Abstract
Background
Recent studies have suggested that genital mycoplasma infections may be associated with male infertility. However, this association remains controversial due to time lapse, sample size, and regional prevalence.
Objectives
This study aimed to systematically evaluate the relationship between genital mycoplasma and male infertility through a meta-analysis and to provide a basis for the clinical management of male infertility.
Methods
We conducted a search on PubMed, EMBASE, the Cochrane Library, and CNKI databases, from January 2000 to June 2023 to identify case–control studies on the interrelationship between genital mycoplasma infection and male infertility. Two independent researchers performed an assessment of the methodological quality of trials according to the Newcastle–Ottawa scale and extracted data strictly based on the inclusion and exclusion criteria, and afterward, we carried out a meta-analysis using Stata 16.0. Pooled odds ratios (OR) with 95% confidence intervals (CI) were used to assess this relationship.
Results
This meta-analysis included 21 studies from seven countries with a total of 53025 infertility cases and 6435 controls; the age range of the participating men was from 20 to 59 years old. The results obtained showed a higher prevalence of M. genitalium, M. hominis and U. urealyticum infections in infertile men than in the controls, with the opposite result for U. parvum (M. genitalium, OR, 3.438 [95% CI: 1.780, 6.643], with P = 0.000; M. hominis, OR, 1.840 [95% CI: 1.013, 3.343], with P = 0.045; U. urealyticum, OR, 3.278 [95% CI: 2.075, 5.180], with P = 0.000; U. parvum, OR, 1.671 [95% CI: 0.947, 2.950], with P = 0.077). Further, two subgroup analyses also showed that M. hominis and U. urealyticum infections were strongly associated with male infertility in China (M. hominis, P = 0.009; U. urealyticum, P = 0.000); however, M. hominis and U. urealyticum infection was not strongly associated with male infertility worldwide (M. hominis, P = 0.553; U. urealyticum, P = 0.050).
Conclusion
This meta-analysis revealed that male infertility was significantly associated with M. genitalium, M. hominis and U. urealyticum infections, while U. parvum infection was not. Further, our study showed that genital mycoplasma infection influences male infertility and provides a basis for future treatment.
Similar content being viewed by others
Introduction
Male infertility is defined as the inability of a male to become pregnant with a fertile female after at least one year of unprotected intercourse [1]. According to the World Health Organization, approximately 15% of couples of reproductive age worldwide have fertility problems, with the male factor accounting for approximately 50% of them [2]. The risk factors contributing to male infertility include lifestyle (drug, alcohol, smoking, and weight etc.), environmental (pesticides and organic solvents, heavy metals like lead, and radiation exposure) and medical conditions (infection, tumor, retrograde ejaculate, surgeries, anti-sperm antibodies, undescended testicles, chromosomal defects, and hormone imbalances etc.) [3,4,5,6]. Reproductive tract infections can cause leukocytosis of semen, the release of inflammatory factors, and altered levels of reactive oxygen species (ROS). They may also lead to secondary vas deferens obstruction, resulting in infertility. Among the many pathogens, although Mycoplasma spp. and Ureaplasma spp. contain bacteria that are natural inhabitants of male urethra, are potentially pathogenic species that play an etiologic role in genital infections and male infertility [7]. However, based on the available data, there is no conclusive evidence on reproductive mycoplasma infections that show their association with male fertility and the pathways through which they elicit their effects.
There are two pathogens of the Mycoplasma spp. that are especially relevant to andrology: Mycoplasma genitalium and Mycoplasma hominis. And andrologically important Mycoplasma spp. are Mycoplasma hominis and Mycoplasma genitalium, both of which are causing urogenital infections [8,9,10]. The prevalence of Mycoplasma spp. infection in the general population is 1.3% in developed countries and 3.9% in developing countries [11]. However, the patients infected with M. genitalium tend to be asymptomatic in their clinical presentation [12]. Only less than 10% of men in the general population with Mycoplasma spp. infections are symptomatic in terms of difficulty urinating, increased penile irritation, urethritis, and infertility. Early in vitro studies found that Mycoplasma spp. infection reduced sperm agglutination and viability, as well as damaged sperm DNA resulting in reduced sperm counts [7]. Because there are no significant clinical symptoms after infection with Mycoplasma spp., patients often ignore relevant laboratory tests and miss the best opportunity for treatment.
