Abstract
Background
Myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) is a complex, heterogenous disease. It has been suggested that subgroups of people with ME/CFS exist, displaying a specific cluster of symptoms. Investigating symptom-based clusters may provide a better understanding of ME/CFS. Therefore, this study aimed to identify clusters in people with ME/CFS based on the frequency and severity of symptoms.
Methods
Members of the Dutch ME/CFS Foundation completed an online version of the DePaul Symptom Questionnaire version 2. Self-organizing maps (SOM) were used to generate symptom-based clusters using severity and frequency scores of the 79 measured symptoms. An extra dataset (n = 252) was used to assess the reproducibility of the symptom-based clusters.
Results
Data of 337 participants were analyzed (82% female; median (IQR) age: 55 (44–63) years). 45 clusters were identified, of which 13 clusters included ≥ 10 patients. Fatigue and PEM were reported across all of the symptom-based clusters, but the clusters were defined by a distinct pattern of symptom severity and frequency, as well as differences in clinical characteristics. 11% of the patients could not be classified into one of the 13 largest clusters. Applying the trained SOM to validation sample, resulted in a similar symptom pattern compared the Dutch dataset.
Conclusion
This study demonstrated that in ME/CFS there are subgroups of patients displaying a similar pattern of symptoms. These symptom-based clusters were confirmed in an independent ME/CFS sample. Classification of ME/CFS patients according to severity and symptom patterns might be useful to develop tailored treatment options.
Similar content being viewed by others
Introduction
Myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) is a serious long-term, multi-system disease, which is often characterized by debilitating fatigue that lasts at least 6 months and cannot be explained by other underlying medical conditions. Globally, about 20 million people are thought to have ME/CFS [1].
The excessive fatigue is often accompanied by a variety of other symptoms, including post-exertional malaise (PEM), sleep problems, pain and cognitive problems [2]. These symptoms seriously affect daily life of people with ME/CFS, as they limit normal daily activities, social routines, work and/or leisure activities [3, 4]. As a result, people with ME/CFS often experience a reduced quality of life [5, 6].
As there is no diagnostic biomarker for ME/CFS, the diagnosis of the disease relies on self-reported symptoms [1]. To date, over twenty case definitions exist, each capturing a different subset of individuals based on their reported symptoms and functioning [2]. The Fukuda CFS Criteria [7], the Canadian ME/CFS Criteria (CCC) [8], the ME International Consensus Criteria (ME-ICC) [9], and the Institute of Medicine Criteria (IOM) [10] are the most commonly used. The lack of a clear case definition emphasizes the heterogeneity of the disease.
Preliminary evidence suggest that subgroups of people with ME/CFS exist, displaying a specific set or cluster of symptoms (i.e. a group of two or more symptoms that occur concurrently and are interrelated) which are relatively independent of other clusters [11]. However, clustering was based on a limited number of symptoms or non-core (i.e. less frequently observed) symptoms [12,13,14,15,16,17], and/or a non-validated questionnaires was used to assess symptoms [16, 18].
Investigating symptom-based clusters may provide a better understanding of the symptom experience of people with ME/CFS. This may contribute to a better understanding of the clinical complexity of ME/CFS, and in turn, may contribute to the development of more tailored symptom management strategies. Therefore, this study aimed to identify clusters in people with ME/CFS based on the frequency and severity of symptoms. In addition, an independent ME/CFS dataset was used to validate the symptom-based clustering.
Methods
Study design and participants
In this cross-sectional study, members of the Dutch ME/CFS Foundation (ME/CVS Stichting; https://mecvs.nl/) were invited to complete a web-based survey between January 26 and February 28 2022. Individuals who did not report being diagnosed with ME/CFS were excluded from the analyses.
Ethical approval for this study was waived by the medical ethics committee of Maastricht University because the Medical Research Involving Human Subjects Act (WMO) did not apply to this study (METC 2021-2797). Digital informed consent was obtained from all respondents at the start of the survey. Without providing informed consent, participants were unable to start the questionnaire.
