Abstract
Background
The presence and extent of late gadolinium enhancement (LGE) has been associated with adverse events in patients with hypertrophic cardiomyopathy (HCM). Signal intensity (SI) threshold techniques are routinely employed for quantification; Full-Width at Half-Maximum (FWHM) techniques are suggested to provide greater reproducibility than Signal Threshold versus Reference Mean (STRM) techniques, however the accuracy of these approaches versus the manual assignment of optimal SI thresholds has not been studied. In this study, we compared all known semi-automated LGE quantification techniques for accuracy and reproducibility among patients with HCM.
Methods
Seventy-six HCM patients (51 male, age 54 ±13 years) were studied. Total LGE volume was quantified using 7 semi-automated techniques and compared to expert manual adjustment of the SI threshold to achieve optimal segmentation. Techniques tested included STRM based thresholds of >2, 3, 4, 5 and 6 SD above mean SI of reference myocardium, the FWHM technique, and the Otsu-auto-threshold (OAT) technique. The SI threshold chosen by each technique was recorded for all slices. Bland-Altman analysis and intra-class correlation coefficients (ICC) were reported for each semi-automated technique versus expert, manually adjusted LGE segmentation. Intra- and inter-observer reproducibility assessments were also performed.
Results
Fifty-two of 76 (68%) patients showed LGE on a total of 202 slices. For accuracy, the STRM >3SD technique showed the greatest agreement with manual segmentation (ICC =0.97, mean difference and 95% limits of agreement =1.6 ± 10.7 g) while STRM >6SD, >5SD, 4SD and FWHM techniques systematically underestimated total LGE volume. Slice based analysis of selected SI thresholds similarly showed the STRM >3SD threshold to most closely approximate manually adjusted SI thresholds (ICC =0.88). For reproducibility, the intra- and inter-observer reproducibility of the >3SD threshold demonstrated an acceptable mean difference and 95% limits of agreement of -0.5 ± 6.8 g and -0.9 ± 5.6 g, respectively.
Conclusions
FWHM segmentation provides superior reproducibility, however systematically underestimates total LGE volume compared to manual segmentation in patients with HCM. The STRM >3SD technique provides the greatest accuracy while retaining acceptable reproducibility and may therefore be a preferred approach for LGE quantification in this population.
Similar content being viewed by others
Background
Late gadolinium enhanced (LGE) cardiovascular magnetic resonance (CMR) has the capacity to identify regional accumulation of myocardial fibrosis in patients with hypertrophic cardiomyopathy (HCM) [1]-[3]. This imaging biomarker is identified in approximately two-thirds of patients with HCM and has been associated with adverse outcomes [4],[5]. However, given the high prevalence of this finding when reported as a binary variable, accurate and reproducible methodologies aimed at LGE burden quantification are required to more adequately estimate risk among this population.
In contrast to ischemic injury, the fibrosis associated with HCM is typically patchy and non-uniform, posing a substantial challenge for manual segmentation. The visual application of a circumferential boundary is therefore an inappropriate reference standard in this population, particularly when being used to validate voxel-based threshold techniques. Despite this, studies to date have largely used manual planimetry as a reference when testing semi-automated LGE segmentation. Such techniques described to date include; i) Signal Threshold versus Reference Mean (STRM) [1],[4],[6], ii) Full Width at Half Maximum (FWHM) [7], and the Otsu Auto Threshold (OAT) techniques [8]. The most notable comparative study inclusive of patients with HCM by Flett et al. included 20 patients with previously established diagnosis. In this study, they reported that the FWHM technique resulted in higher reproducibility metrics than for STRM [9]. However, the accuracy of these techniques to replicate an expert-based segmentation of LGE was not tested. While other studies have attempted to determine the accuracy of various STRM-based signal intensity (SI) thresholds against a visual standard [6],[10], manual LGE planimetry is most commonly considered the reference standard.
In this study we systematically investigated the accuracy and reproducibility of all known semi-automated threshold-based LGE quantification techniques within a large cohort of patients with confirmed HCM. We employed a novel gold standard that represented signal enhancement by expert visual adjustment of a signal intensity threshold for each analyzed image. Based upon our findings we provide recommendations for the optimal selection of signal threshold techniques in reporting total LGE among patients with HCM.
