Abstract
Background
The most common form of treatment for non-metastatic lung cancer is surgery-based combination therapy, which may also include adjuvant radiotherapy or chemotherapy. Second primary malignancies (SPMs) are uncommon but significant radiation side effects in patients with resectable lung cancer, and SPMs have not been adequately investigated. Our study aims to assess the correlations of radiotherapy with the development of SPMs in patients with resectable lung cancer.
Methods
We screened for any primary malignancy that occurred more than five years after the diagnosis of resectable lung cancer. Based on the large cohort of the Surveillance, Epidemiology and End Results database, radiotherapy-correlated risks were estimated using the Poisson regression analysis and the cumulative incidence of SPMs was calculated using Fine-Gray competing risk regression analysis.
Results
Among the 62,435 patients with non-metastatic lung cancer undergoing surgery, a total of 11,341 (18.16%) patients have received radiotherapy. Our findings indicated that radiotherapy was substantially related to a high risk of main second solid malignancies (RR = 1.21; 95%CI, 1.08 to 1.35) and a negligible risk of main second hematologic malignancies (RR = 1.08; 95%CI, 0.84 to 1.37). With the greatest number of patients, the risk of acquiring a second primary gastrointestinal cancer was the highest overall (RR = 1.77; 95 percent CI, 1.44 to 2.15). The cumulative incidence and standardized incidence ratios of SPMs revealed similar findings. Furthermore, the young and the elderly may be more vulnerable, and the highest risk of acquiring most SPMs was seen more than ten years after lung cancer diagnosis. Additionally, more attention should be paid to the second primary gastrointestinal cancer in young individuals with resectable lung cancer.
Conclusion
After receiving radiotherapy, an increased risk of developing second primary solid and gastrointestinal cancers was observed for patients with resectable lung cancer. The prevention of SPMs associated with radiotherapy requires further attention.
Similar content being viewed by others

Background
Lung cancer is one of the most prevalent cancers and the main cause of cancer-specific deaths globally [1, 2]. In 2020, it is anticipated that lung cancer accounts for approximately 1.8 million deaths and 2.2 million new cases worldwide, with the United States accounting for more than ten percent of deaths and new cases [3]. Multiple treatment modalities are utilized in the therapy of lung cancer, including surgical excision, radiotherapy, chemotherapy, immunotherapy and targeted therapy [4,5,6]. Radiotherapy is considered the only treatment strategy that can be used in all stages of lung cancer [7]. In general, to achieve better therapeutic outcomes, radiotherapy is used as the adjuvant therapy after surgery for lung cancer [8]. For instance, Yun et al. found that adjuvant radiotherapy might have had an additional effect on pN2 non-small cell lung cancer (NSCLC) with multiple N2 metastasis or extranodal invasion, resulting in a survival benefit [9]. Compared with adjuvant radiotherapy, preoperative radiotherapy is less common for lung cancer, and its main purpose is to improve prognosis by reducing the risk of local tumor recurrences, which may be particularly helpful in advanced lung cancer [10, 11]. A population-based study found that appropriately using radiotherapy may contribute to an 8.3% improvement in 5-year local control and a 4% increase in survival [12]. However, radiotherapy may also result in a number of adverse effects that could influence how well patients with lung cancer respond to treatment.
Second primary malignancies (SPMs) related to radiotherapy are rare but significant long-term complications that need to be thoroughly evaluated prior to radiation. Emerging studies have demonstrated that radiotherapy may substantially increase the risk of developing SPM in multiple cancers, such as breast cancer [13], nasopharyngeal carcinoma [14] and prostate cancer [15]. And some studies have investigated the optimal doses and theoretical strategies to reduce the risk of SPM for patients receiving radiotherapy [16,17,18]. The annual incidence of developing second primary lung cancer (SPLC) was 1.10% per patient, with an exceptionally high risk among young women with lung cancer [19]. For the treatment of lung cancer, some investigations have revealed that radiotherapy elevated the risk of developing SPMs, whereas others have shown contradictory results. For example, among NSCLC patients, a recent cohort study demonstrated that irradiation increased the risk of developing SPM by 6% [20]. For patients with fully resected NSCLC, another study did not advise routine postoperative radiation [21]. On the other hand, two cohort studies reported that radiotherapy may result in a lower risk of developing SPM, including prostate cancer and thyroid cancer, among lung cancer patients [22]. However, it has not been sufficiently addressed whether developing SPMs is a severe adverse event following radiotherapy for lung cancer, based on the sparse and contradictory findings of earlier research.