In 2001, Robertson proposed the division of Ureaplasma urealyticum into Ureaplasma parvum, while the original Cluster A was retained as Ureaplasma urealyticum [13]. The prevalence of U. urealyticum ranges from 10 to 40% and is thought to be associated with prostatitis, epididymitis, and male infertility [14]. In several studies, the detection rate of U. urealyticum in the semen of infertile men (5–58%) was higher than that of fertile men (3–31%) [15, 16]. Since U. urealyticum plays a dual role, reducing sperm motility at low pH and increasing sperm velocity at high pH, the mechanism by which U. urealyticum causes infertility remains unclear [17]. And in many studies, U. parvum and U. urealyticum have not been studied separately.
Although there is a growing body of research on U. urealyticum, there is still very little research on M. hominis and M. genitalium, and in particular, there are few large-sample studies of semen testing in people infected with M. genitalium. Furthermore, the effects of M. hominis and M. genitalium on male fertility have not been clearly described. Therefore, this study aimed to provide an evidence-based clinical practice approach through a systematic evaluation of clinical literature on the levels of genital mycoplasma infection in infertile and fertile men between January 2000 and June 2023, with the intention of providing insight into the association between U. urealyticum, M. hominis, and M. genitalium infections and male infertility.
Methods
We conducted this review to examine whether genital mycoplasma infection is associated with male infertility by comparing the number of infertility cases in the genital mycoplasma infection group with the number of fertility cases in the control group.
Study selection
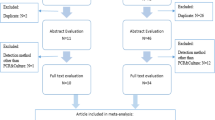
This systematic review and meta-analysis were conducted based on the PRISMA statement [18]. According to the PRISMA flow diagram (Fig. 1), we searched PubMed, EMBASE, the Cochrane Library, and CNKI databases from January 2000 to June 2023, using text and keywords in combination, both as MeSH (Medical Subject Headings) terms and text words. Search terms used in the search strategy: “Mycoplasma,” “Mycoplasma genitalium,” “Mycoplasma hominis,” “Ureaplasma urealyticum,” “U. parvum,” “Infertility,’ “Sterility,” and “Semen quality.” For articles with overlapping data and the same authors, the most recent or comprehensive study was used, and for the same author or duplicate data from different journals, the most recent or comprehensive study was used. Review articles, letters, commentaries, case reports, and preclinical studies were excluded.
Inclusion and exclusion criteria
Eligible studies were included based on the following inclusion criteria: (a) the types of studies included in the literature were all M. genitalium, M. hominis, U. urealyticum, and U. parvum case–control studies; (b) the experimental specimens were from infertile men, and the control specimens were from normal fertile men; (c) the test specimens were from male semen, prostatic fluid, or urethral secretions; (d) studies containing sufficient data to allow for calculation of a pooled odds ratio (OR) and 95% confidence interval (95% CI). The exclusion criteria were as follows: (a) duplicate publications or poor quality information; (b) insufficient primary data and fruitless requests or incomplete study data; (c) reviews, abstracts, commentaries, etc.; (d) infertility or fertility groups where mixed gender and data could not be separated; and (e) animal studies.
Data extraction
Screening and data extraction was carried out by two researchers who first conducted literature screening and data extraction independently, according to the criteria developed. The reasons for excluding articles were also recorded. When a disagreement arises, both parties negotiate or consult with a third-party expert. The established literature screening criteria were as follows: initial screening to exclude articles that clearly did not meet the criteria based on the title and abstract, followed by a detailed reading of the literature to select the final articles for inclusion in this study based on the inclusion and exclusion criteria. The following data were collected from the included articles: first author, country of study, year of publication, sample size, detection methods, and specimen source.