Methods
Patients
Patients were identified from a prospectively enrolled clinical registry of HCM patients undergoing CMR between March 2008 and May 2011 at the Cardiovascular MRI Clinical Research (CMCR) Center at Western University, Canada. Inclusion criteria for this study were adult patients (age ≥18) with echocardiographically diagnosed HCM, defined according to AHA consensus guidelines, including the presence of a hypertrophied (≥15 mm or ≥ 13 mm if a 1st degree relative with HCM) and non dilated left ventricle in the absence of another cardiac or systemic disease capable of producing this magnitude of hypertrophy [11]. Eighteen patients were excluded from analysis due to prior surgical myomectomy or alcohol septal ablation. All patients provided written informed consent and the study protocol was approved by the Western University Research Ethics Board.
CMR protocol
All CMR studies were performed on a clinical 3-T MRI system (TRIO or Verio, Siemens Healthcare, Erlangen, Germany) with a 32 channel cardiac coil using ECG gating. The imaging protocol involved short axis cine imaging and LGE imaging. LGE imaging was performed using inversion recovery gradient echo sequence 10 minutes after the administration of Gadolinium contrast (Magnevist® or Gadovist®, Bayer Inc, Toronto, Ontario, Canada) at a dose of 0.2 mmol/kg. Typical imaging parameters for LGE were: slice thickness 8 mm, gap 2 mm, TE 1.93 ms, flip angle 20 degrees, matrix 256 × 205, TI individually determined to minimize the SI of normal myocardium range 200 to 400 ms.
Image analysis
All images were anonymized and analyzed in random order. Cine images were blindly analyzed using certified software (cvi42, Circle CVI, Calgary, Alberta, Canada) for determination of LV volume, mass and ejection fraction (EF). Papillary muscle was included as part of myocardium. Maximum wall thickness was measured from the short axis views.
LGE images were analyzed by an observer with 7 years of experience in CMR (YM) using the same software (cvi42, Circle CVI.). Magnitude reconstruction images were used for image analysis to maximize clinical generalizability. These were first evaluated for acceptability based upon an appropriate adjustment of the inversion time, and absence of significant motion artifact. Seven patients were excluded from the study due to unacceptable image quality. For each short axis slice, the endocardial and epidcardial boundaries were manually traced with careful attention to avoid visually apparent artifacts or blood pool. These same boundary contours were used for the testing of all LGE quantification techniques to eliminate the influence of contour tracing on threshold-based LGE quantification. Total LGE volume was then quantified using 7 semi-automated techniques and compared to an expert manual adjustment of the signal intensity threshold, as shown in Figure1. Techniques tested included STRM-based thresholds of >2, >3, >4 > 5 and >6 SD above the mean SI of reference myocardium, the FWHM technique, and the Otsu auto-threshold (OAT) technique (Figure2). For STRM-based assessments, the reference myocardium was defined for each slice as the largest contiguous area of myocardium with no visually apparent LGE or artifact. For FWHM-based assessments, the reference region was defined for each slice as an area inclusive of the maximum signal intensity of visually apparent LGE. The resulting SI threshold applied to define LGE for each technique was recorded. Total LGE was determined on a per-patient basis for each semi-automated technique as the sum of LGE area for each slice multiplied by the slice thickness.
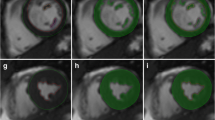
Example of the expert Late Gadolinium Enhancement (LGE) segmentation procedure using a manual adjustment of the signal intensity (SI) threshold for each LGE image. A manual SI threshold (circled) was adjusted using a slide bar until visually identified LGE was segmented in accordance to expert opinion. Panel A shows a raw LGE image prior to application of segmentation. Panel B shows over-representation of LGE at a low SI threshold of 11. Panel C shows "optimal segmentation" at a threshold of 36. Panel D shows under-representation of LGE at a higher threshold of 78. The signal threshold of 36 (arrow) was applied for this image and the corresponding LGE area employed as a reference standard for semi-automated technique comparison.
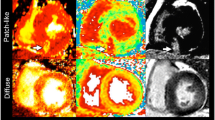
Comparison of; A) Raw LGE image, B) Expert manual segmentation, C) Signal Threshold versus Reference Mean (STRM) threshold of ≥5SD, D), Full Width at Half Maximum (FWHM), and the E) Otsu auto-threshold (OAT) methods applied to the same imaging slice. Reference tissue regions of interest (ROI) for remote myocardium (STRM method) and maximal signal enhancement (FWHM method) are shown in blue and pink, respectively.