In our research, using data from Surveillance, Epidemiology and End Results (SEER) database, we intended to use different approaches to evaluate the radiotherapy-correlated risk of developing individual SPMs for lung cancer, which was rarely studied and had significant implications for radiotherapy in lung cancer treatment.
Methods
Participant cohorts
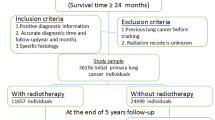
Using the data from nine registries of SEER database, participants diagnosed with lung cancer as the first primary cancer were enrolled from 1975 to 2018. The diagnoses of patients enrolled were pathologically confirmed and defined using the ICD-O-3 site codes (C34.0 to C34.9). The detailed codes for all cancer in this analysis were presented in Table S1. Patients with tumors of localized and regional stages at diagnosis were enrolled. The following were the exclusion criteria: patients under the age of twenty, patients with distant metastasis, patients not receiving the surgery, patients receiving radiotherapy other than external-beam radiotherapy, and those for whom race, tumor stage, age, surgery, radiotherapy, survival time or survival status information were unavailable. Patients with survival time less than five and two years after the diagnosis of the first primary cancer were also excluded in the second solid and hematologic malignancy cohorts, respectively. The survival time cut-off was based on the minimal latency period for radiation-induced tumorigenesis [23].
The use of anonymized, publicly accessible data did not need ethical approval. Access to and use of data from the SEER database did not need patient consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Outcome and follow-up
The primary outcome of this study was defined as the development of SPM (second solid malignancies or hematologic malignancies) five or two years after the diagnosis of the first primary malignancy. We excluded second solid malignancies with fewer than 50 cases in the cohort with at least 5 years of follow-up, and second hematologic malignancies with fewer than 10 cases in a second cohort with at least 2 years of follow-up. In the subsequent analysis, we first evaluated the risk for all the second solid malignancies or hematologic malignancies and separately estimated the risk for each SPM. The SPMs were categorized using the ICD-O-3 site codes and detailed codes were presented in Table S1. Follow-up for second solid and hematologic malignancies began 5 and 2 years after diagnosis of the first primary cancer, respectively. And follow-up ended at all-cause death, the last follow-up, after a 30-year follow-up, SPM diagnosis, or December 31, 2018, whichever occurred first.
Statistical analysis
Poisson regression analysis was conducted to estimate the relative risks (RRs) and 95% CIs of developing SPMs for lung cancer patients according to the administration of radiotherapy (RT vs NRT), and RRs were adjusted for gender and age at diagnosis of the first primary cancer. Next, using the Poisson regression analysis with SEER*Stat (version 8.4.0.1), the standardized incidence ratios (SIRs) and 95% confidence intervals (CIs) were then computed. The SIR was the ratio of the incidence of SPMs among patients with lung cancer to the incidence of corresponding primary malignancies in the general population of the United States. In the subgroup analysis, we stratified patients according to their clinical characteristics and calculated the RRs and SIRs of each subgroup.
With the use of Fine-Gray competing risk regression analysis, we assessed the cumulative incidence of developing SPMs. The competing events for SPM occurrence were all-cause death and non-SPM occurrence. In addition, the corresponding risk model was built to calculate the hazard ratios (HRs) and 95% CIs, which were adjusted for gender and age at diagnosis of the first primary cancer. In this study, the statistically significant threshold was P < 0.05 and all of the statistical analyses were performed with SEER*Stat (version 8.4.0.1) and R software (version 4.0.4).
Results
Patient characteristics
From 1975 to 2018, we have identified 62,435 patients with nonmetastatic lung cancer in this study. Of these, a total of 11,341 (18.16%) patients have received radiotherapy, with larger tumor size, higher tumor grade, and shorter follow-up time than the no-radiotherapy subgroup (Table S2). Overall, the proportion of using radiotherapy reached its maximum rate in 1994 (25.9%) and then declined to 11.1% in 2015. And radiotherapy use proportion of patients with larger tumor sizes was higher than those with smaller sizes (Fig. 1). Next, for the analysis of second solid and hematologic malignancies, we identified 30,290 patients who survived for at least 5 years and 46,202 patients who survived for at least 2 years in two final cohorts, respectively. As for the radiotherapy subgroups in these two final cohorts, the median follow-up time was 118 and 66 months, respectively. The patient characteristics were shown in Table 1. After a latency of 5 years, a total of 264 patients (7.4%) in the radiotherapy group and 1551 patients (5.8%) in the no-radiotherapy subgroup developed main second solid malignancies. After a latency of 2 years, a total of 56 patients (0.9%) in the radiotherapy group and 314 patients (0.8%) in the no-radiotherapy subgroup developed main second hematologic malignancies. The detailed characteristics of patients who developed main second solid and hematologic malignancies were shown in Table S3.
