Abstract
Real-time medication monitoring (RTMM) may potentially enhance adherence to antiretroviral treatment (ART). We describe a participant in an ongoing trial who, shortly after completing trial participation, died of cryptococcal meningitis despite high levels of adherence according to self-report, pill-counts and RTMM (> 99%). However, she evidenced consistently high HIV viral load throughout the 48-week study follow-up. Subsequently, her relatives unsolicitedly returned eight months’ dispensed ART medication that she was supposed to have taken. This brief report illustrates the challenges of adherence measurements including RTMM, and reinforces the need to combine adherence assessments with viral load monitoring in HIV care.
Similar content being viewed by others
Introduction
Sustained adherence to antiretroviral treatment (ART) at levels over 95% among people living with HIV (PLHIV) is required in order to prevent treatment failure and the emergence of drug resistance [1]. Therefore, monitoring of adherence is of paramount importance in the management of PLHIV. In resource-limited settings, self-reported adherence and adherence calculations based on pharmacy refill counts are often used, but have been shown to overestimate actual adherence rates [2]. Several reasons for such over-reporting have been found, including: (1) fear of being expelled from the study and losing the benefits provided by study participation, (2) social distance between study nurses and participants causing mistrust, (3) nursing staff that is perceived as condemning being non-adherent, and (4) counselling provided by study staff that may discourage openness, or alternatively, may be too permissive making misreporting easy [3, 4]. Electronic monitoring devices consisting of either pill bottle caps or pillboxes that record and store the date and time of each opening of the cap or box are generally considered to be the most accurate and reliable adherence assessment method. The assumption underlying electronic medication monitoring is that each pill bottle or pill box opening represents ingestion of medication [5,6,7]. Here we present the case of a woman living with HIV that challenges this assumption. We first describe the ongoing randomized clinical trial in which the subject had participated.
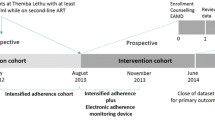
Trial
The trial aims to investigate the effect of two mobile health (mHealth) strategies, real time medication monitoring (RTMM) and use of short message service (SMS), on adherence to treatment among PLHIV in Kilimanjaro, Tanzania. A total of 249 adults on ART who were subjectively judged to be non-adherent by nurse counsellors were recruited from two health centres. The judgement was based on missed clinic visits, reported non-adherence and returning of leftover pills to the clinic. Participants were randomized equally in a ratio of 1:1:1 to receive SMS, RTMM or standard care and followed for 48 weeks. The study was approved by the Kilimanjaro Christian Medical College Research Ethics and Committee (CRERC) and the National Health Research Ethics Committee (NatHRec) of Tanzania.
Our case was randomized to the RTMM arm in which participants use an RTMM-device, the so-called Wisepill device, to take their medication. Each opening of the box is recorded, including the time stamp and status of the battery. This information is instantly sent through mobile data using General Packet Radio Service (GPRS) to a central database which has a secure web-based interface where the usual time of intake (agreed time between patient and healthcare provider) is stored. If the box is not opened on time, the participant receives a short message service (SMS) text on their mobile phone which acts as a reminder to take medication. The RTMM system generates a report that shows pill box openings. This report is then used during bi-monthly consultations with study nurses to discuss adherence and strategies for improvement, if needed. In the trial, adherence in all arms is measured through self-reporting by asking about the number of missed pills during the past month and through pharmacy refill counts by counting the number of leftover pills at each visit. In the RTMM arm, it is also measured through RTMM, in which pill-box openings are considered to indicate medication intakes.
Case
A 38-year old single woman was first diagnosed with HIV in April 2017 and started on ART (tenofovir + lamivudine + efavirenz) in June 2017. Her medical file showed that she had a viral load of 423,000 copies/ml in April 2017 and a CD4-count of 276 cells/µL in June 2017. She was enrolled in our study in February 2018 and at that time was still on the first-line antiretroviral regimen of efavirenz, lamivudine and tenofovir. At enrolment, she had a high viral load of over 200,000 copies/ml, indicating virological failure possibly as a result of treatment non-adherence. As part of participating in the trial (RTMM arm), she reported detailed information about her adherence to treatment during clinic visits. She consistently reported to take all her antiretroviral pills except for one visit where she mentioned to have missed a few pills in the previous month. Her viral load continued to be high (> 200,000 copies/ml) for more than a year during follow-up in our trial despite extensive adherence counselling during study visits. From January 2019 onward, her health gradually deteriorated. She started feeling sick and cryptococcal meningitis was diagnosed. At her last 48 weeks study follow-up visit in February 2019, she looked fatigued and weak. As part of the trial, an exit-interview was scheduled to obtain information about her experience on the usage of RTMM. She did not attend this interview. The patient died of cryptococcal meningitis in July 2019, in spite of having been switched to a second-line antiretroviral regimen of atazanavir, abacavir and lamivudine.
