Abstract
Microparticles (MPs) are 100–1000 nm heterogeneous submicron membranous vesicles derived from various cell types that express surface proteins and antigenic profiles suggestive of their cellular origin. MPs contain a diverse array of bioactive chemicals and surface receptors, including lipids, nucleic acids, and proteins, which are essential for cell-to-cell communication. The tumour microenvironment (TME) is enriched with MPs that can directly affect tumour progression through their interactions with receptors. Liquid biopsy, a minimally invasive test, is a promising alternative to tissue biopsy for the early screening of lung cancer (LC). The diverse biomolecular information from MPs provides a number of potential biomarkers for LC risk assessment, early detection, diagnosis, prognosis, and surveillance. Remodelling the TME, which profoundly influences immunotherapy and clinical outcomes, is an emerging strategy to improve immunotherapy. Tumour-derived MPs can reverse drug resistance and are ideal candidates for the creation of innovative and effective cancer vaccines. This review described the biogenesis and components of MPs and further summarised their main isolation and quantification methods. More importantly, the review presented the clinical application of MPs as predictive biomarkers in cancer diagnosis and prognosis, their role as therapeutic drug carriers, particularly in anti-tumour drug resistance, and their utility as cancer vaccines. Finally, we discussed current challenges that could impede the clinical use of MPs and determined that further studies on the functional roles of MPs in LC are required.
Similar content being viewed by others
Background
Lung cancer (LC) has the highest morbidity and mortality among all types of cancers and accounts for the majority of cancer-related deaths worldwide [1, 2]. The overall 5-year survival rate of LC patients is less than 15% [3]. Currently, carcinoembryonic antigen, fragments of cytokeratin 19, neuron-specific enolase, and pro-gastrin-releasing peptides are the most common tumour markers used in the clinical diagnosis of LC. However, due to the limited sensitivity and specificity of these markers, most LC patients who are diagnosed at an advanced stage usually have a poor prognosis. In addition, despite significant advances in LC research and anticancer therapies, including surgery, radiotherapy, chemotherapy, molecular targeted therapies, and immunosuppressive agents [4], the overall survival rate of LC remains low [3]. Therefore, further studies on the molecular mechanisms, early detection, and targeted therapies of LC are vital.
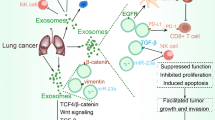
Extracellular vesicles (EVs) are composed of exosomes, microparticles (MPs), and apoptotic bodies (Table 1). These vesicles can be detected in the supernatants of cell cultures and in various biological fluids, such as blood, urine, sputum, breast milk, and synovial, bronchoalveolar lavage, pleural effusion, and ascites fluids [5]. MPs, also called microvesicles, shedding vesicles, or ectosomes, are released into the extracellular space from the surface membranes of cells [6]. In the LC microenvironment, MPs can be found in normal, tumour-infiltrating (e.g., activated platelets, monocytes, and lymphocytes), and cancer cells (Fig. 1). MPs are capable of transferring surface receptors from one cell to another and delivering proteins, mRNA, bioactive lipids, organelles (e.g., mitochondria), and even vaccines based on the delivery of tumour lysates into target cells [7,8,9,10,11,12,13]. MPs shed from various tumour cell lines or tumour cell-related lines have been thought to facilitate extracellular matrix invasion and evasion of the immune response [14], whereas those secreted by normal endothelial cells might exhibit protective effects [15]. Endothelial-derived microparticles (EMPs) enable cells to dispose of potentially harmful and redundant compounds, thereby promoting cellular survival [16,17,18]. Several recent studies have found that MPs may facilitate intercellular communication [19,20,21,22]. MPs have been proposed as indicators of progressive and aggressive LC. The basal values of circulating MPs can serve as an independent predictor of survival outcomes in advanced non-small cell LC (NSCLC) patients. Due to their capacity to pack large amounts of biological information, tumour-derived MPs (TMPs) are ideal candidates for delivering therapeutic agents to tumour cells and may play a crucial role in the development of novel and effective tumour vaccines. Further, TMPs loaded with anti-tumour drugs could reverse drug resistance.
