Abstract
Background
Studies have examined the effect of weight change on osteoporosis, but the results were controversial. Among them, few had looked at weight change over the life span. This study aimed to fill this gap and investigate the association between lifetime body mass index (BMI) trajectories and bone loss.
Methods
In this cross-sectional study, participants at age 50 and above were selected from the National Health and Nutrition Examination Survey (NHANES) 2005–2018. Dual-energy X-ray Absorptiometry was used to measure the bone mineral density at the femoral neck and lumbar spine. Standard BMI criteria were used, with < 25 kg/m2 for normal, 25–29.9 kg/m2 for overweight, and ≥ 30 kg/m2 for obesity. The latent class trajectory model (LCTM) was used to identify BMI trajectories. Multinomial logistic regression models were fitted to evaluate the association between different BMI trajectories and osteoporosis or osteopenia.
Results
For the 9,706 eligible participants, we identified four BMI trajectories, including stable (n = 7,681, 70.14%), slight increase (n = 1253, 12.91%), increase to decrease (n = 195, 2.01%), and rapid increase (n = 577, 5.94%). Compared with individuals in the stable trajectory, individuals in the rapid increase trajectory had higher odds of osteoporosis (OR = 2.25, 95% CI 1.19–4.23) and osteopenia (OR = 1.49, 95% CI 1.02–2.17). This association was only found in the lumbar spine (OR = 2.11, 95% CI 1.06–4.2) but not in the femoral neck. In early-stage (age 25–10 years ago) weight change, staying an obesity and stable weight seemed to have protective effects on osteoporosis (OR = 0.26, 95% CI 0.08–0.77) and osteopenia (OR = 0.46, 95% CI 0.25–0.84). Meanwhile, keeping an early-stage stable and overweight was related to lower odds of osteopenia (OR = 0.53, 95% CI 0.34–0.83). No statistically significant association between recent (10 years ago to baseline) weight change and osteoporosis was found.
Conclusions
Rapid and excess weight gain during adulthood is associated with a higher risk of osteoporosis. But this association varies by skeletal sites. Maintaining stable overweight and obesity at an early stage may have potentially beneficial effects on bone health.
Similar content being viewed by others
Background
Osteoporosis is a systemic skeletal metabolic ailment characterized by decreased bone mass and disturbance of bone architecture, which compromises bone strength and raises the risk of fracture [1]. According to a meta-analysis, the global prevalence of osteoporosis and osteopenia were estimated to be 19.7% and 40.4%, respectively [2]. The National Health and Nutrition Examination Survey (NHANES) data report from 2005 to 2010 showed that 48.3% of individuals aged 65 years and over had osteopenia at the lumbar spine or femoral neck, and 16.2% of the persons had osteoporosis in the United States [3]. However, as the population ages, the prevalence of osteoporosis is expected to increase even further [4].
Body mass index (BMI) and bone mineral density (BMD) are only significantly correlated at certain ages [5, 6]. Yet, studies on the association between BMI and osteoporosis have produced inconsistent results [7, 8], possibly because most studies utilized BMI at a single time point, which ignored the influence of longitudinal weight fluctuation on osteoporosis. Recent studies have focused on the relationship between weight changes and osteoporosis. K E Ensrud et al. demonstrated that late-life weight loss in males was associated with lower total BMD and weaker peripheral bones [9]. A cross-sectional study revealed that the increased likelihood of osteoporosis was significantly affected by attempting to gain weight or loss weight [10]. Nevertheless, most of the research assessed BMI at two time points and did not consider weight changes over a lifetime. Such an approach neglects the course of BMI trajectories over the lifespan, which, given the varying trends, rates, and magnitudes of BMI change, might provide further insight into the complex association of weight change with bone loss.
Based on BMI data from multiple time points, this study used the latent class trajectory model (LCTM) to identify BMI change trajectories during participants’ lifespans. And we studied the association between different BMI trajectories and osteoporosis or osteopenia using multinomial logistic regression among people aged 50 and older from NHANES 2005–2018. From the perspective of weight change trajectories, our present study may provide some strategies for the prevention of osteoporosis in the middle-aged and elderly population.