Virological and immunological outcomes
The participant had a viral load of 231,037 copies/ml at enrolment in February 2018. Three months later in May 2018, her CD4 cell count was 56 cells/μL. When the viral load measurement was repeated in August 2018, it remained high at 262,000 copies/mL and again in January 2019 (232,352 copies/mL) and February 2019 (245,932 copies/mL). At the last study visit in February 2019, her CD4-count had decreased to 16 cells/μL. In May 2019, her viral load had decreased to 44 copies/mL and her CD4 count increased to 160 cells/μL.
Adherence to treatment
The participant’s adherence rates according to self-reporting, pharmacy refill monitoring, and RTMM were recorded at clinic visits during the study period. She reported to only have missed 2 pills in the total follow up of 355 days, which corresponds to an adherence rate of 99%. This was confirmed by pharmacy refill counts, according to which adherence over the total period was likewise above 99%. The high adherence rate of 99% was further confirmed by RTMM. Figure 1 shows the monthly adherence graphs that were generated by the RTMM device. The graphs show that most of the time, the patient consistently opened the RTMM device around 8 pm. In the feedback sessions where she received tailored feedback on her adherence reports, she always confirmed that she took all her medication adequately. Only at one visit in July 2018, she mentioned that her intake was not adequate. In addition, she explained that she used her phone alarm as an extra reminder and that her mother also helped reminding her to take medication. She confirmed that she experienced no problems in taking medication. After finishing her last study visit (end of study follow-up), she was invited for an exit-interview which she was not able to attend (Fig. 2).
Leftover pills
After our participant had died, her relatives came unsolicited to bring back leftover medication. In total, pills dispensed for 151 days (45% of the study period) for the first-line regimen were returned, and pills dispensed for 60 days for the second-line regimen were returned (43% of second-line regimen duration). The production dates of the returned first-line pills ranged from March 2017 to March 2018, indicating that those were the pills she had been supposed to be taking during the study. We had to conclude that despite the excellent adherence rates according to all three measures, i.e. self-report, pharmacy refill monitoring, and RTMM, her adherence had actually been low.
This case report describes a woman living with HIV whose adherence indicators showed excellent adherence but were in sharp contrast with her actual health indices. This case report illustrates that opening of the pillbox, the so-called medication event, does not necessarily mean actual pill intake. We can only speculate what made her open the pillboxes but not ingest the medication. Perhaps she wanted to please the researchers and study nurses, or prevent receiving an SMS reminder post-intake-time, or possibly avoid adverse reactions from those close to her. Despite extensive counselling and discussions on her clinical outcomes and adherence as part of the study, the nurse counsellors who provided feedback on the adherence reports were not aware of signals or information of her non-adherence. In addition, she had disclosed her status to relatives, and reported not to have experienced stigmatization but rather received a lot of social support.
Previous studies have shown that opening the device is a good proxy of real intake of pills at the group level [3, 7], but our case clearly illustrates that exceptions exist at the individual level.
The question arises under what circumstances real-time monitoring is most useful. A study in Uganda found that real-time monitoring was only effective in the early stages of ART treatment, or if patients show medication side-effects [8]. Another study from Botswana also showed that RTMM is more effective in improving adherence for patients novel to ART, compared to experienced and knowledgeable patients [9]. A third study showed that using real-time monitoring alone had a less positive impact than when an SMS reminder was sent before the actual time of intake [10]. Such an SMS is particularly helpful if it contains educational and/or motivational information to stimulate medication intake (Table 1).
Various studies have shown benefits of adherence-enhancing interventions based on electronic medication monitoring (6–8). However, this case helps to remind us that despite high levels of electronically monitored and self-reported adherence, actual adherence may be poor for some individuals. The VOICE-D trial, which is a post-trial study asking participants why their actual product use was lower than they had reported, is worth mentioning. The results suggest that participants, acknowledged the importance of daily monitoring and honest reporting, and yet still over-reported their adherence due to fear of being expelled from the study and worry to be misjudged by nurses for criticizing the interventions [3]. Apparently, our participant refused to take ART but was unwilling or felt uncomfortable to communicate this to her health care providers. It serves as a reminder of the importance of making patients feel free to discuss their concerns and wishes with their healthcare providers, without the fear of being judged or blamed for being non-adherent or refusing medication.
In conclusion, this report illustrates that a valid and accurate measurement of medication adherence remains challenging, and reinforces the importance of combining the assessment of adherence with viral load monitoring as part of HIV care.
Data availability
All the Data are available and will be shared after signing of the Data Transfer agreement between Sender and Receiver .