MPs induced by environmental cues (activation, injury, hypoxia, or apoptosis) are involved in cancer cell initiation, progression, and metastasis; extracellular matrix remodelling; multidrug resistance; and modulation of inflammation [23], thrombosis [24], endothelial dysfunction [25], tissue remodelling [26], angiogenesis [27], and immunological reactions [28]. The levels of circulating MPs are increasingly elevated in many types of cancers, including haematological malignancies [29, 30], breast cancer [31], ovarian cancer [32], and colorectal cancer [33]. Najjar et al. firstly found that increased circulating endothelial cells (CECs) and MPs during or after chemotherapy can act as predictive biomarkers of tumour progression in advanced NSCLC [34]. However, available data on the association between the levels of circulating MPs and LC are limited [35, 36]. In addition, most studies do not distinguish MPs from exosomes and excessively focus on the role of exosomes rather than that of MPs. Thus, this review solely focused on the biogenesis, components, isolation, quantification, and potential clinical implications of MPs in LC.
The biogenesis, components, isolation, and quantification of microparticles
Microparticles biogenesis
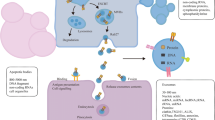
MPs were first described as “platelet-like activity” in 1955 and later as “platelet dust” in 1967. Multiple studies have investigated the composition, origin, and roles of these particles, leading to the gradual replacing of the name "platelet dust" with "microparticles" [37,38,39]. Almost all cell types are capable of producing and shedding MPs [40]. MPs are formed by the outward blebbing of the plasma membrane and subsequently released by the proteolytic cleavage of the cytoskeleton and expression of antigens specific to their parental cells [41]. Moreover, MPs contain many proteins and lipids similar to those found in the membranes of their parental cells and may also contain mRNA. Multiple mechanisms of MP biogenesis have been described; however, the two best-known mechanisms are cell activation and apoptosis [42]. Cell activation causes MP shedding, which starts within minutes of adding the right agonist and is characterised by higher calcium levels in the cytosol [43, 44]. Signs of damage (like injury, hypoxia, or apoptosis) cause the endoplasmic reticulum to release calcium into the cytosol. This causes the cytoskeleton to change shape and the phospholipid asymmetry to flip. When phosphatidylserine moves out of the cell, it causes the cell membrane to bulge outward, which results in a fissure. Consequently, MPs express both phosphatidylserine and surface proteins related to their parental cells on their outer membranes. In apoptosis-dependent MP formation, dynamic membrane blebbing occurs after cell contraction and DNA fragmentation, and it usually lasts for hours [45]. During membrane blebbing, the molecular regulators of MPs release cytosolic calcium, Rho kinases, GTPase, RhoA, mitogen-activated protein kinases, and nuclear factor-κB [46]. The mechanisms by which MPs develop and bud from cell plasma membranes are still largely unknown. Therefore, we must continue to gain more understanding on the underlying mechanisms that allow MPs to carry certain proteins, RNAs, and DNAs.
Molecular components of microparticles
The Vesiclepedia database (www.microvesicles.org) [47] catalogues proteins, lipids, and acids identified in MPs from various sources. MPs contain a broad spectrum of bioactive substances and receptors on their surface, including lipids, nucleic acids, and proteins, that reflect not only their cellular origin but also the stimulus that triggered their biogenesis and secretion. MPs may shuttle these molecules between neighbouring cells via systemic transport or distant anatomic sites where they may induce signalling pathways or directly alter the phenotype of specific recipient cells. As mentioned above, the composition of MPs determines their role in cell communication.