Radiotherapy-correlated risks of second primary malignancies
In the two cohorts with different latency periods, radiotherapy was substantially related to a high risk of main second solid malignancies (RR = 1.21; 95%CI, 1.08 to 1.35) and a negligible risk of main second hematologic malignancies (RR = 1.08; 95%CI, 0.84 to 1.37; Fig. 2). In organ-specific analyses, radiotherapy-correlated risk was especially elevated for cancers of the esophagus (RR = 3.48; 95%CI, 2.35 to 5.07), stomach (RR = 2.27; 95%CI, 1.34 to 3.70) and colon and rectum (RR = 1.28; 95%CI, 0.97 to 1.66; Fig. 2). However, for cancers of breast (RR = 0.97; 95%CI, 0.72 to 1.29) and urinary bladder (RR = 1.04; 95%CI, 0.76 to 1.38), the radiotherapy-correlated risk was nonsignificant. In different hematologic malignancies, radiotherapy was insignificantly associated with the risk of any type of second hematologic malignancies, such as non-Hodgkin lymphoma (RR = 1.19; 95%CI, 0.87 to 1.37; Fig. 2).
Similar results were observed in multivariable competing risk regression analysis. Radiotherapy was also related to an elevated risk of developing main solid malignancies (adjusted HR, 1.16; 95%CI, 1.04 to 1.29; P = 0.03) but not related to the risk of main hematologic malignancies (adjusted HR, 1.04; 95%CI, 0.82 to 1.32; P = 0.78; Table S4). Among each type of solid cancer, the radiotherapy-correlated risk was greatly increased for cancers of the esophagus (adjusted HR, 3.31; 95%CI, 2.25 to 4.87; P < 0.01) and stomach (adjusted HR, 2.18; 95%CI, 1.32 to 3.59; P = 0.01), but the risk was nonsignificant for cancers of colon and rectum (adjusted HR, 1.22; 95%CI, 0.93 to 1.59; P = 0.24; Table S4).
Cumulative incidences of second primary malignancies
Thirty years following the diagnosis of bronchus and lung cancer, the cumulative incidences of main solid malignancies were 9.45% for patients receiving radiotherapy and 8.66% for those without radiotherapy (P = 0.019, Fig. 3A). Specifically, the 30-year cumulative incidences of radiotherapy subgroup and no-radiotherapy subgroup were significantly different for gastrointestinal cancer (3.09% vs 2.06%; P < 0.001; Fig. 3B), esophageal cancer (1.01% vs 0.26%; P < 0.001; Fig. 3C), and stomach cancer (0.52% vs 0.26%; P = 0.022; Fig. 3D). However, no difference in the 30-year cumulative incidences was observed between the two subgroups for colorectal cancer (1.56% vs 1.53%; P = 0.429, Additional file 1: Figure S1).
Radiotherapy-related risks in different subgroups
To analyze the risk of SPMs owing to radiotherapy, patients with lung cancer were subsequently stratified into different subgroups based on different characteristics. In most subgroups, the administration of radiotherapy increased the risk of developing SPMs. For main solid malignancies, the radiotherapy-correlated risk was higher in males (RR = 1.29; 95%CI, 1.13 to 1.47) than in females (RR = 1.07; 95%CI, 0.87 to 1.30; Fig. 4). A higher risk of developing main solid cancers was also observed in patients aged 20 to 49 (RR = 1.35; 95%CI, 0.96 to 1.86) and more than 70 (RR = 1.33; 95%CI, 1.03 to 1.70; Fig. 4). As for patients with different latency periods, the radiotherapy-correlated risk was the highest in the middle latency period (120 to 239 months; RR = 1.42; 95%CI, 1.19 to 1.69) and showed a declining trend in the late latency (240 to 360 months; RR = 1.21; 95%CI, 0.63 to 2.12; Fig. 4).