Measures
Participants completed an online version of the DePaul Symptom Questionnaire version 2 (DSQ-2), which is a self-report measure of ME and CFS symptomatology, demographics, and medical, occupational and social history [19]. The DSQ-2 has demonstrated to have a strong reliability and validity [19, 20] and is able to differentiate individuals with ME/CFS from healthy controls [21] and individuals with other chronic diseases [22, 23]. Furthermore, the DSQ-2 can be used to determine whether individuals meet the criteria for the Fukuda, CCC, ME-ICC and/or IOM case definition [20]. The questionnaire was translated into Dutch using a forward–backward translation procedure.
Participants reported the frequency and severity of 79 symptoms related to the illness over the past 6 months. Frequency of symptoms was rated on a 5-point Likert scale: 0 = none of the time, 1 = a little of the time, 2 = about half the time, 3 = most of the time, and 4 = all of the time. Similarly, severity of symptoms was rated on a 5-point Likert scale: 0 = symptom not present, 1 = mild, 2 = moderate, 3 = severe, and 4 = very severe.
Symptom scores were analyzed in two ways: (1) a composite variable was created by averaging the frequency and severity scores of each symptom and multiplying it by 25; the composite score of each symptom ranged from 0 to 100 points. A higher score indicated a higher symptom burden [20]; and (2) a binary “2/2 threshold” variable was created by examining the frequency and severity scores of each symptom; participants who reported ratings of two or higher for both frequency (i.e. about half the time, most of the time, or all of the time) and severity (i.e. moderate, severe, or very severe) were considered to have the symptom [20]. For all other scores the symptom was not present.
Validation dataset
An extra dataset of 252 people with a self-reported diagnosis of ME/CFS from the United States (US) was used to assess the reproducibility of the symptom-based clusters [24]. Participants for the US database were recruited from email requests to national foundations, posts to online support groups, research forums and social media platforms. All participants provided digital informed consent and subsequently completed an online version of the DSQ-2. Part of these DSQ-2 data were published before [24].
Statistics
Data are presented as median and interquartile ranges (IQR) for continuous data and as frequencies and proportions for categorical data. Moreover, a self-organizing map (SOM) was used to visualize the clustering of the patients. The SOM method can be viewed as a non-parametric regression technique that converts multi-dimensional data spaces into lower dimensional abstractions. A SOM generates a non-linear representation of the data distribution and allows the user to identify homogenous data groups visually.
Severity and frequency scores of the 79 measured symptoms were used. Therefore each participant had 158 features. Clustering was performed on MATLAB (R2022a, MathWorks, MA, USA), following its default SOM setting, except for the number of iterations for training the SOM, which was changed to 1000 [25]. The default random number generation of MATLAB was used to initialize all competitive units of the SOM, meaning that with the same input and SOM settings, the results are always the same. Further details on the clustering method are provided in Additional file 1.
A Kruskal–Wallis test, adjusted for multiple comparisons, was used to test differences in participant characteristics between clusters. Statistical analyses were conducted using SPSS 25.0 (IBM Corporation, NY, USA). A priori, the level of significance was set at p < 0.05. The Venn diagrams were generated with Meta-Chart (https://www.meta-chart.com/venn#/display) and intersection plots were made using the R package ‘UpSetR’ [26]. Finally, the trained SOM was applied to the data from the validation sample.
Results
Participant characteristics
The link to the online questionnaire was send to 1392 members of the Dutch ME/CFS Foundation, of which 367 completed the questionnaire (response rate: 26%). Data from thirty participants were excluded, as they stated to not have been diagnosed with ME/CFS. So, data from 337 participants were used for the analyses (Table 1). In general, participants were mostly middle-aged women [82% female; median (IQR) age: 55 (44–63)] with a normal body mass index [24 (21–28) kg/m2]. Fifty-five percent of the participants were married or living with a partner. The majority of the participants had a medium or high education level and about two-thirds of them were incapacitated for work. Almost 90% of the participants fulfilled the Fukuda case definition, compared to 80%, 59% and 39% fulfilling the IOM, CCC and ME-ICC case definitions, respectively. More than a quarter of the participants met the criteria for all four different case definitions, whilst 5% of the participants met none of the abovementioned case definitions (Table 1, Fig. 1a).