The gold standard of manual threshold assignment was performed for each slice as follows; the SI threshold for definition of LGE was set to zero (100% enhancement) and then manually increased until the segmented signal visually matched the visually identified LGE on each slice (Figure1). The manual signal threshold employed for each slice was recorded.
Inter-observer and intra-observer reproducibility testing was performed by two blinded investigators (YM and JW). This was accomplished by repeating measurements in random order for 15 randomly selected patients. For this analysis the same endocardial and epicardial contours were used, again to focus reproducibility testing on the threshold techniques themselves, inclusive of the application of reference regions.
Statistical analysis
All values were expressed as mean ± SD. Bland Altman analysis and intraclass correlation coefficients (ICC) were reported for each semi-automated technique versus expert, manually adjusted segmentation. Intra and inter-observer reproducibility assessments were similarly performed using Bland Altman analysis and ICC. All analyses were conducted using SPSS for Macintosh, version 19.0 (SPSS, Inc., Chicago, Illinois).
Results
A total of 83 registry patients were available for image analysis. Of these, seven were excluded due to suboptimal image quality related to motion artifact or sub-optimal adjustment of the time from inversion (TI time). This resulted in 76 patients with HCM included in the study, 51 being male and having a mean age of 54 ±13 years. All other patient characteristics are shown in Table1.
Fifty-two patients (68%) were scored as having clear visual evidence of LGE, this finding being identified on a total of 202 out of 742 (27%) available image slices. Manual expert segmentation using slice-based signal threshold assignment resulted in a mean Total LGE burden among the study population of 18.2 ± 20.4 g.
Accuracy of semi-automated LGE quantification techniques
Bland-Altman plots for the semi-automated signal threshold techniques versus expert segmentation are shown in Figure3. Overall, the STRM >3SD technique showed the highest agreement with manual segmentation with an ICC of 0.97 (mean difference and 95% limits of agreement: 1.6 ± 10.7 g). The mean difference of total LGE burden obtained for each of the 7 semi-automated techniques versus the manually adjusted reference standard are shown in Figure4. The STRM >6SD, >5SD, >4SD and FWHM techniques were found to systematically underestimate total LGE burden, as shown in Figures4 and 5. Conversely, the STRM >2SD and OAT techniques were found to overestimate total LGE burden in comparison to the gold standard.
Mean difference in Late Gadolinium Enhancement (LGE) volume (g) and % difference (%) between each semi-automated technique and expert segmentation by manual Signal Intensity (SI) threshold adjustment technique. The Signal Threshold versus Reference Mean (STRM) >3SD technique showed the greatest agreement with manual segmentation while STRM >6SD, >5SD, >4SD and full width at half maximum (FWHM) techniques systematically underestimated total enhanced volume.
Patient example of raw Late Gadolinium Enhanced (LGE) image (Panel A), manual expert segmentation (Panel B) versus full width at half maximum (FWHM) -based segmentation (Panel C) and the Signal Threshold versus Reference Mean (STRM) -based segmentation at >3SD (Panel D), >4SD (Panel E) and >5SD thresholds (Panel F). A significant under-estimation of visually identifiable LGE is evident using the FWHM method.
Using slice-based analysis, the SI threshold applied by each technique was compared to that obtained by manual expert threshold adjustment. This analysis similarly identified the STRM >3SD threshold to most closely approximate the manually adjusted SI threshold with an ICC of 0.88 (mean difference and 95% limits of agreement =2.5 ± 16.8).
Reproducibility of semi-automated LGE quantification techniques
Bland-Altman plots were performed for the assessment of both inter- and intra-observer variability for semi-automated segmentation techniques and for manual expert segmentation. These analyses are shown in Figures6 and 7.
Total LGE assessment by manual signal threshold adjustment was found to be highly reproducible with a mean difference and 95% limits of agreement between observers of -1.3 ± 6.5 g, and 0.3 ± 7.8 g within the same observer. Of the semi-automated techniques, the FWHM method showed the highest intra- and inter-observer reproducibility with a mean difference and 95% limits of agreement between observers of 0.3 ± 2.2 g, and -0.1 ± 0.5 g within the same observer. Corresponding reproducibility values for STRM-based thresholds were found to improve with increasing SI thresholds (Figures6 and 7). For example, for the intra-observer values for >2SD and >6SD thresholds were -0.3 ± 7.5 g and -0.2 ± 4.5 g while the inter-observer values were -0.7 ± 9.0 g and -0.6 ± 4.5 g, respectively.