We then primarily focused on three different gastrointestinal malignancies in the subgroup analyses in light of the results of radiotherapy-correlated risks and cumulative incidences indicated above. Among these three types of cancers and the combined gastrointestinal cancer, females (RR = 6.04; 95%CI, 2.87 to 12.33) had a much higher risk of developing esophageal cancer than males (RR = 2.85; 95%CI, 1.79 to 4.44; Additional file 1: Figure S2), but the risk was barely different between males and females in other gastrointestinal cancers. The radiotherapy-correlated risks for developing gastrointestinal cancers peaked in patients aged 20 to 49 (RR = 2.14; 95%CI, 1.05 to 4.15) and presented a downward trend with increasing age (50–69 years, RR = 1.91; 95%CI, 1.51 to 2.40; > 70 years, RR = 1.13; 95%CI, 0.66 to 1.80; Fig. 5). Specifically, similar patterns were observed in cancer of the stomach (Additional file 1: Figure S3) and colon and rectum (Additional file 1: Figure S4), but patients aged 50 to 69 had the highest risk of developing esophageal cancer (RR = 3.84; 95%CI, 2.49 to 5.84; Additional file 1: Figure S2). As for the latency periods, the increasing risks of developing gastrointestinal cancers were accompanied by an increase in the latency time. Furthermore, the lowest risk of developing gastrointestinal cancer was observed in the subgroup with the shortest latency time (60 to 119 months), which was the same as the risk of developing main solid malignancies (Figs. 4, 5). In addition, the radiotherapy-correlated risk was similar among patients stratified by other characteristics in gastrointestinal cancers, such as race and histology.
Standardized incidence ratios of second primary malignancies
Compared to patients who received no radiotherapy, SIRs increased more for main solid malignancies (1.30; 95%CI, 1.15 to 1.47) and main hematologic malignancies (1.41; 95%CI, 1.10 to 1.79) among patients receiving radiotherapy (Table S5). For gastrointestinal cancers, SIRs were higher in the radiotherapy subgroup (2.08; 95%CI, 1.67 to 2.55) than in the no-radiotherapy subgroup (1.16; 95%CI, 1.05 to 1.28). Specifically, compared to patients who received no radiotherapy, the SIR for esophageal cancer was much higher among patients treated with radiotherapy (8.14; 95%CI, 5.57 to 11.49). And the SIR of developing stomach cancer increased more in the radiotherapy subgroup (2.12; 95%CI, 1.09 to 3.70) than in the other one (1.13; 95%CI, 0.84 to 1.48). The findings of SIRs were similar to RRs and cumulative incidences in our study.
Discussion
In the treatment of lung cancer patients undergoing surgery, as a crucial therapeutic approach, radiotherapy has a well-established place. Although technological advancements have minimized the unintentional irradiation of normal tissues surrounding the tumor, several studies have revealed an elevated risk of SPMs following radiotherapy for some types of cancer. In the present research, using a variety of approaches to analyze data from a large population cohort, we are the first to unveil the comprehensive correlation between radiotherapy and the risk of developing individual SPMs in resectable lung cancer patients. We learned the following significant and fascinating information: First, radiotherapy may significantly raise the chance of second primary solid tumors, particularly gastrointestinal malignancies, among patients with lung cancer who have already undergone surgery. Second, a higher cumulative risk of radiotherapy was particularly observed for developing gastrointestinal cancers, which was similarly higher than that of the general population in the United States. Third, we found that the risk of developing second primary solid cancer after radiotherapy increased with latency and tumor size. Fourth, in contrast to the trends for second primary solid tumors, the radiotherapy-correlated risk of developing second primary gastrointestinal cancers declined with age at diagnosis.