Proteins in microparticles
Many proteins, including selectins, integrins, cluster of differentiation (CD) 40, matrix metalloproteinases, phosphatidylserine, ADP-ribosylation factor 6, and Rho family members, have been indicated as MP-specific [48, 49]. One study identified 910 different proteins in salivary macrovesicles from healthy participants and patients with LC. In particular, 626 proteins were found in salivary MPs from patients with LC [50]. Among these, 243 proteins were identified as dysregulated candidates and 284 as unique to patients with LC, of which 40 were originally from distal organs or tissues, and nine originated from the lungs. In total, 109 proteins were upregulated and 134 were downregulated (Table 2).
Proteins play a key role in LC progression. For example, Ras GTPase-activating-like protein 1 (IQGAP1) acts as a signal interrogator in LC cell proliferation. BPI fold-containing family A member 1 (BPIFA1) takes part in the innate immune response of NSCLC [51]. Cornulin is considered a survival factor related to apoptotic cell death and calcium release [52]. Mucin 1 is cross-processed and presented to antigen-specific CD8+ T cells when carried by MPs. Internalised and soluble mucin 1 is retained in the endolysosomal/HLA-II compartment and does not induce T cell response [53, 54]. Studies have shown that these proteins may be exploited for possible non-invasive detection of LC.
Nucleic acids in microparticles
MicroRNAs (miRNAs) are indispensable for cell differentiation, proliferation, maturation, and apoptosis [55]. miRNAs (e.g., miR-21, miR-19, miR-133, miR-146, miR-126, and miR-223) are detectable in platelet-derived MPs (PMPs) [56]. In platelets and PMPs, miR-223 is the most abundant miRNA [57, 58]. The expression of miR-223 is aberrant in breast cancer, gastric cancer, LC, and ovarian cancer [59,60,61,62]. As such, it is considered to be a member of an emerging family of cancer-promoting miRNAs known as oncomiRs. miR-223 is also the most upregulated miRNA in recurrent tumours [62] because it directly targets the 3′ UTR of erythrocyte membrane protein band 4.1-like 3 (EPB41L3) [61]. PMPs can effectively deliver miR-223 into human LC cells via EPB41L3, promoting tumour invasion. miR-223 not only directly binds to the 3′ UTR of the EPB41L3 mRNA transcript, inhibiting EPB41L3 translation, but also decreases the cellular levels of the EPB41L3 protein. As such, increased motility and decreased adhesion are observed in LC cells, inducing tumour cell invasion. These MP-encapsulated miRNAs can be successfully transported into target cells to silence target genes, hence influencing recipient cell function [63, 64]. Therefore, cell-secreted miRNAs in MPs can serve as a novel class of signalling molecules to mediate intercellular communication from a distance. Some RNA transcripts found in cancer cell-derived MPs can function as messages or biomarkers that can be recognised using available technology or a very sensitive way.
Methods for microparticles isolation and quantification
The Minimal Information for Studies of Extracellular Vesicles provides research guidelines for EVs to promote the transparency and reproducibility of EV studies [65]. Currently, multiple accepted methods, such as ultracentrifugation (including differential centrifugation [DU]), microfluidics, ultrafiltration, immunoaffinity chromatography, and size-exclusion chromatography, have been successfully used for the isolation of MPs [66]. Immunoaffinity chromatography cannot distinguish MPs from exosomes because exclusive markers for each one have not been identified yet. This method often serves as a purification method after isolating MPs from large sample volumes [67]. Traditional ultracentrifugation, described as the most dependable method, consists of a series of centrifugation cycles with varying centrifugal forces and durations to separate EVs based on their density and size differences [66]. DU is an ideal method of EV isolation for many laboratories due to its low-cost and high-throughput properties. Microfluidics is an appealing approach due to its fast and simple operation. Small-volume samples may even be used for disease diagnosis. Combination methods can improve the purity of the collected vesicles [68]. Size-exclusion chromatography can be followed by ultracentrifugation or ultrafiltration to concentrate isolated but diluted MPs [67]. Methods of separation are typically selected with a clinical goal.