Assessment of study quality
The quality of the included studies was assessed using the Newcastle–Ottawa scale. The scale consists of eight items covering three dimensions: (a) selection of study groups (4 points), (b) comparability of groups (2 points), and (c) ascertainment of exposure and outcomes (3 points) for the case–control. The scale assigns a maximum score of nine, which represents a high-quality study [19].
Statistical analysis
The data were processed using Stata 16.0 software. To calculate the relationship between genital mycoplasma and male infertility, the OR was used as an indicator of effect, and the pooled OR and 95% CI were calculated. A P-value of < 0.05 was considered to be statistically significant. Heterogeneity testing of the literature was performed using Higgins’ I2. If I2 is < 50%, multiple similar studies would be considered homogeneous, and a fixed-effects model would be used for the analysis. On the other hand, if I2 is > 50%, multiple similar studies would be considered heterogeneous and combined using a random-effects model. We observed publication bias by plotting funnel plots, and Begg’s rank correlation was used to assess potential publication bias. After which, we then conduct a subgroup analysis of the prevalence between regions.
Results
Characteristics of included studies
The systematic search of the four databases yielded 389 articles. 74 duplicate articles retrieved from different databases were first excluded and, upon further examination, a further 294 articles were excluded based on the inclusion and exclusion criteria established previously, leaving a total of 21 articles. There were 53025 primary and secondary infertility cases and 6435 controls in the 21 studies conducted in seven countries, including China, Korea, Iran, Kuwait, Jordan, Denmark, and Estonia [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. All included studies had been scored based on Newcastle–Ottawa Case–Control Study Scale (Table 1). The data extracted from each study were qualitatively synthesized and are presented in Table 1. All the subjects in these 21 studies were tested or treated at hospitals. Specimens were collected from semen, urethral swabs, or first void urine (FVU) and then tested by polymerase chain reaction (PCR), culture, SAT, or mycoplasma IST. 7 studies focused on M. genitalium infections, comprising 29859 cases and 950 controls. 9 studies were related to M. hominis infection, containing 21,305 cases and 4783 controls. There were 18 studies on Ureaplasma spp. infections, including 14 studies on U. urealyticum and 4 studies on U. parvum, making a total of 23,113 cases and 5536 controls.
Correlation between M. genitalium infection and male infertility
The analysis of the case and control groups for each study outcome regarding M. genitalium infection revealed no statistical heterogeneity among the studies regarding the population with M. genitalium infection, X2 = 5.72, df = 6, P = 0.456, and I2 = 0.0%. The pooled OR of all included studies was 3.438(95% CI: 1.780, 6.643), with Z = 3.68 and P = 0.000 (Fig. 2A), suggesting a statistically significant association between M. genitalium and male infertility.
To detect publication bias, a funnel plot analysis was performed on the included studies (Fig. 2B). Because the symmetry of funnel plots is subjective by observation, Begg’s test and Egger’s test correlation were used to quantify the funnel plots for a more objective evaluation. In Begg’s test, P = 1.000, and in Egger’s test, P = 0.165, suggesting that there may not be a large publication bias.
Correlation between M. hominis infection and male infertility
The analysis of the case and control groups for each study outcome regarding M. hominis infection revealed that there is a statistical heterogeneity among the studies regarding the population with M. hominis infection, X2 = 34.25, df = 8, P = 0.000, I2 = 76.6%. The pooled OR of all included studies was 1.840 (95% CI: 1.013, 3.343), with Z = 2.00 and P = 0.045 (Fig. 3A), which suggests a statistically significant association between M. hominis infection and male infertility.