Previous research has demonstrated that radiotherapy enhanced the probability of particular SPMs in various primary malignancies. For instance, a study enrolling testicular cancer survivors from 1947 to 1991 found that patients with testicular cancer who underwent radiotherapy had a 2.9-fold increased risk of developing pancreatic cancer than those who did not receive radiotherapy, which the risk persisted for over twenty years [24]. Enrolling women with nonmetastatic breast from 1965 to 1989, Kaufman et al. demonstrated that the radiotherapy-correlated risk of developing second primary lung cancer increased in breast cancer patients, especially for ipsilateral lung among ever-smokers [25]. For patients with primary lung cancer, the annual rate of acquiring an SPLC was 1.10% per patient, with young women having an especially high-risk [19]. However, there has been conflicting evidence regarding how radiotherapy affects the emergence of SPMs in patients with primary lung cancer. Gonzalez et al. discovered that radiation enhanced NSCLC patients' likelihood of acquiring SPMs, which concentrated on all patients over 20 years old with lung cancer of all stages and was based on data from 1973 to 2002 [20]. According to a meta-analysis, Burdett et al. have shown that postoperative radiotherapy led to an 18% relative increase in the risk of death for NSCLC patients, indicating a negative effect caused by postoperative radiotherapy [21]. Our investigation, which was based on a sizable population with lung cancer, showed results that were consistent with the idea that radiotherapy was to blame for the occurrence of secondary solid tumors. And we found that the risk of second primary gastrointestinal malignancies was increasing after the implementation of radiotherapy. On the other hand, using the data from 1975 to 2011, Han et al. have revealed that radiotherapy was considerably related to a low risk of developing SPMs in lung cancer patients, especially second primary prostate cancer and thyroid cancer [22]. Han et al. also showed a significantly increased risk for second primary esophageal carcinoma in lung cancer patients, which was similar to our conclusions. The aforementioned studies of primary lung cancer were unable to thoroughly assess the risk of radiation for SPMs in comparison to research examining SPMs in individuals with other primary malignancies. Several factors, including differences in inclusion and exclusion criteria, number of patients, definition of SPMs, and methodology, were assumed to account for the inconsistency of the results in earlier publications.
Currently, the primary treatment for non-metastatic lung cancer is surgery-based combination therapy, followed by adjuvant chemotherapy or radiotherapy [26]. According to the latest ASCO guideline, adjuvant radiotherapy was considered for NSCLC patients with N2 disease but was not recommended for resected stage I or II NSCLC [27]. Our study focused on the effects of radiotherapy on surgically treated patients with non-metastatic lung cancer, aiming to evaluate potential risks of irradiation for developing SPMs. Compared to earlier research that had clear inclusion criteria, we have reached more reliable conclusions and offered stronger evidence in favor of updating guidelines. Because the incidence of developing SPMs was fairly low compared to other adverse events, a broader population was investigated in this investigation than in past studies to increase the trustworthiness of the results [20]. Additionally, radiotherapy was found to be insignificantly linked with second hematologic malignancies in lung cancer, despite being a risk factor for second hematologic malignancies in other cancers, such as prostate cancer [28]. The risk of SPMs was also evaluated using a variety of statistical techniques, such as Poisson regression and competing risk regression. Both approaches consistently showed that the probability of acquiring gastrointestinal cancer and second primary solid cancer was enhanced by radiotherapy. We also computed the SIRs for external validation and compared the incidence of SPMs among lung cancer survivors to the general population of the United States, which may broaden the interpretive perspective on the relationship between irradiation and the development of SPMs.
We divided patients into various subgroups based on their clinical characteristics to precisely assess the potential risk of radiotherapy, and we discovered an elevated risk of acquiring second primary solid and gastrointestinal malignancies in the majority of subgroups. And of all cancers, primary gastrointestinal cancer was the most common. In earlier research, the latency cut-off point varied and the prevalence of SPMs was associated with the latency [29]. We have used the minimal latency period for radiation-induced tumorigenesis in the analysis [23]. The highest radiotherapy-correlated risk was observed after a latency of more than ten years in both primary solid cancer and gastrointestinal cancer, suggesting that long-term follow-up may be essential for resectable lung cancer patients receiving radiotherapy. Next, we examined the radiotherapy-correlated risk in various genders and discovered that men had a higher relative risk of developing second primary solid malignancies following radiotherapy, but women had a higher relative risk of developing second primary esophageal cancer. The prevalence of esophageal cancer was much higher in males than in females, consistent with global statistics, but the proportion of second primary esophageal cancer was higher in females [3]. Moreover, our findings indicated that the risk of developing second primary gastrointestinal cancer decreased with increasing age at diagnosis, whereas the risk of developing second primary solid cancer was higher in patients younger than fifty and older than seventy. More specific screening for second primary solid cancer is advised for both young and elderly people, whereas screening for gastrointestinal cancer is recommended for those under 70 years old.
