Abstract
Cardiovascular Magnetic Resonance (CMR) has become a primary tool for non-invasive assessment of cardiovascular anatomy, pathology and function. Existing contrast agents have been utilised for the identification of infarction, fibrosis, perfusion deficits and for angiography. Novel ultrasmall superparamagnetic particles of iron oxide (USPIO) contrast agents that are taken up by inflammatory cells can detect cellular inflammation non-invasively using CMR, potentially aiding the diagnosis of inflammatory medical conditions, guiding their treatment and giving insight into their pathophysiology. In this review we describe the utilization of USPIO as a novel contrast agent in vascular disease.
Similar content being viewed by others
Introduction
Inflammation is central to many cardiovascular pathophysiological processes including atherosclerosis, myocardial infarction and heart failure. Macrophages are key mediators of these inflammatory pathways, initiating both destructive and reparative processes [1]. Quantification and characterization of tissue macrophage activity may therefore assist in our understanding of the pathogenesis of cardiovascular disease and help determine disease severity and prognosis, as well as providing a biomarker to assess the efficacy of established or novel therapeutic interventions.
Cardiovascular magnetic resonance (CMR) is a well-established clinical imaging modality offering excellent soft tissue contrast and spatial resolution, whilst avoiding ionizing radiation. Standard gadolinium-based contrast agents are paramagnetic and are infused into the blood pool with variable organ extraction rates, although subsequent extravasation and redistribution can be used to identify the interstitial and extracellular spaces. Gadolinium is commonly used as an CMR contrast agent after acute myocardial infarction (MI) to identify areas of tissue infarction and fibrosis [2, 3]. Tissue oedema and rupture of cell membranes with consequent diffusion of gadolinium into the inter- and intra-cellular spaces [2] results in a “delayed gadolinium enhancement” effect in infarcted regions. Recent interest has turned to novel agents that provide additional structural and functional cellular information. Such ‘smart’ contrast agents include iron oxide nanoparticles.
Iron oxide nano-particles
Particles of iron oxide are divided into classes based on their size (Table 1). In this review, we will focus on ultrasmall superparamagnetic particles of iron oxide (USPIOs) that consist of nanoparticles with a diameter of <50 nm and include ferumoxtran-10 (Sinerem, Guerbet) and ferumoxytol (Rienso, Takeda; Feraheme, AMAG Pharmaceuticals). Although Rienso had been authorised for use in European Union, Takeda since has withdrawn it. However Feraheme is clinically available in the United States for the treatment of iron deficiency anemia in adult patients with chronic kidney disease (CKD).
Ferumoxytol is well tolerated by patients with chronic kidney disease and iron deficiency anaemia, and had a similar overall treatment-related adverse event rate to oral iron [4]. This safety data is further supported by additional retrospective observational data from three large haemodialysis clinics in the United States involving more than 8600 patients and more than 33,300 administered doses of ferumoxytol [5, 6]. The only contraindications to use are known hypersensitivity or iron overload. Therefore there is little to limit widespread clinical use as an imaging agent.
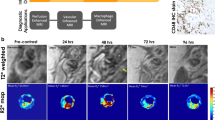
USPIOs can be used as a blood pool contrast agent but it is their ability to be taken up by inflammatory cells that has distinguished them [7]. Cellular uptake of USPIOs occurs through a variety of mechanisms. Phagocytosis and receptor-mediated endocytosis are important for uptake of larger particles, whilst smaller particles are internalized by pinocytosis. Although the avidity of macrophage uptake is strongly influenced by particle size and charge, the surface coating is particularly important [8, 9]. As a result of their smaller size, USPIOs are less readily recognized by phagocytic cells and persist in the circulation for longer than other iron particles (plasma half-life 14–30 h in humans) [10, 11]. They are capable of passing through capillary walls, to be taken up by tissue-resident macrophages and neutrophils (Fig. 1) [12–14]. These characteristics allow USPIOs to detect and highlight cellular inflammation within tissues using CMR.
Imaging methodology
USPIOs induce local magnetic field inhomogeneities that shorten T2 and T2* relaxations times resulting in a signal deficit on magnetic resonance images. USPIOs also have a T1 shortening effect, particularly at low concentrations, and appear bright on T1 weighted images. The T1 shortening effect mainly depends on the strength of the magnetic field, and is higher in lower field strength.