The participants were then divided into a world group and a Chinese group according to their geographical regions for subgroup analysis. The pooled OR of the world group was 1.399 (95% CI: 0.462, 4.241), with Z = 0.59 and P = 0.553, suggesting that there is no statistically significant association between M. hominis and male infertility worldwide. The pooled OR of the Chinese group was 2.323 (95% CI: 1.238, 4.358), with Z = 2.63 and P = 0.009, suggesting a statistically significant association between M. hominis and male infertility in China. Sensitivity analysis was performed because of the high heterogeneity. The Chinese subgroup was found to be significantly less heterogeneous after the exclusion of one study by removing studies one by one; at this point, P = 0.951, I2 = 0.0%. Sensitivity analysis of the world subgroup revealed good stability of the results and no reduction in heterogeneity. In this study, Begg’s test gave a P-value of 0.602, and Egger’s test gave a P-value of 0.907, suggesting that there may not be a large publication bias (Fig. 3B).
Correlation between U. urealyticum infection and male infertility
The analysis of the case and control groups for each study outcome regarding U. urealyticum infection revealed that there is a statistical heterogeneity among the studies regarding the population with U. urealyticum infection, X2 = 87.91, df = 13, P = 0.000, I2 = 85.2%. The pooled OR of all included studies was 3.278 (95% CI: 2.075, 5.180), with Z = 5.09 and P = 0.000 (Fig. 4A), suggesting a significant association between U. urealyticum and male infertility.
The participants were also divided into a world group and a Chinese group. The pooled OR of the world group was 2.250 (95% CI: 0.999, 5.069), with P = 0.050, suggesting no statistically significant association between U. urealyticum and male infertility in the world. The pooled OR of the Chinese group was 3.967 (95% CI: 2.232, 7.050), with P = 0.000, suggesting a statistically significant association between U. urealyticum and male infertility in China. Sensitivity analysis of both subgroups showed no significant reduction in heterogeneity. In this study, Begg’s test gave a P-value of 0.584, and Egger’s test gave a P-value of 0.252, suggesting that there may not be a large publication bias (Fig. 4B).
Correlation between U. parvum infection and male infertility
The analysis of the case and control groups for each study outcome regarding U. parvum infection revealed no statistical heterogeneity among the studies regarding the population with U. parvum infection, X2 = 6.10, df = 3, P = 0.107, I2 = 50.8%. The pooled OR of all included studies was 1.671 (95% CI: 0.947, 2.950), with Z = 1.77 and P = 0.077 (Fig. 5A), suggesting no statistically significant association between U. parvum and male infertility. In this study, Begg’s test gave a P-value of 0.734, and Egger’s test gave a P-value of 0.902, suggesting that there may not be a large publication bias (Fig. 5B).
Discussion
According to the results, our meta-analysis found that male infertility was significantly correlated with M. genitalium, M. hominis and U. urealyticum. Moreover, the infertile men had more M. genitalium, M. hominis and U. urealyticum infections than fertile men. However, male infertility is not significantly associated with U. parvum.
The meta-analysis incorporated the most recent studies and expanded the study population. Based on our results, we drew conclusions that differ from previous studies and provided further evidence to show the relationship between genital mycoplasma infection and male infertility [41]. In contrast to our results, that previous meta-analysis reported that U. urealyticum and M. hominis were significantly associated with male infertility, but U. parvum and M. genitalium were not. We analyzed the possible reasons for the different results, and in this study we expanded the sample size of the study and, following our search criteria, we included eight additional articles published in recent years after that meta-analysis. At the same time, we also took into account the expansion of the study area, which helped to reduce regional or ethnographic effects from a single area. What’s more, we performed a subgroup analysis and considered possible sources of heterogeneity through sensitivity analysis. In addition differences in specimen source and detection methods for genital mycoplasma infections may also contribute to differences in results, with higher detection rates of mycoplasma in semen than urethral swabs in asymptomatic patients with mycoplasma infections. Moreover, the sensitivity of SAT was higher than that of PCR and culture among the detection methods for mycoplasma infection, compared to our meta-analysis, which included more studies in which the source of the specimen was sperm and the detection method was SAT [42].
In the subgroup analysis of M. hominis infection, the results of the world group showed that M. hominis infections were not associated with male infertility, and sensitivity analysis could not exclude studies that affected heterogeneity. However, the Chinese group showed significantly lower heterogeneity after excluding one article [27], so it is possible that the grouping basis and statistical approach in those articles contributed to the high heterogeneity of the studies. Also, in the study conducted on U. urealyticum infection, the exclusion of any of the literature does not reduce the heterogeneity of the study, so the sources of heterogeneity may still be due to the grouping basis.