Methods
Study population and participants selected
The NHANES is a series of cross-sectional national surveys conducted by the National Center for Health Statistics (NCHS) to assess and study the health and nutrition status of American adults and children. Stratified multi-stage sampling techniques and documented designs are used to ensure the sample is representative of the civilian American population [11]. The ethics and data collection protocols were approved by the NCHS Ethics Review Board, and all participants signed informed consent before the interviews and health examinations. The data used in this study were acquired from the NHANES website at https://www.cdc.gov/nchs/nhanes/index.htm.
This study was a cross-sectional study and involved a secondary analysis. Data sets were selected from NHANES 2005–2018. Participants aged 50 and above were enrolled, similar to other studies [12, 13]. We excluded the participants with less than three BMI records and have missing data for both the femoral neck and lumbar spine BMD. We also excluded those with a history of hip and spine fractures, kidney diseases, thyroid disease, and osteoporosis medication to ensure that the results were not confounded by these factors. Finally, a total of 9,706 participants were included in the study analyses (Fig. 1). In the following, we referred to the “baseline” as the time when the survey was conducted and all covariates were collected.
Definition of BMI and weight change
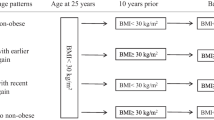
The NHANES Anthropometry section measured participants’ standing height and weight at baseline using standardized examination procedures [14]. The Sample Person Questionnaire retrospectively collected self-reported weight history, including weight one year ago, weight 10 years ago, and weight and height at age 25. We estimated the height 1 year ago and 10 years ago by using the baseline height. BMIs (BMI25y, BMI10prior, BMI1prior, and BMIbaseline) were calculated as weight (kg) at the specific time divided by the corresponding height (m) squared, respectively. For example, BMI25y was defined as weight at age 25 divided by height squared at age 25. BMI data more than three times the interquartile range (IQR) were treated as missing values in our study.
To investigate weight change patterns across different life stages, we evaluated weight changes during the early stage (from 25 years old to 10 years ago) and the recent stage (from 10 years ago to baseline). We considered a weight change of 5% or less as stable and a change of over 5% as significant [15, 16]. Standard BMI criteria were used, with < 25 kg/m2 for normal, 25–29.9 kg/m2 for overweight, and ≥ 30 kg/m2 for obesity. We defined weight change patterns by considering BMI status at the previous time point and the weight change percentage between the two time points. For instance, “normal-stable” in the early stage was defined as having a normal BMI at age 25 and no more than a 5% weight change from age 25 to 10 years ago. The same criterion was applied to define “overweight-stable” and “obesity-stable” weight change patterns. Participants whose BMI changed by more than 5% were assigned to either the “increase” or “decrease” group.
BMD measurement and definition of osteoporosis/osteopenia
Dual-energy X-ray Absorptiometry (DXA) is widely used as the gold standard of bone density assessment due to its validation and the low dose of X-rays [17]. The DXA examination protocol is well-documented in the Body Composition Procedures Manual located on the NHANES website. DXA scans were collected for both the femoral neck and lumbar spine in NHANES 2005–2010, 2013–2014, and 2017–2018. The Lumbar spine was scanned in NHANES 2011–2012 and 2015–2016. The bone mineral density T-score was calculated using the formula (BMDrespondent-mean BMDreference group)/SDreference group. As recommended by the World Health Organization (WHO) and the International Society for Clinical Densitometry (ISCD) [18], we used non-Hispanic white females aged 20–29 years from NHANES III data as the reference group for femoral neck measurements [19], while the reference group for lumbar spine measurements was obtained from the Vital and Health Statistics released by the Centers for Disease Control and Prevention (CDC) [20]. Osteoporosis was defined as a T-score ≤ −2.5 and osteopenia −2.5 < T-score ≤ −1.