References
Bangsberg DR. Less Than 95% Adherence to Nonnucleoside Reverse-Transcriptase Inhibitor Therapy Can Lead to Viral Suppression. Clin Infect Dis. 2006;43(7):939–41. https://doi.org/10.1086/507526.
Sumari-de Boer IM, Ngowi KM, Pima FM, Mmbaga BT, Nieuwkerk PT AR. Pharmacy Refill Counts and Self-reported Adherence Overestimate Adherence to Antiretroviral Treatment among People Living with HIV in Kilimanjaro, Tanzania. In: Poster sessions. Rwanda; 2019.
Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of product adherence in the MTN-003/VOICE Trial for HIV Prevention in Africa: Participants’ Explanations for Dishonesty. AIDS Behav. 2017;21(2):481–91. https://doi.org/10.1007/s10461-016-1609-1.
Corneli AL, McKenna K, Perry B, Ahmed K, Agot K, Malamatsho F, et al. The Science of Being a Study Participant: FEM-PrEP Participants’ Explanations for Overreporting Adherence to the Study Pills and for the Whereabouts of Unused Pills. JAIDS J Acquir Immune Defic Syndr. 2015;68(5). https://journals.lww.com/jaids/Fulltext/2015/04150/The_Science_of_Being_a_Study_Participant__FEM_PrEP.14.aspx
Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. J Clin Pharmacol. 2005;45(4):461–7.
Vrijens B, Goetghebeur E. Statistical Methods in Medical Research The impact of compliance in pharmacokinetic studies. Stat Methods Med Res. 1999;8:247.
Tu W, Nyandiko WM, Liu H, Slaven JE, Scanlon ML, Ayaya SO, et al. Pharmacokinetics-based adherence measures for antiretroviral therapy in HIV-infected Kenyan children. J Int AIDS Soc. 2017;20(1):21157.
Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–6.
Reid MJA, Steenhoff AP, Thompson J, Gabaitiri L, Cary MS, Steele K, et al. Evaluation of the effect of cellular SMS reminders on consistency of antiretroviral therapy pharmacy pickups in HIV-infected adults in Botswana: a randomized controlled trial. Heal Psychol Behav Med. 2017;5(1):101–9.
Haberer JE, Musiimenta A, Atukunda EC, Musinguzi N, Wyatt MA, Ware NC, et al. Short message service (SMS) reminders and real-time adherence monitoring improve antiretroviral therapy adherence in rural Uganda. Aids. 2016;30(8):1295–9.
Acknowledgements
We thank the participants for their involvement in our study and for allowing us to study potentially sensitive data. Furthermore, we thank the administration of KCMC for allowing us to conduct this study in the hospital and the nurse counselors for recruiting the participants.
Funding
We thank European and Developing Countries Clinical Trials Partnership (EDCTP) for their financial support of this study (Grant No. TMA972).
Author information
Authors and Affiliations
Contributions
KN: Involved in the project as study coordinator and developed the study protocol. Responsible on designing and writing the whole manuscript include compiling of inputs from the listed authors and responding the queries. Also involved in data collection, analysis and interpretation and identify relevant literature. LM: Involved in the study as study pharmacist. Engaged in follow-up process of the enrolled participants and interviewed them. Also participated in data collection and interpretation of the data. FL: Involved as Study Doctor who seeing the participants during clinic visits as well translating the clinic data during manuscript writing. EM: Involved as Principal pharmacist of the study and accountable to interpret the adherence data. BTM: Support to the study team and administrative issue relating to the study. Furthermore, review of the manuscript before submission. MS: Provided expertise inputs on presentation of the data and recommend relevant scientific writing courses. Critically reviewed the final manuscript before submission. PN: Involved in development of the study protocol. Supervised weekly meetings during the manuscript writing and provided technical inputs on shaping the manuscript to fit the journal criteria. REA: Involved in development of the study protocol, provided support during the entire study and review of the manuscript before submission. PR: Critically Reviewed the final manuscript before submission. MSB: Developed the study protocol and oversaw the study procedures as principal investigator. Also, organized the manuscript methodology. Involved in critical review of initial and final drafts of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Kilimanjaro Christian Medical College Research Ethics and Review Committee (CRERC) and the National Health Research Ethics Sub-Committee (NathRec) of Tanzania. Written informed consent was requested from study participants before enrolment.
Competing interests
The authors declare that they have no competing interests. All authors have read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ngowi, K.M., Masika, L., Lyamuya, F. et al. Returning of antiretroviral medication dispensed over a period of 8 months suggests non-adherence despite full adherence according to real time medication monitoring. AIDS Res Ther 17, 57 (2020). https://doi.org/10.1186/s12981-020-00313-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12981-020-00313-z