Studies have shown that approximately 15% of male infertility is currently associated with genital infections, and that damage caused by mycoplasma such as non-gonococcal urethritis (NGU), orchitis, and prostatitis tends to be moderate compared to the severe symptoms caused by Chlamydia trachomatis and gonococcus in the reproductive tract, including testicular atrophy and obstructive azoospermia [43,44,45]. Genital mycoplasma is closely associated with male genitourinary infections, and NGU is the most common disease of the genital tract in men [46]. M. hominis and U. urealyticum can commensally exist in the urethra, due to their low inflammatory characteristics, most patients are asymptomatic [47]. Antibiotics are the main treatment for genital mycoplasma infections, and recent studies have shown that moxifloxacin is an effective treatment for M. genitalium, but antimicrobial resistance limits its oral treatment options [48]. Azithromycin may be used instead of moxifloxacin if macrolide susceptibility can be ascertained using molecular resistance tests [49]. And so far, there has been considerable disagreement on the exact association of M. genitalium with male infertility [50]. Similarly, the evidence for Ureaplasma spp. as pathogens causing infertility is less conclusive than that for Chlamydia trachomatis and Neisseria gonorrhoeae [51]. The results of our study indicate that urogenital infections caused by M. genitalium, M. hominis and U. urealyticum are correlated with male infertility, which provide new basis for further revealing the relationship between urogenital mycoplasmas and male infertility.
Laboratory diagnosis of genital mycoplasma is important in the prevention of infertility. Culture approach to detect bacterial infections have shown good sensitivity and specificity, but nucleic acid amplification tests have significantly higher sensitivity for asymptomatic infections with low organisms’ load, which is a significantly relevant advantage [52]. Multiplex assays of PCR offer an option for patients with pathogen co-infection, this technologies are available for the detection of up to eighteen different organisms, and their sensitivity and specificity are comparable with their respective singleplex assays, which help solve the problem of U. urealyticum and U. parvum that cannot be diferentiated in culture [53]. Another test for the identification of genital mycoplasma (M. genitalium, M. hominis and U. urealyticum) showed consistent results with PCR on clinical samples, but with higher sensitivity at lower DNA concentrations [54]. These more economical and convenient methods facilitate the spread of mycoplasma diagnosis.
This meta-analysis has some limitations. First, the literature studies were sourced from only seven countries, most of which were from China and the Middle East, with only three studies from Europe, and no relevant studies were reported from the Americas; thus making it impossible to determine whether there are environmental or geographical differences in the content of our study [55, 56]. Second, risk factors for male infertility vary over time and there have been a few case–control studies on mycoplasma infections and male infertility in recent years [57], therefore, more recent studies are needed to determine whether this correlation is influenced by temporal changes. Third, the detection of co-infections with pathogens that may cause male infertility, which was not part of our study, was also not represented in some studies [7, 58].
Currently, many patients with genital mycoplasma infections have mild symptoms and variability in the samples collected for evaluation, which may increase the risk of infertility if left undiagnosed and untreated for long periods of time [59]. Despite some limitations, this meta-analysis provides some evidence for the clinical management of male infertility due to genital tract mycoplasma infections. We also expect future statistical analyses of genital mycoplasma collection methods to determine optimal samples and sampling sites [60]. A more extensive study of mycoplasma globally will help to better understand its epidemiology and pathogenesis and to develop appropriate strategies for the treatment and prevention of male infertility.
Conclusions
In summary, our meta-analysis suggested M. genitalium, M. hominis and U. urealyticum, but not U. parvum, were associated with male infertility. We looked forward to larger sample sizes of different ethnic populations required to confirm our findings. In addition, in further studies, an assessment of U. parvum is needed to demonstrate the association with male infertility.