Covariates
Various sociodemographic information was obtained, including age, sex, ethnicity, education, marital status, and poverty-income ratio (PIR). PIR was calculated by dividing family income by poverty guideline, considering the family size, year, and state. A higher PIR reflects a relatively higher socioeconomic status [21]. We categorized PIR as ≤ 1.30, 1.31–3.50, and > 3.50 [22]. Smoking cigarettes was categorized as never, former, and current. Alcohol consumption was classified as non-drinker and ever-drinker, and ever-drinker was further divided into current drinker and social drinker based on drinking frequency [23]. Physical activity was categorized into inactive, insufficient, moderate, and high by the cut-off values of 600 and 1200 metabolic equivalents of task (MET) minutes per week in conformity with the Global Physical Activity Questionnaire Analysis [24]. We categorized sleep status into ≥ 7 h and < 7 h as suggested by the National Sleep Foundation [25]. Other covariates included cancer, diabetes, systolic/diastolic blood pressure (BP), total cholesterol, and baseline BMI. Cancer and diabetes were categorized into “yes” and “no” based on the questions “Ever told you had cancer or malignancy” and “Doctor told you to have diabetes”. BP (mmHg), total cholesterol (mmol/L), and baseline BMI (Kg/m2) were defined as continuous variables. In the NHANES study, BP was measured after the participants had been seated for five minutes, and three consecutive readings were obtained. A fourth BP reading was taken if the data was interrupted or incomplete. BP values were averaged across all measurements in our study.
Statistical analysis
We used LCTM to identify the trajectories of BMI over time [26, 27]. The LCTM is a specialized type of finite mixture modeling that aims to find latent classes of people that exhibit similar trends in a determinant with time [28]. The optimal number of trajectories was chosen based on the minimum Bayesian information criteria (BIC) while maintaining the mean posterior probability over 70% in each class and the class size ≥ 2% of the population [29]. The model selection process also considered the clinical significance of the trajectories [30]. We assumed different BMI trajectories for men and women, due to sex differences in the factors that influence BMI [31]. Therefore, the LCTMs were fitted for men and women separately and used for subgroup analyses subsequently.
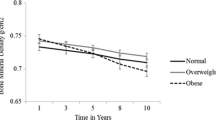
Baseline characteristic data were grouped by BMI trajectories and presented as weighted mean and standard error (SE) for continuous variables, and frequencies and weighted percentages for categorical variables. Analysis of variance (ANOVA) and chi-square tests were used for group comparisons of continuous and categorical variables, respectively. T-score comparisons of the femoral neck and lumbar spine across different BMI trajectories were presented using violin plots. Age and sex-adjusted partial correlation coefficients between the T-score and baseline BMI of the two skeletal sites were also calculated. We fitted three multinomial logistic regression models to evaluate the impact of different BMI trajectories on osteoporosis or osteopenia. To rule out the positive correlations between baseline BMI and BMD, baseline BMI was adjusted in all three models: Model 1: adjusted for age, sex, ethnicity, and baseline BMI; Model 2: Model 1 plus education, smoking, alcohol drinking, physical activity, and sleep status; Model 3: Model 2 plus diabetes, cancer, and total cholesterol. Results were reported as odds ratios (OR), 95% confidence intervals (95% CI), and P-values.
In sensitivity analyses, we excluded participants with extreme BMI values (< 15 and > 50 kg/m2) at any time point, as these values were deemed biologically implausible [26]. Furthermore, we calculated the E-value to gauge the potential influence of unobserved confounding on the observed association. To account for the complex sampling techniques and study design, all analyses used a weighted approach [32]. Statistical significance was indicated by a bidirectional P-value < 0.05. Data analyses were conducted using R software Version 4.2.1.
Results
The BMI trajectories and characteristics of participants
Four BMI trajectories were identified using LCTM (Fig. 2). The values of the BIC parameter, the mean posterior probability, and the sample size of each trajectory in the model, which we used to determine the number of clusters, were presented in Additional file 1: Table S1. The majority of the participants remained on a stable trajectory during their lifetime (79.14%), while some experienced a slight increase in BMI (12.91%). 5.94% followed a moderately to rapidly increasing BMI trajectory throughout the time, and 2.01% had a trajectory of increasing to decreasing. These four trajectories were labeled as “stable,” “slight increase,” “rapid increase,” and “increase to decrease,” respectively. LCTM results for different sexes were presented in Additional file 1: Tables S2–S3 and Figure S1.