A range of approaches have been used to evaluate USPIO accumulation in tissues. Most simply, images may be qualitatively assessed for signal deficits. However this approach is subjective, and signal deficits due to calcification or other artefacts may be misinterpreted. Manually drawn regions of interest have been used to allow comparison of signal intensity of the target tissue with that of control tissue although discrete focal areas of USPIO accumulation, and thus focal inflammation, may be missed.
Tissue properties, such as the presence of oedema or haemorrhage, can alter image intensities on T2* sequences, and so pre- and post-contrast images need to be compared to delineate the impact of USPIO accumulation. This requires accurate co-registration of these paired scans and adjustments for differences in baseline intensity. A specific region of interest (ROI) map can be drawn and subsequently transferred to each subsequent co-registered image, thus ensuring the signal intensity can be compared for identical sample regions in different scans from the same patient.
Rather than assessing focal image brightness at a single echo time, the T2* time constant can be calculated from the exponential decay curve using multiple echo times (Fig. 2). This method provides greater reproducibility, broad applicability throughout the field of view, and independence from T1 effects and a range of imaging variables. In the presence of USPIO, the T2* relaxation rate is increased thus giving a lower T2* value, or higher R2* value (R2* is the inverse of T2*, R2* = 1/T2*). Calculation of these values permits the generation of T2* or R2* maps indicative of USPIO accumulation (Fig. 3).
Theoretical T2* exponential decay curves. The T2* curve can be plotted using signal intensities from a region of interest (green crosses) for specific echo times (TEs). In this case, a line of best fit is plotted using the known equation for T2* decay. A T2* map is created from these derived T2* values giving pixel-by-pixel measurements of T2* reported in units of milliseconds, rather than signal intensity of raw images. The red curve describes the decay from pre-USPIO tissue, and the green curved indicated a faster decay due to presence of USPIO. The blue line describes the time constant, T2*
Cardiac T2* Imaging. Multiple images obtained from increasing echo time points (3 time points shown from the left) can be combined to create a T2* map (final image on the right). This map includes the spleen and liver (yellow arrows) and the myocardium (white arrow). These tissues are dark indicating low T2* values consistent with higher USPIO uptake
Various authors have used different techniques to calculate USPIO uptake in tissues, and have reported results using T2, T2* or R2*. This can cause confusion since higher values infer diminished USPIO uptake in T2/T2* weighted images, but higher uptake in R2* maps. For the purposes of this paper, imaging techniques will be described but results reported in terms of “increased USPIO uptake.” In order to account for native R2* values, various authors have used the delta increase in R2* value from successive scans, or factor increase. When pre-USPIO scans have not been performed, it must be assumed that non-inflamed tissue has similar R2* values to pre-USPIO native R2* values.
Finally, it must be noted that USPIO imaging can be affected by artefact. USPIO also shorten T1, and so cause signal enhancement of T1 weighted imaging [15]. However, at high concentration USPIO can cause signal loss with such imaging limiting its use with T1 weighted sequences [16]. The superparamagnetic nature of the particles means that they generate strong local magnetic field inhomogeneities, and it is this magnetic susceptibility that is being imaged by CMR. However this can cause loss of distinction of anatomical borders and distort normal tissues (“blooming artefact”). USPIO will accumulate in the reticulo-endothelial system including the liver and spleen. This accumulation can affect neighbouring structures, and care must be taken not mistake blooming artefact for USPIO uptake.
Cardiovascular applications
Atherosclerotic plaque
Given the central role of macrophage biology in the pathogenesis of atherosclerosis, USPIOs have an obvious application in the investigation of atherosclerotic disease. In pre-clinical studies, uptake of USPIOs is demonstrable within numerous atherosclerotic models including aortic plaques of hyperlipidemic rabbits [17, 18] and mice [19] as well as the neointimal hyperplasia following balloon injury [12, 20], and is proportional to plaque macrophage content.
Modulation of inflammation within atherosclerotic plaques can be assessed by USPIO imaging [21]. P38 Mitogen-activated protein kinase (MAPK) is an inflammatory signalling pathway activated by angiotensin II in various vascular cell types [22, 23]. Angiotensin II infusion leads to macrophage accumulation and UPSIO uptake in atherosclerotic plaques of mice [21, 24] that can be inhibited by co-administration of a p38 MAPK pathway inhibitor. Interestingly, this effect was predominantly manifested by a reduction in USPIO uptake by macrophages rather than a reduction in macrophage numbers, suggesting an effect on macrophage activity rather than recruitment. In contrast, the angiotensin II type 1 receptor antagonist, irbesartan, decreased both USPIO uptake and macrophage content in the apolipoprotein E deficient mouse model [25].