Particle number can be measured by light-scattering technologies (e.g., nanoparticle tracking analysis [NTA]), standard flow cytometry (FCM) [69,70,71,72], tunable resistive pulse sensing (TRPS) [73], cryogenic electron microscopy [74], platform combining surface plasmon resonance with atomic force microscopy (AFM) [75], or dynamic light scattering (DLS). AFM can be used to study the size, antigenic properties, and number of defined subsets of MPs [76]. Single-particle analyses like NTA, TRPS, and DLS are now widely used to measure the number and size of EVs. However, they do not give enough information about phenotype and are not the best way to measure vesicles that are larger than 200 nm. Until recently, FCM could analyse only large EVs or the population of smaller EVs captured on beads prior to analysis [77]. FCM remains the most extensively used technique for the enumeration and characterisation of MPs [78, 79]. To achieve better specificity of EV subtype separation, two or more methods are used for EV characterisation. Taken together, essential technologies need to be investigated further to ensure the reliable isolation of disease-specific MPs from body fluid and tissue samples, as well as to rigorously discriminate these vesicles from those formed by non-diseased cells. Further, it is important to develop the necessary methods for high-sensitivity identification of specific cargo proteins, RNAs, or miRNAs.
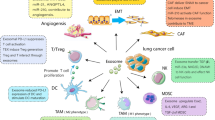
Different microparticles in the lung cancer microenvironment
Cells can release MPs derived from many sources, including leukocytes, platelets, erythrocytes, endothelial cells, macrophages, and tumour cells, at each stage of their lifecycle. In LC, MPs can play a role in inflammation, thrombus formation [24, 26, 29, 80], and angiogenesis [24, 29,30,31]. Furthermore, PMPs exhibit pro-angiogenic activity, which can promote capillary-like structure formation and pro-angiogenic factor production [14, 20, 23, 25]. Conversely, EMPs can be either pro- or anti-angiogenic, depending on exposure to factors stimulating their production [24].
Platelets release more MPs when various inflammatory factors are upregulated and under disease conditions, such as malignancy [81], sepsis [82], thrombocytopenia [83], arterial thrombosis [26], thrombotic thrombocytopenia [84], uraemia [85], and rheumatoid arthritis [86]. PMPs are activated in a calcium flux-calpain-dependent manner [87]. TMPs regulate tumour microenvironment (TME); increase tumour invasion, metastasis, and angiogenesis [88]; and even escape immune surveillance. In the airway, alveolar macrophages are a major source of bronchoalveolar lavage fluid cellular components and have a significant influence on inflammation. After interacting with different cells in a pathological state, macrophage-derived MPs (MMPs) are transported to various types of respiratory cells, such as lung epithelial cells, endothelial cells, fibroblasts, and monocytes, ultimately leading to cellular homeostasis and differentiation [89]. EMPs can carry a wide range of transcripts and have angiogenic activity mainly in quiescent endothelial cells by promoting endothelial cell proliferation, organising capillary-like structures, and preventing apoptosis. Elevated levels of circulating lymphocyte-derived MPs (LMPs) are associated with disease progression in advanced NSCLC [90]. The total MPs, PMPs, and LMPs increased significantly with disease progression in patients with advanced NSCLC who were treated with immune checkpoint inhibitors. The participation of different MPs in the key steps of cancer progression through different functions has been considered. The surface antigens that characterise and used to enumerate the functions of different MPs are summarised in Table 3.
Clinical applications of microparticles for diagnosis, prognosis, and therapy
Liquid biopsy, a minimally invasive test, is a promising alternative to tissue biopsy for the early screening of LC [90, 91]. MPs can be found in blood, urine, sputum, breast milk, synovial, and bronchoalveolar lavage. For high stability, biological fluids can be regarded as ideal materials for liquid biopsies. The composition of MPs mirrors the contents of donor cells and bears the hallmarks of the regulated sorting mechanisms of these cells, providing diagnostic utility for LC. The diverse biomolecular information from MPs, including that on proteins, lipids, various metabolites, and nucleic acids, provides prospective biomarkers for LC risk assessment, early detection, diagnosis, prognosis, and surveillance.