The STRM >3SD threshold, identified among the techniques as optimal with respect to precision, demonstrated acceptable inter- and intra-observer ICC values of 0.992 and 0.995. The corresponding mean difference and 95% limits of agreement were -0.5 ± 6.8 g and -0.9 ± 5.6 g, respectively.
Discussion
This is the largest analysis performed to date comparing both the accuracy and reproducibility of semi-automated SI threshold-based LGE quantification. In this study we focused solely on patients with HCM, a cohort recognized to pose challenge for LGE characterization due to a patchy and non-uniform distribution. Uniquely, the reference standard used in this analysis provided a more appropriate comparator of total LGE burden compared to the convention of manual planimetry.
The potential importance of LGE quantification in patients with HCM has been highlighted by a number of recent studies [4],[5],[12]-[16], each emphasizing a role for the identification of patients at elevated risk of future cardiovascular events. Despite this, only limited data is available regarding the accuracy of LGE quantification in this population [6],[9]. Specifically, in contrast to ischemia-mediated injury, the distribution of collagen deposits in HCM appears highly variable in both density and distribution, ranging from diffusely dispersed patches of collagen fibers to dense and irregular islands of mature scar [1],[17],[18]. As such, the manual application of a linear boundary to quantify this phenomenon appears to be an inappropriate gold standard. In the current study we attempted to mitigate this limitation by employing a manually-adjusted signal threshold, a technique most congruent with the threshold-based segmentation techniques being tested. This provided a more appropriate expert-adjudicated reference standard for the assessment of accuracy.
When compared to expert opinion, our results identified the STRM > 3SD technique to provide the closest estimate of LGE burden among the population. STRM-based thresholds below this cut-off (i.e.: >2SD) systematically over-estimated LGE burden compared to the reference standard, while thresholds above this cut-off (i.e.: >4SD, >5SD and >6SD) systematically under-estimated LGE burden. The FWHM approach was shown to under-estimate LGE burden, as illustrated in Figure4, while the OAT method systematically over-estimated LGE burden. With respect to reproducibility we identified, similar to the report by Flett et al., that the FWHM method provides superior reproducibility than STRM-based methods [9].
Based upon our findings it appears that no "ideal" segmentation technique currently exists for the quantification of LGE among patients with HCM. And, depending upon a preference towards accuracy or reproducibility, an informed decision must be made to best answer the question posed. However, when weighing the relative performance metrics of each technique, we conclude that the STRM > 3SD technique may provide the most optimal result for general use with accuracy that best represents the LGE burden identified by expert visual adjudication and provides sufficiently robust reproducibility. While the FWHM method remains attractive for reproducibility it must be used in recognition of a significant under-estimation of total LGE burden. The OAT method cannot be recommended for use in this population.
LGE has been shown to be a predictor of worse outcomes in HCM, inclusive of worse LV systolic dysfunction [12], ventricular arrhythmias [5],[13],[14], sudden cardiac death [13] and both all cause and cardiac mortality [4]. However, a particular challenge for its use for risk stratification in this population is a high prevalence of LGE when described as a binary finding; as high as 78% among those with genetically confirmed disease with LVH [19]. As such, attention towards quantification of LGE burden has emerged, and this is reliant upon provision of both accuracy and reproducibility. While the lack of any ideal approach has led to appropriate exploration for alternate techniques of fibrosis quantification through T1 mapping [20],[21], and extra cellular volume (ECV) fraction estimation [19],[22], it is anticipated that conventional LGE imaging will remain an important diagnostic and prognostic tool in clinical practice for the foreseeable future. As such, the standardization of LGE reporting in this population is required.