USPIO uptake occurs in human carotid atherosclerotic plaques and appears to correlate with the number of iron-laden macrophages on histology [26]. Consistent with the inflammatory cell infiltrate associated with vulnerable plaques, 75 % of ruptured or rupture-prone lesions show USPIO uptake compared to only 7 % of apparently stable lesions. Determining the overall macrophage burden is challenging because of a number of factors. There is a curvilinear relationship between area of signal intensity reduction and USPIO concentration. Signal intensity is also dependent on density of particle accumulation, and a heterogeneous population of macrophages would be expected to have differing degrees of USPIO uptake. The amount of USPIO infused, and by extrapolation perfusion of target tissue, will also determine the magnitude of CMR changes [27].
USPIO uptake and inflammation does not correlate with plaque volume or the degree of luminal stenosis [28], and as already stated USPIO maybe useful in investigating USPIO activity in contrast to concentration [24]. This raises the possibility of using USPIO uptake to monitor disease activity in carotid stenosis rather than using conventional anatomical measurements. For instance, statins reduce inflammation within atherosclerotic plaques as well as systemic markers of inflammation [29, 30] and this has been assessed using USPIO uptake. The ATHEROMA study (Atorvastatin THerapy: Effects on Reduction Of Macrophage Activity) compared the effect of high-dose (80 mg daily) versus low-dose (10 mg daily) atorvastatin on plaque inflammation [31]. Patients underwent UPSIO-enhanced CMR at baseline, 6 weeks and 12 weeks of therapy. Although there were no differences in USPIO uptake over the course of the study in the low-dose group, there was a reduction in USPIO uptake in the high-dose group at both 6 and 12 weeks. This correlated with a reduction in LDL cholesterol and a reduction in micro-emboli count on trans-cranial Doppler [32].
Abdominal aortic aneurysms
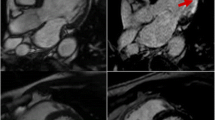
Macrophages are intimately involved in the development, expansion and ultimately rupture of abdominal aortic aneurysms. Preliminary evidence of USPIO uptake in human abdominal aortic aneurysms (AAA) has been described [33, 34]. In a pilot study, we demonstrated that just under half of patients with AAA had focal mural uptake of USPIOs. The aneurysm expansion rate was three-fold higher in patients with USPIO uptake in the aneurysm wall (0.66 versus 0.22 cm/year) [35]. Histology of tissue excised at the time of elective surgical repair confirmed co-localization of USPIOs with CD68 immunostaining for macrophages. Thus USPIO-enhanced CMR appears to identify those patients with more rapid disease progression requiring earlier preventative surgical or endovascular intervention to prevent rupture.
Cerebrovascular disease
Stroke results from an acute disruption to the cerebral blood supply leading to tissue ischemia and eventually necrosis. Inflammatory cells are recruited to the infarct zone, but may extend the injury by interacting with “at risk” cells in the penumbra of the infarct [36, 37]. In a murine model of middle cerebral artery occlusion, USPIO uptake is detected in this penumbra region of infarction [38, 39]. By 7 days the USPIO is confined to the infarct itself, and histology confirms a large population of iron-containing macrophages in the infarcted tissue consistent with migration of macrophages from the penumbra. Further work has indicated that in the setting of established stroke, USPIO leaks through the injured blood–brain barrier accounting for the initial accumulation at the periphery of the infarct and intravascular trapping rather than macrophage uptake [40]. In addition there is widespread uptake resulting from leakage of USPIO into the cerebro-spinal fluid with delivery of nanoparticals to more remote areas. Thus the application of USPIO in such settings is limited although it is possible to track focal USPIO uptake associated with macrophage/microglial infiltration 6 days after cerebral ischaemia, identifying a subacute pathological process [41, 42].