Microparticles as diagnostic biomarkers for lung cancer
Profiling proteomics has revealed a variety of EV-associated protein cargoes, including receptors, transcription factors, enzymes, signalling proteins, lipid raft proteins, cytoskeletal and extracellular proteins, vesicle-trafficking proteins, and immune-interacting proteins [92, 93]. BPIFA1, Mucin 5B, and Ras GTPase-activating-like protein can prove useful as non-invasive biomarkers of LC [50]. Moreover, SPARC-like protein 1 (SPARCL1), IQGAP1, BPIFA1, and cornulin are potential candidate proteins abnormally expressed in multiple types of cancers, especially LC. SPARCL1 is classified as a member of a larger family of secreted acidic and cysteine-rich matricellular proteins [94]. According to Isler et al., SPARCL1 is downregulated in human NSCLC and thus can be effectively identified as a predictive factor. A survey suggested that SPARCL1 downregulation is mediated by transacting factors that bind to its exon 1 [95]. IQGAP1 participates in multiple cellular actions (i.e., transcription, cell–cell adhesion, and cytoskeleton regulation) by targeting calmodulin, cell division control protein 42, Ras-related C3 botulinum toxin substrate 1, actin, β-catenin, and E-cadherin. BPIFA1 predominantly exists in the upper respiratory tract and salivary glands of both mice and humans and participates in the lung immune response. Cornulin is a newly discovered member of the “fused gene” family and the product of the novel gene c1orf10, an oesophageal-specific and cancer-associated gene located on 1q21. The c1orf10 gene encodes a Ca2+-binding protein in the upper layer of squamous epithelia that plays an important role in epidermal differentiation and is a marker of late epidermal differentiation.
Microparticles as prognostic biomarkers for lung cancer
MPs have been proposed as indicators of progression and aggressiveness of NSCLC [96]. For example, the level of EMPs is a useful diagnostic marker for LC [97]. The basal value of circulating MPs serves as an independent predictor of 1-year clinical outcomes in patients with advanced NSCLC [98]. A level of circulating EMPs ≥ 1100.5 count/mL is one of the most important predictors of 1-year mortality in patients with end-stage NSCLC, with sensitivity and specificity rates of 77.6% and 56.9%, respectively. In addition, patients with small-cell LC who initially responded to chemotherapy exhibited low basal MP numbers.
EMPs activate matrix metalloproteases, which are involved in the degradation of the extracellular matrix and the release of growth factors that are essential for tissue remodelling, angiogenesis, and metastasis [99]. Moreover, Tseng et al. found that circulating EMPs are more closely associated with small cell carcinoma than squamous cell carcinoma [36]. Squamous cell carcinoma tends to have a slower growth rate and spread later in the course of the disease than small cell carcinoma and adenocarcinoma [100]. As a result, squamous cell carcinoma displays a slower rate of metastasis and lower degree of angiogenesis in the host microenvironment than other types of LC, leading to a lower level of EMPs.
Najjar et al. found that before chemotherapy, the total MPs in patients with stage IV NSCLC are significantly higher than those in patients with stage III NSCLC. Further, the rate of change in total MPs after chemotherapy can predict disease progression [34]. Elevated levels of circulating LMPs are associated with disease progression in advanced NSCLC [90]. According to this study, total MPs, PMPs, and LMPs increased significantly with disease progression in advanced NSCLC with treatment. Due to their significance as prospective lung cancer biomarkers and biological communication carriers, MPs have drawn the attention of the scientific community. MPs have the potential to be used as a specimen for liquid biopsy with a higher sensitivity and accuracy.