Availability of data and materials
The datasets employed in the current study can be available from the corresponding author upon the reasonable request.
References
Krausz C, Riera-Escamilla A. Genetics of male infertility. Nat Rev Urol. 2018;15(6):369–84.
Inhorn MC, Pasquale P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;4:411.
Batiha O, Al-Deeb T, Al-zoubi E, Alsharu E. Impact of COVID-19 and other viruses on reproductive health. Andrologia. 2020;52(9):e13791.
Nassan FL, Chavarro JE, Cigdem T. Diet and men’s fertility: does diet affect sperm quality? Fertil Steril. 2018;110(4):570–7.
Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I. Diet and nutritional factors in male (in) fertility—underestimated factors. J Clin Med. 2020;9(5):1400.
Wu C, Lipshultz LI, Kovac JR. The role of advanced paternal age in modern reproductive medicine. Asian J Androl. 2016;3:1.
Farsimadan M, Motamedifar M. Bacterial infection of the male reproductive system causing infertility. J Reprod Immunol. 2020;142: 103183.
Dessì D, Margarita V, Cocco AR, Marongiu A, Fiori PL, Rappelli P. Trichomonas vaginalis and Mycoplasma hominis: new tales of two old friends. Parasitology. 2019;146(9):1150–5.
Horner P, Donders G, Cusini M, Gomberg M, Jensen J, Unemo M. Should we be testing for urogenital Mycoplasma hominis, Ureaplasma parvum and Ureaplasma urealyticum in men and women?–A position statement from the European STI Guidelines Editorial Board. J Eur Acad Dermatol Venereol. 2018;32(11):1845–51.
Jensen J, Cusini M, Gomberg M, Moi H, Wilson J, Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022;36(5):641–50.
Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review andmeta-analysis. Sex Transm Infect. 2018;94(4):255–62.
Horner PJ, Martin DH. Mycoplasma genitalium infection in men. J Infect Dis. 2017;216(suppl_2):S396.
Robertson JA, Stemke GW, Davis JW, Harasawa R, Robertson JA. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol. 2002;52:587–97.
Reichart M, Levi H, Kahane I, Bartoov B. Dual energy metabolism‐dependent effect of Ureaplasma urealyticum infection on sperm activity. J Androl. 2001;22(3).
Andrade-Rocha FT. Ureaplasma urealyticum and Mycoplasma hominis in men attending for routine semen analysis. Urol Int. 2003;71(4):377–81.
Pellati D, Mylonakis I, Bertoloni G, et al. Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol. 2008;140(1):3–11.
Knox CL, Allan JA, Allan JM, et al. Ureaplasma parvum and Ureaplasma urealyticum are detected in semen after washing before assisted reproductive technology procedures. Fertil Steril. 2003;80(4):921–9.
Page MJ, Mckenzie JE, Bossuyt PM, Boutron I, Moher D. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;(10)
Zhang L, Hu P, Chen X, Bie P. Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized trials. 2014;
Zhang Q, Xioa Y, Zhuang W, Cheng B, Zheng L, Cai Y, Zhou H, Wang Q. Effects of biovar I and biovar II of Ureaplasma urealyticum on sperm parameters, lipid peroxidation, and deoxyribonucleic acid damage in male infertility. Urology. 2014;84:87.
Abusarah EA, Awwad ZM, Charvalos E, Shehabi AA. Molecular detection of potential sexually transmitted pathogens in semen and urine specimens of infertile and fertile males. Diagn Microbiol Infect Dis. 2013;77(4):283–6.
Ahmadi MH, Mirsalehian A, Gilani MAS, Bahador A, Talebi M. Asymptomatic infection with Mycoplasma hominis negatively affects semen parameters and leads to male infertility as confirmed by improved semen parameters after antibiotic treatment. Urology. 2017;100:97–102.
Al-Sweih NA, Al-Fadli AH, Omu AE, Rotimi VO. Prevalence of Chlamydia trachomatis, Mycoplasma hominis, Mycoplasma genitalium, and Ureaplasma urealyticum infections and seminal quality in infertile and fertile men in Kuwait. J Androl. 2012;33(6):1323–9.