Proportional Venn diagrams and UpSet plots showing the overlap of different ME/CFS case definitions in the Dutch (a) and US (b) ME/CFS population. The different case definitions are displayed as horizontal bars on the lower left corner of the UpSet plot. Paired intersections are displayed as black dots; gray dots indicate the case definitions that are not part of the intersection. Black lines connecting 2 or more black dots indicate which case definitions form the intersections. The heights of the vertical bars indicate the intersection size (number of number of patients with indicated case definitions)
Symptoms
The vast majority of the participants were experiencing fatigue (90.7% fulfilled the 2/2 threshold). Furthermore, PEM, sleep-related problems, neurocognitive problems and pain were frequently reported, whilst on average autonomic, neuroendocrine, and immune symptoms were less prevalent (Table 2). Participants reported a median (IQR) of 27 (19–37) symptoms (using the 2/2 threshold, Additional file 2).
Symptom-based clusters
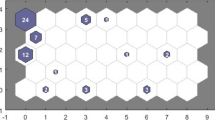
Forty-five clusters were identified, of which 13 clusters included ≥ 10 patients (Fig. 2a, b). In general, key features of these 13 clusters can be summarized as follows:
Symptom-based clusters using self-organizing maps. All clusters of patients are displayed in the direction of left to right and bottom to top. Each hexagon represents a cluster, and the number within a hexagon shows the number of patients in the cluster. The x-axis and y-axis indicate the number of clusters, starting from 0. In particular, coordinate (0,0) corresponds to Cluster 1, coordinate (1,0) corresponds to Cluster 2, etc. The 13 clusters including at least 10 patients are shown in purple. a Symptom-based clusters in Dutch ME/CFS population. b Connection between symptom-based clusters. The blue hexagons represent the clusters. The colors in the regions containing the red lines indicate the distances between clusters: darker colors represent larger distances (less similarity between the two clusters), lighter colors represent smaller distances (more similarity between the two clusters). c Symptom-based clusters in US population
-
Participants in Cluster 2 (n = 24) were characterized by low frequency and severity scores for problems related to dizziness/fainting, stomach ache and problems staying asleep.
-
Participants in Cluster 4 (n = 23) were characterized by low frequency and severity scores for urinary problems and higher frequency scores for increased heart rate by standing.
-
Participants in Cluster 7 (n = 30) were characterized by increased frequency scores for cognitive impairments.
-
Participants in Cluster 9 (n = 26) were characterized by high frequency and severity scores for dizziness/fainting, feeling unsteady on their feet and sensitivity/intolerance to smell and alcohol.
-
Participants in Cluster 11 (n = 17) were characterized by high frequency and severity scores for impaired day–night rhythm but low frequency and severity scores for muscle weakness and coordination problems.
-
Participants in Cluster 19 (n = 19) were characterized by low frequency scores for physical fatigue, symptoms after exercise and irritable bowel problems.
-
Participants in Cluster 24 (n = 43) were characterized by high frequency and severity scores for sensitivity to sound, sleeping problems and symptoms after exercise.
-
Participants in Cluster 26 (n = 18) were characterized by a relatively high symptom burden combined with high frequency and severity scores for temperature related symptoms and pressure pain.
-
Participants in Cluster 28 (n = 38) were characterized by a relatively low symptom burden and low frequency and severity scores for muscle related problems.
-
Participants in Cluster 31 (n = 15) were characterized by high frequency and severity scores for sensitivity/intolerance to mold and temperature and stomach/bowel problems.
-
Participants in Cluster 36 (n = 10) had the highest symptom burden (i.e. highest frequency and severity of symptoms).
-
Participants in Cluster 37 (n = 18) had the lowest symptom burden (i.e. lowest frequency and severity of symptoms).
-
Participants in Cluster 40 (n = 20) were characterized by low frequency and severity scores for symptoms after physical and mental exercise, muscle fatigue and problems with focus on one thing.
Participants in Clusters 28 and 37 reported a significantly lower number of symptoms compared to participants in Clusters 4, 7, 9, 24, 26, 31 and 36, whilst the number of symptoms in Clusters 36 was significantly higher compared to all other clusters, except for Clusters 7, 9 and 26 (p < 0.05; Table 3).