Clinical studies have utilized ferumoxtran-10 in patients 4–5 days after stroke, with imaging at 24–36 h and repeated 48–72 h later [43]. T1- and T2/T2*-weighted imaging reveals parenchymal enhancement that increases between the 2 scans, corresponding to the expected macrophage distribution. These USPIO induced changes do not correspond to conventional gadolinium-enhanced changes, suggesting they occurred independently of blood–brain-barrier breakdown. It could be speculated that these changes may have been due to differences in blood pooling effects due to perfusion changes rather than USPIO inflammatory cell uptake. It would be expected that ischemic volume would correlate with inflammatory burden and CMR changes if USPIOs were being taken up by inflammatory cells. Nighoghossian et al. found no such correlation six days after stroke [44] although the study had a number of limitations including imperfect timing of the scans and the completion of only 5 patients using the more sensitive T2* imaging protocol.
Despite these limitations, a pre-clinical model of the investigation of anti-inflammatory medication in the treatment of stroke has major potential [45]. Using a murine model, the anti-inflammatory agent minocycline can be evaluated after middle cerebral artery occlusion [45]. Minocycline treatment reduced USPIO uptake within the infarct, and was associated with reductions in infarct size, blood–brain barrier permeability and microglia/macrophage counts.
Future applications
The application of USPIOs to study myocardial inflammation has translational application where the pathology involves substantial monocyte influx into the plaque or tissue [46] (Table 2).
Targeted iron oxide particles
Conjugating iron oxide particles with antibodies allows targeted imaging. Pre-clinical imaging to date has employed 9.4 Tesla CMR. This would be more sensitive in detecting USPIO than clinical CMR systems (1.5 or 3-tesla). In addition, injected unconjugated USPIOs injected directly into the blood stream concentrate within macrophages resulting in high local distribution. It remains to be seen if antibody-labelled USPIOs will be sufficiently concentrated at their target site to allow detection in clinical CMR systems. Specific subsets of monocytes or other cell types could be tracked with successful application of this method. This would allow delineation of the temporal dynamics of cellular and immunological processes by repeated scanning. This has been demonstrated in a pre-clinical model of cerebral ischaemia using USPIOs grafted with a specific peptide targeting vascular cellular adhesion molecule-1 (VCAM-1). This study indicated the potential of VCAM-1 to assess vascular injury.
E-selectin is an adhesion molecule between the endothelium and leukocytes that plays a critical role in the pathogenesis of inflammation [47]. An E-selectin monoclonal antibody-USPIO conjugate has been used to track vascular inflammation in a murine model of contact hypersensitivity [48]. More recently, USPIOs have been conjugated with a scavenger receptor to identify inflammation in atherosclerotic plaques [49].
Another potential confounding factor is that macrophages of different subsets or with different activation status take up USPIO at different rates. This could result in false positive or negative CMR enhancement. Direct labeling of cells with USPIO would avoid this error but published data are limited. Although directly labeling of macrophages with USPIO and delivery through the carotid artery has been successful in producing T2* hypo-enhancement after transient ischaemia, it is associated with increased mortality in a rat model [50].
USPIO cell labelling and monitoring cell trafficking
The ability to track cells non-invasively in vivo would be a valuable technique with a number of potential applications that include inflammatory cell tracking and evaluation of engraftment of cells administered as part of cell-based therapies.
USPIOs can be used to label cells in vitro for subsequent in vivo tracking. Smooth muscle cells labelled with iron nanoparticles can be imaged when directly injected into either healthy or infarcted myocardium in a pre-clinical model [51]. This technique can be utilized to label human aortic smooth muscle cells incorporated into tissue engineered vascular grafts and implanted into mice [52]. We have also demonstrated that cell tracking can be achieved in vivo in humans using similar approaches with the larger SPIOs [53].
Summary
USPIOs are taken up by macrophages, and can be identified in vivo by CMR scanning. T2 and T2*-weighted scanning provide a sensitive method of assessing USPIO accumulation.
USPIO-enhanced magnetic resonance imaging is a promising method for assessing inflammatory processes associated with a range of cardiovascular diseases including those affecting the atherosclerotic plaque and large arteries. Potential clinical applications are under evaluation and include assessing the effects of novel pharmacological agents and in vivo cell tracking to determine the fate of cells administered as part of cell therapy.