Among all participants, the weighted mean age was 60.99 (0.16) years and 4,230 (45.24%) were female. The characteristics of the population were compared by trajectory groups in Table 1. Significant differences were observed in all variables except for education, diastolic blood pressure (DBP), and total cholesterol among the four groups. The stable trajectory group had the highest prevalence of osteoporosis and osteopenia but the lowest baseline BMI. And this group also exhibited the lowest T-scores for the femoral neck and lumbar spine (Fig. 3). We further calculated the partial correlation coefficients adjusted for age and sex between the T-score and baseline BMI of the two skeletal sites, resulting in partial correlation coefficients of 0.39 and 0.31 (both P < 0.001), respectively.
Association between BMI trajectories and osteoporosis/osteopenia
We used multinomial logistic regression to assess the association between BMI trajectories and osteoporosis/osteopenia. As shown in Table 2, after adjustment for covariates, individuals in the rapid increase trajectory had 225% odds of developing osteoporosis (OR = 2.25, 95% CI 1.19–4.23, P = 0.012) and 149% odds of developing osteopenia (OR = 1.49, 95% CI 1.02–2.17, P = 0.040) compared with individuals in the stable trajectory. However, we found no statistically significant association between either a slight increase or increase to decrease trajectory and osteoporosis or osteopenia when compared to the stable trajectory. Sex-stratified analysis was consistent with that in the total population, where the odds of osteoporosis in the rapid increase trajectory were 2.69 (OR = 2.69, 95% CI 1.03–7.07, P = 0.044) and 2.62 (OR = 2.62, 95% CI 1.17–5.86, P = 0.019) times higher than the odds in the stable trajectory, for male and female, respectively. Participants were also stratified by age, and the statistically significant association between rapid increase trajectory and osteoporosis or osteopenia was only observed in the group ≤ 65 years. The odds ratios were 2.32 (95% CI 1.11–4.83, P = 0.025) for osteoporosis and 1.57 (95% CI 1.02–2.43, P = 0.043) for osteopenia.
Different skeletal locations have different associations
Multinomial logistic regression analyses were also performed and adjusted for the same covariates in different skeletal sites. The results in Table 3 indicated that the association between BMI trajectory and osteoporosis varied depending on the bone site. In the femoral neck, we can only find a modest and positive association between rapid increase trajectory and osteopenia (OR = 1.45, 95% CI 1–2.09, P = 0.048) in model 3. As for the lumbar spine, the odds of osteoporosis were much higher in the rapid increase trajectory compared to the stable trajectory, across all models.
Effect of weight change on osteoporosis/osteopenia at different life stages
As seen in Table 4, there were no statistically significant results regarding recent weight change and the presence of osteoporosis or osteopenia. However, during the early stage, staying an obesity-stable body weight had a protective effect on osteoporosis (OR = 0.26, 95% CI 0.08–0.77, P = 0.016) and osteopenia (OR = 0.46, 95% CI 0.25–0.84, P = 0.011). We also observed a beneficial association between overweight-stable and osteopenia (OR = 0.53, 95% CI 0.34–0.83, P = 0.005). Moreover, increasing weight during the early stage was also found to decrease the likelihood of developing osteoporosis by approximately 30% (OR = 0.71, 95% CI 0.51–0.97, P = 0.033).
The trajectory graph (Fig. 2) clearly showed that individuals in the rapid increase trajectory had substantial weight gains, especially after the age of 45. Therefore, we further separated these individuals and also named the “rapid increase” in the analysis of recent weight change (Additional file 1: Table S4). Compared to the normal-stable group, those in the rapid increase group had higher odds of osteoporosis (OR = 2.34, 95% CI 1.12–4.92, P = 0.024).