The merits of this study have been distinctly observed. Our study was based on a large population with relatively homogenous treatment exposure, which could make the results reliable and solid. Furthermore, we have evaluated the radiotherapy-correlated risk of developing individual SPMs for lung cancer, which was rarely studied and had significant implications in lung cancer treatment. Besides, different statistical methods were used in the study to comprehensively validate the results. Certainly, there are some limitations in this study. First, due to the incomplete records of the SEER database, it was unclear whether delayed radiotherapy could lead to the underestimation of the radiotherapy-correlated risk. Second, as a population-based study, potential biases may be caused by the lack of randomization of the first treatment. And the development of SPMs might be affected by other risk factors, including other treatments, environmental factors and genetic characteristics [30]. Third, the SEER database lacked detailed information on radiotherapy, such as the number of doses administered, which may limit the analysis of the specific relationship between radiotherapy and SPMs.
Conclusions
To summarize, SPMs are uncommon but important adverse events after receiving radiotherapy, which is rarely investigated in lung cancer treatment. Based on a large population with resectable lung cancer, our study used different methodologies to comprehensively evaluate the relationship between radiotherapy and SPMs, assessing the elevated risk of developing second primary solid and gastrointestinal cancers. Our findings could serve as a meaningful reference for the early detection and treatment of SPMs in patients with resectable lung cancer receiving radiotherapy. Randomized controlled trials should be conducted in the future to further validate our conclusion, and further subgroup analysis is needed to evaluate the radiotherapy-correlated risk of different subpopulations.
Availability of data and materials
All the data are available in a public, open-access repository.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- SPM:
-
Second primary malignancy
- SEER:
-
Surveillance, epidemiology and end results
- RR:
-
Relative risk
- SIR:
-
Standardized incidence ratio
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
References
Zhou B, Zang R, Zhang M, Song P, Liu L, Bie F, Peng Y, Bai G, Gao S. Worldwide burden and epidemiological trends of tracheal, bronchus, and lung cancer: a population-based study. EBioMedicine. 2022;78: 103951.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40:611–25.
Zhou B, Bie F, Zang R, Zhang M, Song P, Liu L, Peng Y, Bai G, Zhao J, Gao S. RNA modification writer expression profiles predict clinical outcomes and guide neoadjuvant immunotherapy in non-small cell lung cancer. EBioMedicine. 2022;84: 104268.
Zhang F, Guo W, Zhou B, Wang S, Li N, Qiu B, Lv F, Zhao L, Li J, Shao K, et al. Three-year follow-up of neoadjuvant programmed cell death protein-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol. 2022;17:909–20.
Barton MB, Jacob S, Shafiq J, Wong K, Thompson SR, Hanna TP, Delaney GP. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112:140–4.
Salazar MC, Rosen JE, Wang Z, Arnold BN, Thomas DC, Herbst RS, Kim AW, Detterbeck FC, Blasberg JD, Boffa DJ. Association of delayed adjuvant chemotherapy with survival after lung cancer surgery. JAMA Oncol. 2017;3:610–9.
Yun JK, Lee GD, Choi S, Kim HR, Kim YH, Park SI, Kim DK. The addition of radiotherapy to adjuvant chemotherapy has a combinatorial effect in pN2 non-small cell lung cancer only with extranodal invasion or multiple N2 metastasis. Lung Cancer. 2021;155:94–102.
Blumenthal GM, Bunn PA Jr, Chaft JE, McCoach CE, Perez EA, Scagliotti GV, Carbone DP, Aerts H, Aisner DL, Bergh J, et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol. 2018;13:1818–31.
Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, Escriu C, Peters S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv1–21.
Shafiq J, Hanna TP, Vinod SK, Delaney GP, Barton MB. A population-based model of local control and survival benefit of radiotherapy for lung cancer. Clin Oncol (R Coll Radiol). 2016;28:627–38.
Grantzau T, Overgaard J. Risk of second non-breast cancer among patients treated with and without postoperative radiotherapy for primary breast cancer: a systematic review and meta-analysis of population-based studies including 522,739 patients. Radiother Oncol. 2016;121:402–13.
Chow JCH, Tam AHP, Cheung KM, Lee VHF, Chiang CL, Tong M, Wong ECY, Cheung AKW, Chan SPC, Lai JWY, et al. Second primary cancer after intensity-modulated radiotherapy for nasopharyngeal carcinoma: a territory-wide study by HKNPCSG. Oral Oncol. 2020;111: 105012.