Several recent studies have reported on the value of volume-based quantification rather than dichotomous reporting of LGE in patients with HCM. The extent of LGE has been shown to be related to larger LV mass [23] and reduced systolic function [12],[23] in this population. In a study of 217 HCM patients, the extent of LGE was associated with risk of heart failure admissions, deterioration to New York Heart Association functional class III or IV, or heart failure-related death over a mean of 3.1 years [14]. Another study assessed progression of LGE between 2 CMR examinations (mean interval of 719 days among 55 HCM patients) where a greater interval increases in LGE was associated with worsening of NYHA class [24]. Ismail et al. recently published a study of 711 HCM patients diagnosed by standard clinical criteria and followed them for a median of 3.5 years [15]. Thirty-two patients reached the primary endpoint of sudden cardiac death (SCD) or aborted SCD. The extent of LGE quantified using the FWHM method was a predictor of the primary endpoint by univariable analysis (HR per 5% LGE 1.24). However, this failed to remain predictive by multivariable analysis. The second study, published by Chan et al., included 1293 HCM patients defined by CMR findings and followed for a median of 3.3 years [16]. Thirty-seven patients experienced the primary outcomes of SCD or appropriate defibrillator therapy. The extent of LGE was quantified by a manual adjustment of gray scale threshold (similar to that used as the reference standard in the current study). Using this approach, %LGE was a significant predictor of the primary outcome in both univariate and multivariate analysis, the latter inclusive of relevant conventional risk factors (adjusted HR of 1.46/10% increase in LGE). A LGE burden ≥15% was associated with a HR of 2.14 and an estimated 5-year event rate of 6.3%. Whether differences in LGE quantification methodology are adequate to explain differences in predictive utility between the Chan et al., and Ismail et al. studies remains uncertain. However, such differences highlight a need to adopt a common, standardized approach to LGE quantification.
Appropriate debate exists, and will continue to exist, regarding optimal metrics of LGE quantification for the accuracy of cardiovascular events in specific disease cohorts. Whether a single LGE threshold is optimal to predict arrhythmic events, or whether intermediate LGE signal (ie: "border-zone") is more discriminative among patients with ischemic cardiomyopathy remains uncertain [25]-[27]. This same debate could extend to the HCM population, a recent study by Appelbaum et al. similarly suggesting LGE signal between the range of 4SD and 6SD providing higher predictive value for non sustained ventricular arrhythmia [28]. Irrespective, we must first acknowledge fundamental strengths and weaknesses of each segmentation technique, and only then select that which is best suited to answer the question being posed.
Study limitations
This study was designed to assess the accuracy and reproducibility of LGE quantification in patients with echocardiographically confirmed HCM at a single academic institution. Therefore, generalization of our findings beyond this referral population cannot be recommended.
We recognize that, while the employed reference standard was chosen as the best possible solution, it remains imperfect in that subjectivity is still introduced when applying manual thresholds. Histologic correlation, while desirable, was not available in this clinical cohort. However, a recently reported histologic study by Moravsky et al. provides complementary findings by comparing histological findings from septal myectomy samples to regionally matched LGE segmentation. While this study could not assess total LGE burden (i.e.: limited to the myomectomy sample itself), the STRM technique was similarly favoured for accurate estimates of total fibrosis burden [29]. Specifically, they found a >4SD threshold most closely approximated percent collagen deposition within the septal myomectomy sample - a threshold highly consistent with our current findings. Similar to our findings, they identified that the FWHM technique systematically under-represented histologically based measurements of fibrosis.
Finally, the process by which segmentation boundaries and reference tissues are identified is a dominant harbinger of variability for LGE quantification. The reproducibility reported in this study is based upon rigorous attention to standard operating procedures established within a core-laboratory environment and therefore represents ideal operating conditions. It is strongly advised that efforts be made to follow an established set of rules when executing LGE quantification, such as those outlined in the methods section of this study.
Conclusions
In this large cohort study, the STRM >3SD LGE segmentation technique provided greatest accuracy and an acceptable reproducibility for total LGE burden quantification versus the reference standard of expert, slice-by-slice adjustment of a SI threshold to quantitate extent of LGE. In contrast, FWHM-based segmentation is highly reproducible but provides a systematic under-representation of total LGE burden versus expert opinion. Consensus opinion regarding the preferred LGE segmentation methodology is required to facilitate the clinical translation of LGE quantification in the risk stratification of patients with HCM.