Abbreviations
- CABG:
-
Coronary artery bypass grafting
- CMR:
-
Cardovascular magnetic resonance
- FDG-PET:
-
Fluorodeoxyglucose-positron emission tomography
- MAPK:
-
Mitogen-activated protein kinase
- MCP-1:
-
Monocyte chemotactic protein-1
- MI:
-
Myocardial infarction
- RAS:
-
Renin-angiotensin system
- ROI:
-
Region of interest
- SPIO:
-
Super-paramagnetic iron oxide
- TNF-α:
-
Tumour necrosis factor alpha
- USPIO:
-
Ultrasmall super-paramagnetic iron oxide
- VCAM-1:
-
Vascular cell adhesion molecule 1
References
Nahrendorf M, Pittet MJ, Swirski FK. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121:2437.
Lima JA, Judd RM, Bazille A, Schulman SP, Atalar E, Zerhouni EA. Regional heterogeneity of human myocardial infarcts demonstrated by contrast-enhanced MRI. Potential mechanisms. Circulation. 1995;92:1117–25.
Schelbert EB, Hsu LY, Anderson SA, Mohanty BD, Karim SM, Kellman P, et al. Late gadolinium-enhancement cardiac magnetic resonance identifies postinfarction myocardial fibrosis and the border zone at the near cellular level in ex vivo rat heart. Circ Cardiovasc Imaging. 2010;3:743–52.
Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, et al. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–605.
Schiller B, Bhat P, Sharma A, Li Z, Fortin G, McLaughlin J, et al. Safety of Feraheme¬Æ(Ferumoxytol) in hemodialysis patients at 3 dialysis chains over a 1-year period. J Am Soc Nephrol. 2011;22:477A–8.
Sharma A, Bhat P, Schiller B, Fortin G, McLaughlin J, Li Z, et al. Efficacy of Feraheme®(Ferumoxytol) administration on target hemoglobin levels and other iron parameters across 3 dialysis chains. J Am Soc Nephrol. 2011;22:485A.
Christen T, Ni W, Qiu D, Schmiedeskamp H, Bammer R, Moseley M, et al. High-resolution cerebral blood volume imaging in humans using the blood pool contrast agent ferumoxytol. Magn Reson Med. 2013; 70(3):705-10.
Saito S, Tsugeno M, Koto D, Mori Y, Yoshioka Y, Nohara S, et al. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int J Nanomedicine. 2012;7:5415–21.
Tsuchiya K, Nitta N, Sonoda A, Otani H, Takahashi M, Murata K, et al. Atherosclerotic imaging using 4 types of superparamagnetic iron oxides: new possibilities for mannan-coated particles. Eur J Radiol. 2013;82:1919–25.
Landry R, Jacobs PM, Davis R, Shenouda M, Bolton WK. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am J Nephrol. 2005;25:400–10.
Hunt MA, Bago AG, Neuwelt EA. Single-dose contrast agent for intraoperative MR imaging of intrinsic brain tumors by using ferumoxtran-10. AJNR Am J Neuroradiol. 2005;26:1084–8.
Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–22.
Dousset V, Delalande C, Ballarino L, Quesson B, Seilhan D, Coussemacq M, et al. In vivo macrophage activity imaging in the central nervous system detected by magnetic resonance. Magn Reson Med. 1999;41:329–33.
Gellissen J, Axmann C, Prescher A, Bohndorf K, Lodemann KP. Extra- and intracellular accumulation of ultrasmall superparamagnetic iron oxides (USPIO) in experimentally induced abscesses of the peripheral soft tissues and their effects on magnetic resonance imaging. Magn Reson Imaging. 1999;17:557–67.
Small WC, Nelson RC, Bernardino ME. Dual contrast enhancement of both T1- and T2-weighted sequences using ultrasmall superparamagnetic iron oxide. Magn Reson Imaging. 1993;11:645–54.
Fananapazir G, Marin D, Suhocki PV, Kim CY, Bashir MR. Vascular artifact mimicking thrombosis on MR imaging using ferumoxytol as a contrast agent in abdominal vascular assessment. J Vasc Interv Radiol. 2014;25:969–76.
Schmitz SA, Coupland SE, Gust R, Winterhalter S, Wagner S, Kresse M, et al. Superparamagnetic iron oxide-enhanced MRI of atherosclerotic plaques in Watanabe hereditable hyperlipidemic rabbits. Invest Radiol. 2000;35:460–71.
Schmitz SA, Taupitz M, Wagner S, Coupland SE, Gust R, Nikolova A, et al. Iron-oxide-enhanced magnetic resonance imaging of atherosclerotic plaques: postmortem analysis of accuracy, inter-observer agreement, and pitfalls. Invest Radiol. 2002;37:405–11.