Please see Additional file 3 for symptom scores of the different clusters. Please see Additional file 4 for the symptom scores of the remaining 42 clusters with < 10 patients.
Clinical characteristics
Participant characteristics of the 13 largest symptom-based clusters are displayed in Table 3. On average, participants in cluster 4 were significantly younger compared to participants in clusters 7, 19, 24, 28, 37 and 40. Cluster 19 had a significantly higher proportion of males compared to Clusters 28. Prevalence of work disability was significantly lower in Cluster 37 compared to Clusters 4, 7, 24, 26 and 36. Please see Additional file 5 for the characteristics of the remaining 42 clusters with < 10 patients.
Case definitions
Distribution across the different case definitions and number of case definitions that were met were significantly different between the 13 largest clusters. Generally, the proportion of patients fulfilling the different case definitions was highest in Cluster 9 and lowest in Cluster 19, 28 and 37. None of the participants in Cluster 37 fulfilled the CCC and ME-ICC case definition, and also the proportion of patients fulfilling the IOM case definition was lowest in Cluster 37. The proportion of patients fulfilling the Fukuda CFS Criteria was lowest in Cluster 36. In addition, Clusters 9, 26 and 36 had the highest proportion of participants fulfilling the ME-ICC case definition.
Validation of symptom-based clusters
Participant characteristics of the US database are listed in Table 1. In general, these participants were slightly younger, were more often widower or divorced, had a higher education level and were less often on disability compared to the Dutch participants (all p < 0.05). The proportion of patients fulfilling the different case definitions and the total number of case definitions met was significantly higher in the US database compared to the Dutch database. Furthermore, US participants generally experienced a higher symptom burden compared to the Dutch participants (Table 2). Applying the trained SOM to the data of the validation sample, resulted in a similar symptom pattern compared the Dutch dataset (Fig. 2c).
Discussion
This study demonstrated that people with self-reported ME/CFS can suffer from a variety of symptoms, besides severe fatigue. Indeed, these symptoms co-occurred in multiple specific patterns. Moreover, 5% of the people with ME/CFS did not meet the criteria of the most common case definitions. These findings were corroborated in an independent second sample of people with ME/CFS.
As expected, fatigue and PEM, which are considered key symptoms of ME/CFS, were reported across all of the symptom-based clusters. However, the clusters were defined by a distinct pattern of other symptoms. For example, Cluster 7 was predominately characterized by cognitive symptoms, whilst Cluster 26 was characterized by more temperature-related symptoms and pressure pain. Importantly, the different clusters are identified by a specific pattern of symptom severity and frequency, but this does not indicate that these symptoms are not present in other clusters.
Remarkably, 11% of the patients could not be classified into one of the 13 largest clusters (≥ 10 people), indicating that a considerable proportion of people with ME/CFS present an unique symptom pattern.
The current findings emphasize the large heterogeneity in symptoms in a sample of people with ME/CFS, and the complexity for clinicians to adequately monitor and treat these patients. Which symptom needs to be addressed first? Can different symptoms be addressed at the same time? Are symptoms responsive to pharmacological and/or non-pharmacological treatment?
Interestingly, when applying the identified clusters to the validation dataset, similar symptom patterns were found. This suggests that the symptom-based clusters may be valid for samples of people with ME/CFS in different parts of the world.
Our findings were built on earlier attempts to identify symptom-based clusters. For example, Hickie et al. showed that in CFS a distinction can be made between high and low symptomatic people [16]. Furthermore, using five common symptoms listed in the diagnostic criteria for CFS (i.e. muscle pain, joint pain, headaches, painful lymph nodes, sore throat) Collin et al. identified three phenotypes in a UK secondary care cohort (i.e. high symptomatic, low symptomatic and pain-only), which were replicated in another UK patient cohort and a Dutch tertiary care cohort [13, 27]. The existence of a highest symptomatic and lowest symptomatic clusters in ME/CFS was confirmed by our analyses.
Similar to Huber et al. we not only included the most commonly reported symptoms in ME/CFS, but we also included non-core symptoms [12]. In addition to the high and low symptomatic subgroups, they were able to identify symptom-specific subgroups, including one subgroup with primarily gastro-intestinal symptoms, one subgroup with primarily circulatory symptoms, one subgroup with gastro-intestinal and circulatory symptoms, and one subgroup with circulatory symptoms and orthostatic Intolerance [12].