Sensitivity analysis
After excluding the participants with BMI of < 15 and > 50 kg/m2, the multinomial logistic regression results remained the same (Additional file 1: Table S5). The E-value for the point estimate and the lower confidence interval were 3.93 and 1.67. Following the suggestion from VanderWeele and Ding [33], the large E-value means that only the unmeasured confounders have a strong association with both the BMI trajectories and incident osteoporosis could the observed OR of 2.25 be explained away, but weak confounders could not do so.
Discussion
This study examined the association between four BMI trajectories and bone health among individuals aged 50 and above in NHANES 2005–2018. We found that a rapid increase in BMI trajectory was moderately and positively associated with osteoporosis and osteopenia. This association was consistent across different sexes and the age group of ≤ 65 years. Additionally, we found that the impact of rapid weight gain on bone loss varied depending on the skeletal site, with the lumbar spine being more affected. When examining the impact of different life-stage weight changes on bone loss, we discovered that maintaining a stable obesity or overweight BMI during early stages was associated with lower odds of osteoporosis and osteopenia compared to those with a normal-stable BMI.
Despite the positive correlation between BMI and BMD, an increasing number of research have revealed that obesity may not be a protective factor against osteoporosis and may even be harmful to bone health [34, 35]. The inconsistent results may be due to the interaction between body weight, lean mass, and fat mass, as well as the complicated relationship between obesity and bone. This includes the positive impact of increased mechanical loading on BMD and the negative impact of excess fat on bone metabolism [8]. Therefore, the relationship between obesity and bone health is not straightforward and requires further investigation. Our study results indicate that individuals who experienced a rapid and extensive increase in BMI trajectory during adulthood may have a higher risk of developing osteoporosis. This finding is consistent with a previous study that found extreme obesity to be associated with reduced BMD in postmenopausal women [36]. Influenced by age-related declines in physical activity and hormone levels, weight gain in middle-aged and elderly people is primarily manifested as an increase in adipose tissue rather than lean tissue [37]. However, excessive adipose tissue is hazardous for bones. A population-based cohort study of Australians aged 45 to 70 found that visceral fat mass was adversely related to BMD after adjusting for body mass and lifestyle factors [38]. This negative association between fat mass and BMD was also confirmed by Zhang et al. [39]. Excess fat mass leads to an imbalance between the pro-inflammatory and anti-inflammatory adipokines, rendering the body in a state of chronic inflammation [40]. High levels of inflammatory factors such as tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) in fat tissue can promote the differentiation and activation of osteoclasts, inhibit the activity of osteoblasts, and finally lead to the decrease in bone density [41].
Different from the individuals who had rapid and extensive weight gain, those who maintained a stable weight in the early stage, whether overweight or obese, showed a lower risk of osteoporosis or osteopenia. Regardless of the reasons mentioned above, biological homeostasis may play a role. Homeostasis refers to the body’s ability to maintain relative stability in the internal environment despite external stimuli [42]. As individuals age, weight gain, muscle loss, and body fat redistribution can cause an imbalance in homeostasis, ultimately leading to adverse health outcomes. One study found that women who maintain a stable body weight can reduce bone loss after menopause [43]. In our analysis of the early-stage weight change, we found the increase group also had a lower risk of osteoporosis. This may be because most people in this group followed moderate weight gain and had a higher BMD.
Li’s research revealed an inverted U-shaped relationship between BMI and lumbar BMD, indicating that excessive BMI may have negative effects on the lumbar spine [6]. Our study reported that rapid and excessive weight increase affected the lumbar spine, but not the femoral neck. Previous pharmacological investigations have shown that the spine is more responsive to drug therapy than the hip [44]. Similarly, the lumbar spine may be more sensitively influenced by weight change or fat tissue than the femoral neck, since the spine bone has a higher turnover rate and is more sensitive to hormone and endocrine changes [45]. Additionally, a 14-year longitudinal study concluded that body mass index was adversely correlated with bone loss in the lumbar spine, but not in the femoral neck [46]. Yang’s prospective cohort study also found a significant association between whole body fat mass and bone loss only in the lumbar spine [47].