Sigovan M, Bessaad A, Alsaid H, Lancelot E, Corot C, Neyran B, et al. Assessment of age modulated vascular inflammation in ApoE−/− mice by USPIO-enhanced magnetic resonance imaging. Invest Radiol. 2010;45:702–7.
Hyafil F, Laissy JP, Mazighi M, Tchetche D, Louedec L, Adle-Biassette H, et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler Thromb Vasc Biol. 2006;26:176–81.
Morris JB, Olzinski AR, Bernard RE, Aravindhan K, Mirabile RC, Boyce R, et al. p38 MAPK inhibition reduces aortic ultrasmall superparamagnetic iron oxide uptake in a mouse model of atherosclerosis: MRI assessment. Arterioscler Thromb Vasc Biol. 2008;28:265–71.
Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999;5:439–47.
Ju H, Nerurkar S, Sauermelch CF, Olzinski AR, Mirabile R, Zimmerman D, et al. Sustained activation of p38 mitogen-activated protein kinase contributes to the vascular response to injury. J Pharmacol Exp Ther. 2002;301:15–20.
Brasier AR, Recinos A, 3rd, Eledrisi MS. Vascular inflammation and the renin-angiotensin system. Arterioscler Thromb Vasc Biol. 2002;22:1257–66.
Sigovan M, Kaye E, Lancelot E, Corot C, Provost N, Majd Z, et al. Anti-inflammatory drug evaluation in ApoE−/− mice by ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. Invest Radiol. 2012;47:546–52.
Trivedi RA, Mallawarachi C, JM UK-I, Graves MJ, Horsley J, Goddard MJ, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–6.
Lutz AM, Weishaupt D, Persohn E, Goepfert K, Froehlich J, Sasse B, et al. Imaging of macrophages in soft-tissue infection in rats: relationship between ultrasmall superparamagnetic iron oxide dose and MR signal characteristics. Radiology. 2005;234:765–75.
Tang TY, Howarth SP, Miller SR, Graves MJ, JM UK-I, Li ZY, et al. Correlation of carotid atheromatous plaque inflammation using USPIO-enhanced MR imaging with degree of luminal stenosis. Stroke. 2008;39:2144–7.
Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65.
Tahara N, Kai H, Ishibashi M, Nakaura H, Kaida H, Baba K, et al. Simvastatin attenuates plaque inflammation: evaluation by fluorodeoxyglucose positron emission tomography. J Am Coll Cardiol. 2006;48:1825–31.
Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, JM UK-I, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–50.
Patterson AJ, Tang TY, Graves MJ, Muller KH, Gillard JH. In vivo carotid plaque MRI using quantitative T2* measurements with ultrasmall superparamagnetic iron oxide particles: a dose–response study to statin therapy. NMR Biomed. 2011;24:89–95.
Sadat U, Taviani V, Patterson AJ, Young VE, Graves MJ, Teng Z, et al. Ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging of abdominal aortic aneurysms--a feasibility study. Eur J Vasc Endovasc Surg. 2011;41:167–74.
Howarth SP, Tang TY, Graves MJ, JM UK-I, Li ZY, Walsh SR, et al. Non-invasive MR imaging of inflammation in a patient with both asymptomatic carotid atheroma and an abdominal aortic aneurysm: a case report. Ann Surg Innov Res. 2007;1:4.
Richards JM, Semple SI, MacGillivray TJ, Gray C, Langrish JP, Williams M, et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ Cardiovasc Imaging. 2011;4:274–81.
Braun JS, Jander S, Schroeter M, Witte OW, Stoll G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol. 1996;92:255–63.
del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112.
Rausch M, Sauter A, Frohlich J, Neubacher U, Radu EW, Rudin M. Dynamic patterns of USPIO enhancement can be observed in macrophages after ischemic brain damage. Magn Reson Med. 2001;46:1018–22.
Wiart M, Davoust N, Pialat JB, Desestret V, Moucharrafie S, Cho TH, et al. MRI monitoring of neuroinflammation in mouse focal ischemia. Stroke. 2007;38:131–7.