In this study, symptoms were derived from a validated measure of ME/CFS symptomatology [19, 20], and both symptom frequency and severity scores were used in the analyses. Therefore, in contrast to earlier studies [12, 15, 18], our clusters were not only based on the presence of specific symptoms, but we were also able to identify subgroups of people with ME/CFS that were characterized by lower severity or frequency scores for specific symptoms.
This study used case definitions that are commonly used for the diagnosis of ME/CFS. The large majority of the patients (90%) fulfilled the Fukuda criteria, and there is a considerable overlap between the case definitions. Indeed, 29% of the patients met the criteria for all four different case definitions. However, there is a subset of patients that represent a symptom pattern which is only captured by specific case definitions. For example, 18 patients (5%) only fulfilled the ME-ICC case definition. Interestingly, another 5% of the participants did not meet the criteria of any of the four common case definitions. The lack of consensus in diagnostic criteria for ME/CFS underlines the difficulty in diagnosing the disease, and limits the external validity of ME/CFS studies using a specific case definition as inclusion criterion. In addition, different core symptoms are required for the different case definitions, which is also reflected in the identified symptom-based clusters. For example, none of the participants in Cluster 37 fulfilled the CCC or ME-ICC, explained by the fact that in these participants fatigue was not the result of exertion and they experienced no sensitivities, and a lack of neurosensory, flu-like, gastrointestinal and/or cardiovascular symptoms. In contrast, in Cluster 36 all participants met the ME-ICC, but only half of them met the Fukuda, CCC and IOM criteria, as half of the participants reported to experience lifelong fatigue [2].
It has been already been suggested that, instead of using the different ME/CFS case definitions, classification of patients according to severity and symptom patterns might be more useful to predict differences in prognosis or expected effects of therapy [28]. Additionally, identification of clusters of ME/CFS patients with distinct symptom patterns can provide more insight in the disease burden and may be useful for developing treatment strategies tailored to individual needs of patients.
Strengths and limitations
A clear strength of this study is the use of two datasets with a considerable amount of participants with ME/CFS to identify and apply the symptom-based clusters. This supports the external validity of our findings. Furthermore, using the SOM approach, we were able to cluster a large dataset and visualize it on a two-dimensional map, in which similar datapoints are clustered into the same group or nearby groups.
This study has the following methodological limitations. First, the possibility of selection bias is present, as it is reasonable to assume that participants with a higher symptom burden are more likely to complete the questionnaire. On the other hand, patients with cognitive or concentration related problems are less likely to participate. To account for concentration related problems, participants were allowed to take breaks while completing the online questionnaire. Second, similar to earlier studies, the used populations predominantly consisted of females [29, 30], though it has been recognized that prevalence of ME/CFS is higher in women compared to men [31]. Furthermore, almost all participants were white, whilst however the prevalence of ME/CFS has suggested to be higher in ethnic minority populations [32, 33]. Third, the DSQ-2 only captures the frequency and severity of symptoms, but doesn’t account for within-day and between-day variation in symptoms. Future studies should consider the use of ecological momentary assessment, which involves repeated measurements of the participant’s symptoms, behavior and context in vivo and in real time [34]. Fourth, clusters were based on self-reported symptoms and did not include clinical variables, such as inflammatory markers, physical functioning, anxiety, depression, common comorbidities and/or current treatment. Moreover, the online survey did not allow us to identify whether symptom-based clusters were associated with relevant patient-reported outcomes, including functional status and quality of life.