Abbreviations
- CMR:
-
Cardiovascular magnetic resonance
- HCM:
-
Hypertrophic cardiomyopathy
- LV:
-
Left ventricular
- LGE:
-
Late gadolinium enhancement
- STRM:
-
Signal threshold versus reference mean
- FWHM:
-
Full width at half maximum
- OAT:
-
Otsu auto threshold
- SI:
-
Signal intensity
- ICC:
-
Intraclass correlation coefficient
- ROI:
-
Region of interest
- SCD:
-
Sudden cardiac death
References
Moon JC, Reed E, Sheppard MN, Elkington AG, Ho SY, Burke M, Petrou M, Pennell DJ: The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2004, 43: 2260-2264. 10.1016/j.jacc.2004.03.035.
Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, Lytle BW, Lever HM, Desai MY: Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. 2009, 54: 242-249. 10.1016/j.jacc.2009.04.026.
Papavassiliu T, Schnabel P, Schröder M, Borggrefe M: CMR scarring in a patient with hypertrophic cardiomyopathy correlates well with histological findings of fibrosis. Eur Heart J. 2005, 26: 2395-10.1093/eurheartj/ehi518.
Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert E-M, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, Mahrholdt H: Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010, 56: 875-887. 10.1016/j.jacc.2010.05.007.
Adabag AS, Maron BJ, Appelbaum E, Harrigan CJ, Buros JL, Gibson CM, Lesser JR, Hanna CA, Udelson JE, Manning WJ, Maron MS: Occurrence and frequency of arrhythmias in hypertrophic cardiomyopathy in relation to delayed enhancement on cardiovascular magnetic resonance. J Am Coll Cardiol. 2008, 51: 1369-1374. 10.1016/j.jacc.2007.11.071.
Harrigan CJ, Peters DC, Gibson CM, Maron BJ, Manning WJ, Maron MS, Appelbaum E: Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular MR imaging. Radiology. 2011, 258: 128-133. 10.1148/radiol.10090526.
Amado LC, Gerber BL, Gupta SN, Rettmann DW, Szarf G, Schock R, Nasir K, Kraitchman DL, Lima JA: Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol. 2004, 44: 2383-10.1016/j.jacc.2004.09.020.
Vermes E, Childs H, Carbone I, Barckow P, Friedrich MG: Auto-threshold quantification of late gadolinium enhancement in patients with acute heart disease. J Magn Reson Imaging. 2013, 37: 382-390. 10.1002/jmri.23814.
Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC: Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011, 4: 150-156. 10.1016/j.jcmg.2010.11.015.
Spiewak M, Malek LA, Misko J, Chojnowska L, Milosz B, Klopotowski M, Petryka J, Dabrowski M, Kepka C, Ruzyllo W: Comparison of different quantification methods of late gadolinium enhancement in patients with hypertrophic cardiomyopathy. Eur J Radiol. 2010, 74: e149-e153. 10.1016/j.ejrad.2009.05.035.
Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW: 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011, 58: e212-e260. 10.1016/j.jacc.2011.06.011.
Maron MS, Appelbaum E, Harrigan CJ, Buros J, Gibson CM, Hanna C, Lesser JR, Udelson JE, Manning WJ, Maron BJ: Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008, 1: 184-191. 10.1161/CIRCHEARTFAILURE.108.768119.
Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ: Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010, 3: 51-58. 10.1161/CIRCHEARTFAILURE.109.854026.
O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, Chandrasekaran B, Bucciarelli-Ducci C, Pasquale F, Cowie MR, McKenna WJ, Sheppard MN, Elliott PM, Pennell DJ, Prasad SK: Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010, 56: 867-874. 10.1016/j.jacc.2010.05.010.
Ismail TF, Jabbour A, Gulati A, Mallorie A, Raza S, Cowling TE, Das B, Khwaja J, Alpendurada FD, Wage R, Roughton M, McKenna WJ, Moon JC, Varnava A, Shakespeare C, Cowie MR, Cook SA, Elliott P, O'Hanlon R, Pennell DJ, Prasad SK. Role of late gadolinium enhancement cardiovascular magnetic resonance in the risk stratification of hypertrophic cardiomyopathy.Heart. 2014; Published Online First: Jun 24. doi:10.1136/heartjnl-2013-305471.,
Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, Udelson JE, Rowin E, Lombardi M, Cecchi F, Tomberli B, Spirito P, Formisano F, Biagini E, Rapezzi C, De Cecco CN, Autore C, Cook EF, Hong SN, Gibson CM, Manning WJ, Appelbaum E, Maron MS: Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014, 130: 484-495. 10.1161/CIRCULATIONAHA.113.007094.