Desestret V, Brisset JC, Moucharrafie S, Devillard E, Nataf S, Honnorat J, et al. Early-stage investigations of ultrasmall superparamagnetic iron oxide-induced signal change after permanent middle cerebral artery occlusion in mice. Stroke. 2009;40:1834–41.
Yang YM, Feng X, Yin LK, Li CC, Jia J, Du ZG. Comparison of USPIO-enhanced MRI and Gd-DTPA enhancement during the subacute stage of focal cerebral ischemia in rats. Acta Radiol. 2014;55(7)864–73.
Yang YM, Feng XY, Yin le K, Li CC, Li AN, Jia J, et al. In vivo USPIO-enhanced MR signal characteristics of secondary degeneration in the ipsilateral substantia nigra after middle cerebral artery occlusion at 3 T. J Neuroradiol. 2013;40:198–203.
Saleh A, Schroeter M, Jonkmanns C, Hartung HP, Modder U, Jander S. In vivo MRI of brain inflammation in human ischaemic stroke. Brain. 2004;127:1670–7.
Nighoghossian N, Wiart M, Cakmak S, Berthezene Y, Derex L, Cho TH, et al. Inflammatory response after ischemic stroke: a USPIO-enhanced MRI study in patients. Stroke. 2007;38:303–7.
Marinescu M, Chauveau F, Durand A, Riou A, Cho TH, Dencausse A, et al. Monitoring therapeutic effects in experimental stroke by serial USPIO-enhanced MRI. Eur Radiol. 2013;23(1):37–47.
Alam SR, Shah AS, Richards J, Lang NN, Barnes G, Joshi N, et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ Cardiovasc Imaging. 2012;5:559–65.
Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259–87.
Reynolds PR, Larkman DJ, Haskard DO, Hajnal JV, Kennea NL, George AJ, et al. Detection of vascular expression of E-selectin in vivo with MR imaging. Radiology. 2006;241:469–76.
Segers FM, den Adel B, Bot I, van der Graaf LM, van der Veer EP, Gonzalez W, et al. Scavenger receptor-AI-targeted iron oxide nanoparticles for in vivo MRI detection of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2013;33:1812–9.
Riou A, Chauveau F, Cho TH, Marinescu M, Nataf S, Nighoghossian N, et al. MRI assessment of the intra-carotid route for macrophage delivery after transient cerebral ischemia. NMR Biomed. 2013;26(2):115-23.
Riviere C, Boudghene FP, Gazeau F, Roger J, Pons JN, Laissy JP, et al. Iron oxide nanoparticle-labeled rat smooth muscle cells: cardiac MR imaging for cell graft monitoring and quantitation. Radiology. 2005;235:959–67.
Nelson GN, Roh JD, Mirensky TL, Wang Y, Yi T, Tellides G, et al. Initial evaluation of the use of USPIO cell labeling and noninvasive MR monitoring of human tissue-engineered vascular grafts in vivo. FASEB J. 2008;22:3888–95.
Richards JM, Shaw CA, Lang NN, Williams MC, Semple SI, MacGillivray TJ, et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ Cardiovasc Imaging. 2012; 5(4): 509-17.
Shapiro EM, Skrtic S, Koretsky AP. Sizing it up: cellular MRI using micron-sized iron oxide particles. Magn Reson Med. 2005;53:329–38.
McAteer MA, von Zur Muhlen C, Anthony DC, Sibson NR, Choudhury RP. Magnetic resonance imaging of brain inflammation using microparticles of iron oxide. Methods Mol Biol. 2011;680:103–15.
Yang Y, Yanasak N, Schumacher A, Hu TC. Temporal and noninvasive monitoring of inflammatory-cell infiltration to myocardial infarction sites using micrometer-sized iron oxide particles. Magn Reson Med. 2010;63:33–40.
Boutry S, Brunin S, Mahieu I, Laurent S, Vander Elst L, Muller RN. Magnetic labeling of non-phagocytic adherent cells with iron oxide nanoparticles: a comprehensive study. Contrast Media Mol Imaging. 2008;3:223–32.
Margolis DJ, Hoffman JM, Herfkens RJ, Jeffrey RB, Quon A, Gambhir SS. Molecular imaging techniques in body imaging. Radiology. 2007;245:333–56.
Di Marco M, Sadun C, Port M, Guilbert I, Couvreur P, Dubernet C. Physicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. Int J Nanomedicine. 2007;2:609–22.
