked as red. This group consists of papers that contain a very limited amount of information. In yellow are scores from 3 to 6. These papers report on minimal and/or maximal parameters or the goals that therapists pursue. In green, papers scored with 7–8 are marked. These papers report the actual therapy parameters in a way that they could potentially be used for a meta-analysis.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
van Dellen, F., Labruyère, R. Settings matter: a scoping review on parameters in robot-assisted gait therapy identifies the importance of reporting standards. J NeuroEngineering Rehabil 19, 40 (2022). https://doi.org/10.1186/s12984-022-01017-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-022-01017-3