Conclusion
This study demonstrated that in ME/CFS there are subgroups of patients displaying a similar pattern of symptoms. These symptom-based clusters were confirmed in an independent ME/CFS sample. Classification of ME/CFS patients according to severity and symptoms patterns might be useful to develop tailored treatment options. Future studies are needed to investigate the relation between the identified symptom clusters and clinically relevant outcomes in patients with ME/CFS, including health-related quality of life and daily functioning.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ME:
-
Myalgic encephalomyelitis
- CFS:
-
Chronic fatigue syndrome
- PEM:
-
Post-exertional malaise
- CCC:
-
Canadian ME/CFS criteria
- ME-ICC:
-
ME International Consensus Criteria
- IOM:
-
Institute of Medicine
- WMO:
-
Medical Research Involving Human Subjects Act
- METC azM/UM:
-
Medical ethics committee of the University Hospital Maastricht and Maastricht University
- DSQ-2:
-
DePaul Symptom Questionnaire version 2
- IQR:
-
Interquartile ranges
- SOM:
-
Self-organizing map
References
https://blogs.cdc.gov/global/2020/05/12/cdc-team-takes-me-cfs-around-the-world/. Accessed 24 Oct 2022.
Lim EJ, Son CG. Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2020;18(1):289.
Conroy K, Bhatia S, Islam M, Jason LA. Homebound versus bedridden status among those with myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare. 2021;9(2):106.
Pemberton S, Cox DL. Experiences of daily activity in chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) and their implications for rehabilitation programmes. Disabil Rehabil. 2014;36(21):1790–7.
Falk Hvidberg M, Brinth LS, Olesen AV, Petersen KD, Ehlers L. The health-related quality of life for patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). PLoS ONE. 2015;10(7): e0132421.
Eaton-Fitch N, Johnston SC, Zalewski P, Staines D, Marshall-Gradisnik S. Health-related quality of life in patients with myalgic encephalomyelitis/chronic fatigue syndrome: an Australian cross-sectional study. Qual Life Res. 2020;29(6):1521–31.
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–9.
Carruthers BM, Jain AK, De Meirleir KL, Peterson DL, Klimas NG, Lerner AM, et al. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chronic Fatigue Syndr. 2003;11(1):7–115.
Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. 2011;270(4):327–38.
Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington, DC: The National Academies Press; 2015. p. 304.
Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28(4):270–82 (quiz 83–4).
Huber KA, Sunnquist M, Jason LA. Latent class analysis of a heterogeneous international sample of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue. 2018;6(3):163–78.
Collin SM, Nikolaus S, Heron J, Knoop H, White PD, Crawley E. Chronic fatigue syndrome (CFS) symptom-based phenotypes in two clinical cohorts of adult patients in the UK and The Netherlands. J Psychosom Res. 2016;81:14–23.
Williams TE, Chalder T, Sharpe M, White PD. Heterogeneity in chronic fatigue syndrome—empirically defined subgroups from the PACE trial. Psychol Med. 2017;47(8):1454–65.
Aslakson E, Vollmer-Conna U, Reeves WC, White PD. Replication of an empirical approach to delineate the heterogeneity of chronic unexplained fatigue. Popul Health Metr. 2009;7:17.
Hickie I, Lloyd A, Hadzi-Pavlovic D, Parker G, Bird K, Wakefield D. Can the chronic fatigue syndrome be defined by distinct clinical features? Psychol Med. 1995;25(5):925–35.
Asprusten TT, Sletner L, Wyller VBB. Are there subgroups of chronic fatigue syndrome? An exploratory cluster analysis of biological markers. J Transl Med. 2021;19(1):48.
Sullivan PF, Smith W, Buchwald D. Latent class analysis of symptoms associated with chronic fatigue syndrome and fibromyalgia. Psychol Med. 2002;32(5):881–8.
Jason LA, Sunnquist M. The development of the DePaul symptom questionnaire: original, expanded, brief, and pediatric versions. Front Pediatr. 2018;6:330.
Bedree H, Sunnquist M, Jason LA. The DePaul symptom questionnaire-2: a validation study. Fatigue. 2019;7(3):166–79.
Jason LA, Sunnquist M, Brown A, Evans M, Vernon SD, Furst J, et al. Examining case definition criteria for chronic fatigue syndrome and myalgic encephalomyelitis. Fatigue. 2014;2(1):40–56.
Klebek L, Sunnquist M, Jason LA. Differentiating post-polio syndrome from myalgic encephalomyelitis and chronic fatigue syndrome. Fatigue. 2019;7(4):196–206.