Wallis CJ, Mahar AL, Choo R, Herschorn S, Kodama RT, Shah PS, Danjoux C, Narod SA, Nam RK. Second malignancies after radiotherapy for prostate cancer: systematic review and meta-analysis. BMJ. 2016;352: i851.
Karimi AH, Mirian SF, Mahmoudi F, Geraily G, Vega-Carrillo HR, Mohiuddin M. Feasibility of 18-MV grid therapy from radiation protection aspects: unwanted dose and fatal cancer risk caused by photoneutrons and scattered photons. Comput Methods Programs Biomed. 2022;213: 106524.
Geraily G, Elmtalab S, Mohammadi N, Alirezaei Z, Martinez-Ovalle SA, Jabbari I, Vega-Carrillo HR, Karimi AH. Monte Carlo evaluation of out-of-field dose in 18 MV pelvic radiotherapy using a simplified female MIRD phantom. Biomed Phys Eng Expr. 2021;8:1.
Elmtalab S, Abedi I, Alirezaei Z, Choopan Dastjerdi MH, Geraily G, Karimi AH. Semi-experimental assessment of neutron equivalent dose and secondary cancer risk for off-field organs in glioma patients undergoing 18-MV radiotherapy. PLoS ONE. 2022;17: e0271028.
Thakur MK, Ruterbusch JJ, Schwartz AG, Gadgeel SM, Beebe-Dimmer JL, Wozniak AJ. Risk of second lung cancer in patients with previously treated lung cancer: analysis of surveillance, epidemiology, and end results (SEER) data. J Thorac Oncol. 2018;13:46–53.
de Gonzalez A, Curtis RE, Kry SF, Gilbert E, Lamart S, Berg CD, Stovall M, Ron E. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12:353–60.
Burdett S, Rydzewska L, Tierney J, Fisher D, Parmar MK, Arriagada R, Pignon JP, Le Pechoux C. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2016;10:CD002142.
Han C, Wu Y, Kang K, Wang Z, Liu Z, Zhang F. Long-term radiation therapy-related risk of second primary malignancies in patients with lung cancer. J Thorac Dis. 2021;13:5863–74.
Council NR: Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. The National Academy of Sciences: Washington, DC, USA 2006:1–406.
Hauptmann M, Børge Johannesen T, Gilbert ES, Stovall M, van Leeuwen FE, Rajaraman P, Smith SA, Weathers RE, Aleman BM, Andersson M, et al. Increased pancreatic cancer risk following radiotherapy for testicular cancer. Br J Cancer. 2016;115:901–8.
Kaufman EL, Jacobson JS, Hershman DL, Desai M, Neugut AI. Effect of breast cancer radiotherapy and cigarette smoking on risk of second primary lung cancer. J Clin Oncol. 2008;26:392–8.
Zhou B, Zang R, Zhang M, Song P, Liu L, Bie F, Peng Y, Bai G, Gao S. A new N descriptor for non-small cell lung cancer: the classification based on anatomic location, number and ratio of metastatic lymph nodes. Transl Lung Cancer Res. 2022;11:1540–54.
Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, Lin SH, Pass HI, Seth R, Shepherd FA, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non–small-cell lung cancers: American society of clinical oncology/cancer care Ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–74.
Wang R, Zeidan AM, Yu JB, Soulos PR, Davidoff AJ, Gore SD, Huntington SF, Gross CP, Ma X. Myelodysplastic syndromes and acute myeloid leukemia after radiotherapy for prostate cancer: a population-based study. Prostate. 2017;77:437–45.
Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64.
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin P, Travis LB. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734–45.
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFC2500900), National Natural Science Foundation of China (82273129), Medical and Health Science-Technology Innovation Project of the Chinese Academy of Medical Sciences (2021-I2M-1-015), Central Health Research Key Projects (2022ZD17), and Research Project of the Institute (LC2019L01).
Author information
Authors and Affiliations
Contributions
BZ, YH and SG designed and supervised the study. BZ, RC, MZ, PS and FB analyzed the data. BZ wrote the original draft. GB, YL, QH, YH and SG edited the draft. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not required for the use of anonymized publicly available data.
Consent for publication
All authors agree to the publication of this article.
Competing interests
The authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional figures and tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, B., Zang, R., Song, P. et al. Association between radiotherapy and risk of second primary malignancies in patients with resectable lung cancer: a population-based study. J Transl Med 21, 10 (2023). https://doi.org/10.1186/s12967-022-03857-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03857-y