To our knowledge, this is the first study to assess the association between BMI trajectories and osteoporosis and osteopenia. The use of BMI at multiple time points to identify weight change patterns provided further and novel insight into the study of the BMI's impacts on bone. However, our study has some limitations. First, four trajectories of BMI change were identified in this study, but other trajectories may exist. Further identification of potential patterns of BMI change is needed. Second, the small number of people in the increase to decrease group may lead to increased instability of the results. Third, in the cross-sectional study of NHANES study, causality could not be established. Follow-up cohort studies or randomized clinical trials are needed for causal research. Meanwhile, weight history was collected retrospectively so that recall bias may exist. Last, we conducted this research in the American population, further validation in other populations is needed.
Conclusion
The observational study from NHANES 2005–2018 showed that rapid and excess weight gain throughout adulthood may increase the risk of osteoporosis. The lumbar spine BMD is more responsive to quick and excessive weight increase than the femoral neck. Maintaining an early-stage stable and overweight BMI is a protective factor against osteoporosis. This study suggests that individuals should avoid fast weight increases and extreme obesity to promote bone health and prevent osteoporosis.
Availability of data and materials
The NHANES database is publicly accessible and contains deidentified data for all participants utilized in this study. This dataset can be accessed, queried, and downloaded using this link: https://www.cdc.gov/nchs/nhanes/index.htm.
Abbreviations
- BMI:
-
Body mass index
- BMD:
-
Bone mineral density
- NHANES:
-
National Health and Nutrition Examination Survey
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- LCTM:
-
Latent class trajectory model
- NCHS:
-
The National Center for Health Statistics
- IQR:
-
Interquartile range
- DXA:
-
Dual-energy X-ray Absorptiometry
- WHO:
-
World Health Organization
- ISCD:
-
The International Society for Clinical Densitometry
- CDC:
-
Centers for disease control and prevention
- MET:
-
Metabolic equivalents of task
- BP:
-
Blood pressure
- SE:
-
Standard error
- ANOVA:
-
Analysis of variance
- DBP:
-
Diastolic blood pressure
- TNF-α:
-
Tumor necrosis factor α
- IL-6:
-
Interleukin-6
References
Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am. 2020;104:873–84.
Xiao P-L, Cui A-Y, Hsu C-J, Peng R, Jiang N, Xu X-H, et al. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: a systematic review and meta-analysis. Osteoporos Int. 2022;33:2137–53.
Looker AC. Percentage of Adults Aged 65 and Over With Osteoporosis or Low Bone Mass at the Femur Neck or Lumbar Spine: United States, 2005–2010. https://www.cdc.gov/nchs/data/hestat/osteoporsis/osteoporosis2005_2010.pdf. Accessed 19 Feb 2023
Becker DJ, Kilgore ML, Morrisey MA. The societal burden of osteoporosis. Curr Rheumatol Rep. 2010;12:186–91.
Cherukuri L, Kinninger A, Birudaraju D, Lakshmanan S, Li D, Flores F, et al. Effect of body mass index on bone mineral density is age-specific. Nutr Metab Cardiovasc Dis NMCD. 2021;31:1767–73.
Li Y. Association between obesity and bone mineral density in middle-aged adults. J Orthop Surg. 2022;17:268.
Lloyd JT, Alley DE, Hawkes WG, Hochberg MC, Waldstein SR, Orwig DL. Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos. 2014;9:175.
Zhu K, Hunter M, James A, Lim EM, Cooke BR, Walsh JP. Discordance between fat mass index and body mass index is associated with reduced bone mineral density in women but not in men: the Busselton healthy ageing study. Osteoporos Int. 2017;28:259–68.
Ensrud KE, Vo TN, Burghardt AJ, Schousboe JT, Cauley JA, Taylor BC, et al. Weight loss in men in late life and bone strength and microarchitecture: a prospective study. Osteoporos Int. 2018;29:1549–58.
Choi H-G, Kwon B-C, Yim S-H, Youk H, Lee J-W. Weight change is associated with osteoporosis: a cross sectional study using the Korean community health survey. Int J Environ Res Public Health. 2021;18:13368.
Curtin LR, Mohadjer LK, Dohrmann SM, Montaquila JM, Kruszan-Moran D, Mirel LB, et al. The national health and nutrition examination survey: sample design, 1999–2006. Vital Health Stat. 2012;2:1–39.