Jason LA, Ohanian D, Brown A, Sunnquist M, McManimen S, Klebek L, et al. Differentiating multiple sclerosis from myalgic encephalomyelitis and chronic fatigue syndrome. Insights Biomed. 2017;2(2):11.
Ohanian D, Brown A, Sunnquist M, Furst J, Nicholson L, Klebek L, et al. Identifying key symptoms differentiating myalgic encephalomyelitis and chronic fatigue syndrome from multiple sclerosis. Neurology (ECronicon). 2016;4(2):41–5.
Bullinaria JA. Self organizing maps: fundamentals. In: Introduction to neural networks. Burlington: Elsevier; 2004. p. 1–15.
Conway JR, Lex A, Gehlenborg N. UpSetR: an R package for the visualization of intersecting sets and their properties. Bioinformatics. 2017;33(18):2938–40.
Collin SM, Heron J, Nikolaus S, Knoop H, Crawley E. Chronic fatigue syndrome (CFS/ME) symptom-based phenotypes and 1-year treatment outcomes in two clinical cohorts of adult patients in the UK and The Netherlands. J Psychosom Res. 2018;104:29–34.
Brurberg KG, Fønhus MS, Larun L, Flottorp S, Malterud K. Case definitions for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review. BMJ Open. 2014;4(2): e003973.
Zdunek M, Jason LA, Evans M, Jantke R, Newton JL. A cross cultural comparison of disability and symptomatology associated with CFS. Int J Psychol Behav Sci. 2015;5(2):98–107.
Bhatia S, Olczyk N, Jason LA, Alegre J, Fuentes-Llanos J, Castro-Marrero J. A cross-national comparison of myalgic encephalomyelitis and chronic fatigue syndrome at tertiary care settings from the US and Spain. Am J Soc Sci Humanit. 2020;5(1):104–15.
Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, Son CG. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med. 2020;18(1):100.
Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. 1999;159(18):2129–37.
Dinos S, Khoshaba B, Ashby D, White PD, Nazroo J, Wessely S, et al. A systematic review of chronic fatigue, its syndromes and ethnicity: prevalence, severity, co-morbidity and coping. Int J Epidemiol. 2009;38(6):1554–70.
Maes IH, Delespaul PA, Peters ML, White MP, van Horn Y, Schruers K, et al. Measuring health-related quality of life by experiences: the experience sampling method. Value Health. 2015;18(1):44–51.
UNESCO Institute for Statistics. International standard classification of education: ISCED 2011. Montreal: UIS. p. 85.
Funding
This study did not receive any funding for its design, analysis, and interpretation of data, or for writing the manuscript.
Author information
Authors and Affiliations
Contributions
MAS, JMD and AWV designed the study. AWV, MVH, QD, JMD, and MAS were responsible for the data collection. QD was responsible for data analysis. AWV, MVH and JMD drafted the manuscript. All authors critically reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was waived by the medical ethics committee of Maastricht University because the Medical Research Involving Human Subjects Act (WMO) did not apply to this study (METC 2021-2797). Digital informed consent was obtained from all respondents at the start of the survey. Without providing informed consent, participants were unable to start the questionnaire.
Consent for publication
Not applicable.
Competing interests
MAS reports grants from Netherlands Lung Foundation, Stichting Astma Bestrijding, AstraZeneca, TEVA, Chiesi and Boehringer Ingelheim; consultancy fees from AstraZeneca and Boehringer Ingelheim for advisory boards, all outside the submitted work. No other disclosures were reported.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional details on the clustering method.
Additional file 2: Figure S1.
Number of reported symptoms in Dutch (n = 337) and US (n = 252) database using the 2/2 threshold for frequency and severity.
Additional file 3: Table S1.
Symptom severity and frequency scores of the 13 largest clusters.
Additional file 4: Table S2.
Symptom severity and frequency scores of the remaining 42 clusters with < 10 participants.
Additional file 5: Table S3.
Participant characteristics of all clusters.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Vaes, A.W., Van Herck, M., Deng, Q. et al. Symptom-based clusters in people with ME/CFS: an illustration of clinical variety in a cross-sectional cohort. J Transl Med 21, 112 (2023). https://doi.org/10.1186/s12967-023-03946-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-023-03946-6