Taupitz M, Wagner S, Schnorr J, Kravec I, Pilgrimm H, Bergmann-Fritsch H, et al. Phase I clinical evaluation of citrate-coated monocrystalline very small superparamagnetic iron oxide particles as a new contrast medium for magnetic resonance imaging. Invest Radiol. 2004;39:394–405.
Schnorr J, Taupitz M, Schellenberger EA, Warmuth C, Fahlenkamp UL, Wagner S, et al. Cardiac magnetic resonance angiography using blood-pool contrast agents: comparison of citrate-coated very small superparamagnetic iron oxide particles with gadofosveset trisodium in pigs. Rofo. 2012;184:105–12.
Wagner M, Wagner S, Schnorr J, Schellenberger E, Kivelitz D, Krug L, et al. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: initial experience in humans. J Magn Reson Imaging. 2011;34:816–23.
Ludwig A, Poller WC, Westphal K, Minkwitz S, Lattig-Tunnemann G, Metzkow S, et al. Rapid binding of electrostatically stabilized iron oxide nanoparticles to THP-1 monocytic cells via interaction with glycosaminoglycans. Basic Res Cardiol. 2013;108:328.
Herborn CU, Vogt FM, Lauenstein TC, Dirsch O, Corot C, Robert P, et al. Magnetic resonance imaging of experimental atherosclerotic plaque: comparison of two ultrasmall superparamagnetic particles of iron oxide. J Magn Reson Imaging. 2006;24:388–93.
Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–8.
Tang TY, Howarth SP, Miller SR, Graves MJ, JM UK-I, Trivedi RA, et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques contralateral to symptomatic carotid stenosis: an ultra small superparamagnetic iron oxide enhanced magnetic resonance study. J Neurol Neurosurg Psychiatry. 2007;78:1337–43.
Tang TY, Howarth SP, Miller SR, Graves MJ, JM UK-I, Li ZY, et al. Comparison of the inflammatory burden of truly asymptomatic carotid atheroma with atherosclerotic plaques in patients with asymptomatic carotid stenosis undergoing coronary artery bypass grafting: an ultrasmall superparamagnetic iron oxide enhanced magnetic resonance study. Eur J Vasc Endovasc Surg. 2008;35:392–8.
Rausch M, Baumann D, Neubacher U, Rudin M. In-vivo visualization of phagocytotic cells in rat brains after transient ischemia by USPIO. NMR Biomed. 2002;15:278–83.
Krombach GA, Wendland MF, Higgins CB, Saeed M. MR imaging of spatial extent of microvascular injury in reperfused ischemically injured rat myocardium: value of blood pool ultrasmall superparamagnetic particles of iron oxide. Radiology. 2002;225:479–86.
Montet-Abou K, Daire JL, Hyacinthe JN, Jorge-Costa M, Grosdemange K, Mach F, et al. In vivo labelling of resting monocytes in the reticuloendothelial system with fluorescent iron oxide nanoparticles prior to injury reveals that they are mobilized to infarcted myocardium. Eur Heart J. 2010;31:1410–20.
Kanno S, Wu YJ, Lee PC, Dodd SJ, Williams M, Griffith BP, et al. Macrophage accumulation associated with rat cardiac allograft rejection detected by magnetic resonance imaging with ultrasmall superparamagnetic iron oxide particles. Circulation. 2001;104:934–8.
Acknowledgements
The authors are supported by grants from the British Heart Foundation (RE/08/001 and FS/12/83), Medical Research Council (G1001339), National Institute for Health Research (EME 11/20/03), Chest Heart and Stroke Scotland (R11/A135) and Chief Scientist Office (ETM/266). DEN is supported by the British Heart Foundation (CH/09/002). PAH is supported by NHS Research Scotland Fellowship.
Support
Dr Shirjel Alam was supported by a Scholarship grant from the British Heart Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SA conceived and wrote the review paper. GT provided Fig. 1, SS provided Fig. 2. CA, JR, SM,PH and DN reviewed and edited the paper. All authors read and approved the manuscript.
Peter Henriksen and David E. Newby contributed equally to this work.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Alam, S.R., Stirrat, C., Richards, J. et al. Vascular and plaque imaging with ultrasmall superparamagnetic particles of iron oxide. J Cardiovasc Magn Reson 17, 83 (2015). https://doi.org/10.1186/s12968-015-0183-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-015-0183-4