Ma M, Feng Z, Liu X, Jia G, Geng B, Xia Y. The saturation effect of body mass index on bone mineral density for people over 50 years old: a cross-sectional study of the US population. Front Nutr. 2021;8:763677.
Jepsen DB, Thomsen K, Hansen S, Jørgensen NR, Masud T, Ryg J. Effect of whole-body vibration exercise in preventing falls and fractures: a systematic review and meta-analysis. BMJ Open. 2017;7:e018342.
Hyattsville. Anthropometry Procedures Manual. 2017. https://wwwn.cdc.gov/nchs/data/nhanes/2017-2018/manuals. Accessed 19 Feb 2023.
Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, et al. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004.
Liu C-T, Sahni S, Xu H, McLean RR, Broe KE, Hannan MT, et al. Long-term and recent weight change are associated with reduced peripheral bone density, deficits in bone microarchitecture, and decreased bone strength: the framingham osteoporosis study: LONG-TERM AND SHORT-TERM WEIGHT LOSS AND PERIPHERAL BONE MEASURES. J Bone Miner Res. 2018;33:1851–8.
Baran DT, Faulkner KG, Genant HK, Miller PD, Pacifici R. Diagnosis and management of osteoporosis: guidelines for the utilization of bone densitometry. Calcif Tissue Int. 1997;61:433–40.
Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–81.
Looker AC, Orwoll ES, Johnston CC, Lindsay RL, Wahner HW, Dunn WL, et al. Prevalence of low femoral bone density in older U.S. adults from NHANES III. J Bone Miner Res. 1997;12:1761–8.
Looker AC, National Center for Health Statistics (U.S.), National Health and Nutrition Examination Survey (U.S.), editors. Lumbar spine and proximal femur bone mineral density, bone mineral content, and bone area, United States, 2005–2008: data from the National Health and Nutrition Examnination Survey (NHANES). Hyattsville, Md: U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012.
Zheng Y, Wang J, Wang Y, Xu K, Chen X. The hidden dangers of plant-based diets affecting bone health: a cross-sectional study with U.S. National Health and Nutrition Examination Survey (NHANES) data from 2005–2018. Nutrients. 2023;15:1794.
Lan T, Liu B, Bao W, Thorne PS. BMI modifies the association between dietary intake and serum levels of PCBs. Environ Int. 2021;156:106626.
Alharthi SSY, Natto ZS, Midle JB, Gyurko R, O’Neill R, Steffensen B. Association between time since quitting smoking and periodontitis in former smokers in the National Health and Nutrition Examination Surveys (NHANES) 2009 to 2012. J Periodontol. 2019;90:16–25.
WHO. Surveillance and Population-Based Prevention, Prevention of Noncommunicable Diseases Department, World Health Organization. Global Physical Activity Questionnaire (GPAQ) Analysis Guide. 2014. https://cdn.who.int/media/docs/default-source/ncds/ncd-surveillance/gpaq-analysis-guide.pdf?sfvrsn=1e83d571_2. Accessed 19 Feb 2023.
Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National sleep foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–3.
Tumin D, Frech A, Lynch JL, Raman VT, Bhalla T, Tobias JD. Weight gain trajectory and pain interference in young adulthood: evidence from a longitudinal birth cohort study. Pain Med. 2020;21:439–47.
Kelly Y, Patalay P, Montgomery S, Sacker A. BMI development and early adolescent psychosocial well-being: UK millennium cohort study. Pediatrics. 2016;138:e20160967.
Jones BL, Nagin DS. A note on a stata plugin for estimating group-based trajectory models. Sociol Methods Res. 2012;42:608–13.
Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3:628–35.
Lennon H, Kelly S, Sperrin M, Buchan I, Cross AJ, Leitzmann M, et al. Framework to construct and interpret latent class trajectory modelling. BMJ Open. 2018;8:e020683.
Dastgheib SA, Rezaianzadeh A, Maharlouei N, Rahimikazerooni S, Lankarani KB. Gender difference in determinant factors of being overweight among the 40–70-year-old population of Kharameh cohort study, Iran. BMC Public Health. 2021;21:746.