Huang C, Long X, Jing S, et al. Ureaplasma urealyticum and Mycoplasma hominis infections and semen quality in 19,098 infertile men in China. World J Urol. 2016;34(7):1–6.
Lee JS, Kim KT, Lee HS, Yang KM, Seo JT, Choe JH. Concordance of Ureaplasma urealyticum and mycoplasma hominis in infertile couples: impact on semen parameters. Urology. 2013;6:81.
Li WN, Zhu WB, Liu G. Correlation of Mycoplasma genitalium infection with male infertility. Natl J Androl. 2018;24(11):999–1004.
Liu J, Wang Q et al. Prevalence of Ureaplasma urealyticum, Mycoplasma Hominis, Chlamydia Trachomatis infections, and semen quality in infertile and fertile men in China. Urology. 2014;83(4).
Ma XP, Gao XQ. The effect of Ureaplasma urealyticum on the level of P34H expression, the activity of hyaluronidase, and DNA fragmentation in human spermatozoa. Am J Reprod Immunol. 2017;77:e12600.
Peerayeh SN, Yazdi RS, Zeighami H. Original Article Association of Ureaplasma urealyticum infection with Varicocele-related infertility. 2008;
Plecko V, Zele-Starcevic L, Tripkovic V, et al. Unusually low prevalence of Mycoplasma genitalium in urine samples from infertile men and healthy controls: a prevalence study. BMJ Open. 2014;4(8): e005372.
Punab M. Mycoplasma genitalium provokes seminal inflammation among infertile males. Int J Mol Sci. 2021;22.
Safavifar F, Bandehpour M, Hosseiny SJ, Khorramizadeh MR, Shahverdi A, Kazemi B. Mycoplasma infection in pyospermic infertile and healthy fertile men. Novelty Biomed. 2015;3(1).
Wang T, WM, Wang ZJ. Research on relationship between sperm quality and reproductive tract infections in 176 cases with male infertility. Chin J Health Lab Technol. 2013;
Zeighami H, Peerayeh SN, Safarlu M. Detection of Ureaplasma urealyticum in semen of infertile men by PCR. Pak J Biol. 2007;10(21):3960–3.
Zeng L ZQ, Jing W, Luo X. Analysis of mycoplasma infection and drug susceptibility result in male infertile patients. J Mod Med Health. 2013;
Zeng W CQ, Pi H, Mei Y, Wang L, Cao Y. The investigation of two biovars of Ureaplasma urealyticum and antisperm antibody in infertile men. Shandong Med J. 2011.
Zhang Z, Zhang H, Dong Y, Han R, Dai R, Liu R. Ureaplasma urealyticum in male infertility in Jilin Province, North-east China, and its relationship with sperm morphology. J Int Med Res. 2011;39(1):33–40.
Zhou YH, Ma HX, Shi XX, Liu Y. Ureaplasma spp. in male infertility and its relationship with semen quality and seminal plasma components. J Microbiol Immunol Infect. 2018;51(6):778–83.
Ahmadi MH, Mirsalehian A, Gilani MAS, Bahador A, Talebi M. Improvement of semen parameters after antibiotic therapy in asymptomatic infertile men infected with Mycoplasma genitalium. Infection. 2018;46(1):31–8.
Zhang ZQZJ, Chen WB. Research of male infertility and urogenital mycoplasma infection. Heilongjiang Med J. 2014;38:451–2.
Huang C, Zhu HL, Xu KR, Wang SY, Fan LQ, Zhu WB. Mycoplasma and ureaplasma infection and male infertility: a systematic review and meta-analysis. Andrology. 2015;3(5):809–16.
Liang Y, Jin X, Yuan F, Li Z, Chen S. Comparison of rRNA-based and DNA-based nucleic acid amplifications for detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and Ureaplasma urealyticum in urogenital swabs. BMC Infect Dis. 2018;18(1):651.