Anderson KR, Sutton MG, Lie JT: Histopathological types of cardiac fibrosis in myocardial disease. J Pathol. 1979, 128: 79-85. 10.1002/path.1711280205.
Melacini P, Basso C, Angelini A, Calore C, Bobbo F, Tokajuk B, Bellini N, Smaniotto G, Zucchetto M, Iliceto S, Thiene G, Maron BJ: Clinicopathological profiles of progressive heart failure in hypertrophic cardiomyopathy. Eur Heart J. 2010, 31: 2111-2123. 10.1093/eurheartj/ehq136.
Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, Cirino AL, Lakdawala NK, Orav EJ, González A, López B, Díez J, Jerosch-Herold M, Kwong RY: T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013, 6: 415-422. 10.1161/CIRCIMAGING.112.000333.
Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, Watkins H, Neubauer S: Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012, 5: 726-733. 10.1161/CIRCIMAGING.112.976738.
Bull S, Majno GJI, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, Francis JM, Karamitsos TD, Prendergast BD, Robson MD, Neubauer S, Moon JC, Myerson SG: Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013, 99: 932-937. 10.1136/heartjnl-2012-303052.
Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE: Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012, 14: 64-10.1186/1532-429X-14-64.
Ho CY, López B, Coelho-Filho OR, Lakdawala NK, Cirino AL, Jarolim P, Kwong R, González A, Colan SD, Seidman JG, Díez J, Seidman CE: Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N Engl J Med. 2010, 363: 552-563. 10.1056/NEJMoa1002659.
Todiere G, Aquaro GD, Piaggi P, Formisano F, Barison A, Masci PG, Strata E, Bacigalupo L, Marzilli M, Pingitore A, Lombardi M: Progression of myocardial fibrosis assessed with cardiac magnetic resonance in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2012, 60: 922-929. 10.1016/j.jacc.2012.03.076.
Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY: Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006, 114: 32-10.1161/CIRCULATIONAHA.106.613414.
Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, Gerstenblith G, Weiss RG, Marban E, Tomaselli GF, Lima JA, Wu KC: Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007, 115: 2006-10.1161/CIRCULATIONAHA.106.653568.
Roes SD, Borleffs CJ, Van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, De Roos A, Schalij MJ, Bax JJ: Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009, 2: 183-10.1161/CIRCIMAGING.108.826529.
Appelbaum E, Maron BJ, Adabag S, Hauser TH, Lesser JR, Haas TS, Riley AB, Harrigan CJ, Delling FN, Udelson JE, Gibson CM, Manning WJ, Maron MS: Intermediate-signal-intensity late gadolinium enhancement predicts ventricular tachyarrhythmias in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2012, 5: 78-85. 10.1161/CIRCIMAGING.111.963819.
Moravsky G, Ofek E, Rakowski H, Butany J, Williams L, Ralph-Edwards A, Wintersperger BJ, Crean A: Myocardial fibrosis in hypertrophic cardiomyopathy: accurate reflection of histopathological findings by CMR. JACC Cardiovasc Imaging. 2013, 6: 587-596. 10.1016/j.jcmg.2012.09.018.
Acknowledgements
The authors are greatly appreciative of the technical expertise of Kim Krueger, MRT and John Butler, MRT for image acquisition. We also thank Guanmin Chen, Ph. D for his help in statistical analysis.
Funding
This study was funded in part by the Imaging in Cardiovascular Therapeutics grant from the Ontario Research Fund.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
James White receives in-kind research contributions from Bayer, Inc in the form of MRI contrast agents. There are no other conflicts of interest or financial relationships to disclose.
Authors' contributions
YM: study design, image analysis, statistical analysis, manuscript drafting. LK and SJ: image analysis, manuscript revising. CL, SW, AH: study design and manuscript drafting, JS, DS, MR: acquisition of the data, manuscript revising. JW: study design, manuscript revising and finalizing, guarantor of this work. All authors have made revisions to the manuscript and have read and approved the final version.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mikami, Y., Kolman, L., Joncas, S.X. et al. Accuracy and reproducibility of semi-automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 16, 85 (2014). https://doi.org/10.1186/s12968-014-0085-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-014-0085-x