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;2:1–24.
VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74.
Zhao L-J, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, et al. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res. 2007;23:17–29.
Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg. 2011;6:30.
Núñez NP, Carpenter CL, Perkins SN, Berrigan D, Jaque SV, Ingles SA, et al. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obes Silver Spring Md. 2007;15:1980–7.
Lee CG, Boyko EJ, Nielson CM, Stefanick ML, Bauer DC, Hoffman AR, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–40.
Zhu K, Hunter M, James A, Lim EM, Cooke BR, Walsh JP. Relationship between visceral adipose tissue and bone mineral density in Australian baby boomers. Osteoporos Int. 2020;31:2439–48.
Zhang P, Peterson M, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr. 2015;101:337–43.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97.
Wang T, He C. TNF-α and IL-6: the link between immune and bone system. Curr Drug Targets. 2020;21:213–27.
Goldstein DS. How does homeostasis happen? Integrative physiological, systems biological, and evolutionary perspectives. Am J Physiol Regul Integr Comp Physiol. 2019;316:R301–17.
Blain H, Carrière I, Favier F, Jeandel C, Papoz L. Body weight change since menopause and percentage body fat mass are predictors of subsequent bone mineral density change of the proximal femur in women aged 75 years and older: results of a 5 year prospective study. Calcif Tissue Int. 2004;75:32–9.
Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, et al. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the fracture intervention trial long-term extension. J Bone Miner Res Off J Am Soc Bone Miner Res. 2004;19:1259–69.
Naessén S, Carlström K, Glant R, Jacobsson H, Hirschberg AL. Bone mineral density in bulimic women–influence of endocrine factors and previous anorexia. Eur J Endocrinol. 2006;155:245–51.
Reeve J, Walton J, Russell LJ, Lunt M, Wolman R, Abraham R, et al. Determinants of the first decade of bone loss after menopause at spine, hip and radius. QJM Mon J Assoc Physicians. 1999;92:261–73.
Yang S, Center JR, Eisman JA, Nguyen TV. Association between fat mass, lean mass, and bone loss: the Dubbo osteoporosis epidemiology study. Osteoporos Int. 2015;26:1381–6.
Acknowledgements
We would like to thank the staff and participants in NHANES for their hard work and dedication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding
We acknowledge financial support from the Natural Science Foundation of Shanghai, China (23ZR1414000), the National Key Research and Development Program of China (Grant Numbers: 2022YFC3400700), the Science and Technology Innovation 2030 Major Projects (2022ZD0211600).
Author information
Authors and Affiliations
Contributions
Drs KX and XC had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: JW, KX, XC. Acquisition, analysis, or interpretation of data: JW, YZ, YW, CZ. Drafting of the manuscript: JW, YZ, YW, CZ. Critical revision of the manuscript for important intellectual content: JW, YZ, YW, CZ, YJ, CS, MC, TZ, XC, KX. Statistical analysis: JW, YZ, KX. Obtained funding: KX, XC. Administrative, technical, or material support: KX, XC. Supervision: YJ, CS, MC, TZ, XC, KX.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethics and data collection protocols were approved by the NCHS Ethics Review Board and all participants signed informed consent before the interviews and health examinations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Model adequacy assessments of latent class trajectory models for the total population. Table S2. Model adequacy assessments of latent class trajectory models for male. Table S3. Model adequacy assessments of latent class trajectory models for female. Table S4. The association between recent weight change and osteoporosis/osteopenia. Table S5. The association between BMI trajectories and osteoporosis/osteopenia after excluding participants with BMI < 15 and > 50 kg/m2. Figure S1. BMI trajectories during adulthood of different sexes. Figure A for male and B for female.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Zheng, Y., Wang, Y. et al. BMI trajectory of rapid and excessive weight gain during adulthood is associated with bone loss: a cross-sectional study from NHANES 2005–2018. J Transl Med 21, 536 (2023). https://doi.org/10.1186/s12967-023-04397-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-04397-9