Goulart ACX, Farnezi HCM, França JPBM, Dos Santos A, Ramos MG, Penna MLF. HIV, HPV and Chlamydia trachomatis: impacts on male fertility. JBRA Assisted Reprod. 2020;24(4):492.
Henkel R, Offor U, Fisher D. The role of infections and leukocytes in male infertility. Andrologia. 2021;53(1): e13743.
Li W, Shi L, Long X, Li Y, Zhu W, Liu G. Mycoplasma genitalium incidence, treatment failure, and resistance: a retrospective survey of men of infertile couples from a hospital in China. Andrology. 2020;8(1):91–100.
Plummer EL, Ratten LK, Vodstrcil LA, Murray GL, Danielewski JA, Fairley CK, Garland SM, Chow EPF, Bradshaw CS. The urethral microbiota of men with and without idiopathic urethritis. MBio. 2022;13(5):e0221322.
Sarier M, Kukul E. Classification of non-gonococcal urethritis: a review. Int Urol Nephrol. 2019;51(6):901–7.
Dionne-Odom J, Geisler WM, Aaron KJ, Waites KB, Westfall AO, Van Der Pol B, Xiao L. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in alabama. Clin Infect Dis. 2018;66(5):796–8.
Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and treatment of sexually transmitted infections: a review. JAMA. 2022;327(2):161–72.
Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev. 2011;24(3):498–514.
Gimenes F, Souza RP, Bento JC, Teixeira JJ, Maria-Engler SS, Bonini MG, Consolaro ME. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol. 2014;11(12):672–87.
Caruso G, Giammanco A, Virruso R, Fasciana T. Current and future trends in the laboratory diagnosis of sexually transmitted infections. Int J Environ Res Public Health. 2021;18(3):1038.
Choe H-S, Lee DS, Lee S-J, et al. Performance of Anyplex™ II multiplex real-time PCR for the diagnosis of seven sexually transmitted infections: comparison with currently available methods. Int J Infect Dis. 2013;17(12):e1134–40.
Wang Y, Cheng Y, Xie J, Liu Y. Establishment of multiplex loop-mediated isothermal amplification for rapid detection of genitourinary mycoplasma. Clin Lab. 2018;64(7):1217–24.
Machalek DA, Tao Y, Shilling H, Jensen JS, Unemo M, Murray G, Chow EPF, Low N, Garland SM, Vodstrcil LA, Fairley CK, Hocking JS, Zhang L, Bradshaw CS. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(11):1302–14.
Martin DH, Manhart LE, Workowski KA. Mycoplasma genitalium from basic science to public health: summary of the results from a national institute of allergy and infectious diseases technical consultation and consensus recommendations for future research priorities. J Infect Dis. 2017;216(suppl_2):S427–30.
Cina M, Baumann L, Egli-Gany D, Halbeisen FS, Ali H, Scott P, Low N. Mycoplasma genitalium incidence, persistence, concordance between partners and progression: systematic review and meta-analysis. Sex Transm Infect. 2019;95(5):328–35.
Ko EY, Sabanegh ES Jr, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102(6):1518–27.
Sarier M, Sepin N, Duman I, et al. Microscopy of Gram-stained urethral smear in the diagnosis of urethritis: which threshold value should be selected? Andrologia. 2018;50(10): e13143.
Overmann J, Abt B, Sikorski J. Present and future of culturing bacteria. Annu Rev Microbiol. 2017;71:711–30.
Acknowledgements
This study was funded by National Natural Science Funds of China (82171594)
Funding
National Natural Science Funds of China, Grant/Award Number: 82171594.
Author information
Authors and Affiliations
Contributions
CC and XC designed the study and analyzed the data. YS, SW, and YP revised the images. SN, RW and LL performed the literature search and collected data for the manuscript. XL revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cheng, C., Chen, X., Song, Y. et al. Genital mycoplasma infection: a systematic review and meta-analysis. Reprod Health 20, 136 (2023). https://doi.org/10.1186/s12978-023-01684-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12978-023-01684-y