Therapeutic applications of microparticles for lung cancer
Microparticles as a novel mode of drug delivery
Remodelling the TME, which profoundly influences immunotherapy and clinical outcomes [101, 102], is an emerging strategy to improve immunotherapy [103]. Due to their capacity to package large amounts of biological information, TMPs are ideal for delivering therapeutic agents (e.g., oncolytic adenoviruses, chemotherapeutic drugs, nucleic acids, antibodies, and antigens) to tumour cells, effectively killing the cancer cells [104,105,106]. Drug MPs can be directly injected into superficial solid tumours or delivered to target tumour cells through a drainage tube in cases of malignant pleural effusion and ascites. Drug MPs can also be used to target tumour-associated macrophages, key players in tumour immunosuppression, cancer stemness, and metastasis [107]. M1-like macrophages remodel the TME by reducing the number of immunosuppressive cells and augmenting T cell infiltration, thereby promoting effective antitumor T cell immunity [107]. Drug-packaging TMPs efficiently mobilise endogenous neutrophils and induce intrinsic antitumour activities. The attracted neutrophils display a mature CD11b+/CD15b+ phenotype and kill tumour cells by releasing reactive oxygen species and NO into the TME [108].
Autologous TMPs packaged with chemotherapeutic agents have been approved as a new biological therapy for malignant tumours due to their demonstrated safety and tolerability [106]. According to our previous studies, TMPs packed with methotrexate, a chemotherapeutic drug, markedly restrict the growth of malignant pleural effusion and provide a survival benefit in both animal and human experiments [109, 110]. Ran et al. found that TMPs can act effectively deliver oncolytic adenoviruses to tumours and induce highly efficient cytolysis [111]. Additionally, Chen et al. proposed a donor cell-assisted membrane biotinylation strategy to achieve biocompatible quantum dot labelling of TMPs, thereby creating a novel method for nanocarrier preparation [112]. MPs are nontoxic and stable in body fluids; however, their efficacy for drug delivery to target cells still requires more research before they can be exploited. Efforts should be made to load isolated MPs with specific therapeutic cargos (drugs, RNAs, or DNAs) and then employ them to effectively deliver therapy to diseased or injured target cells.
Microparticles and drug resistance
Therapeutic resistance is the leading cause of a poor prognosis for cancer. Progression of cancer is a complicated process dependent on interactions between the tumour and TME [113]. Although TMPs play important roles in promoting the formation of tumour drug resistance, increasing studies have focused on therapeutic applications of MPs to reverse drug resistance.
Drug resistance of microparticles in lung cancer
TMPs are capable of conferring resistance to chemotherapy. Two mechanisms are involved in MP-induced drug resistance. In the first mechanism, TMPs transport functional plasma membrane transporter proteins, including P-glycoprotein (P-gp), breast cancer resistance protein [114], and multidrug resistance (MDR)-associated protein 1 (MRP1) [115] or resistance-associated miRNAs, from drug-resistant cancer cells to drug-sensitive cancer cells [116]. MDR is innately present in tumours that arise from epithelium with a high constitutive P-gp expression [117, 118]. MDR development in cancer is clinically associated with the overexpression of the efflux transporter P-gp (P-gp, ABCB1) or MRP1 (MRP1, ABCC1) in numerous malignancies, including lung, breast, neuroblastoma, and prostate cancers [119,120,121]. P-gp and MRP1 belong to the ATP-binding cassette (ABC) transporter superfamily. ABC-transporters hydrolyse ATP to drive the extrusion of chemotherapeutic drugs against a concentration gradient from otherwise drug-sensitive cells. MRP1 and functional P-gp are transferred into recipient cells by MPs, imposing a donor dominant ABCC1 trait on drug-sensitive cells [116, 122, 123]. In addition to functional P-gp, MPs can also transport RNA, which can re-template recipient cells to ensure the acquisition of the donor cell MDR trait [122,123,124]. Some miRNAs, such as miR-27a, miR-326, and miR-451, have a potent ability to regulate ABC transporters [120, 123,124,125,126]. In the second mechanism, chemotherapeutic agents are directly expelled from cancer cells [127].
Microparticles and reversing drug resistance
Drug resistance remains a formidable hurdle in cancer therapy [128]. It may result from decreased drug uptake, increased drug efflux and expression of drug efflux pumps, drug inactivation/detoxification, more efficient DNA repair, and dysregulation of apoptotic pathways [129,130,131]. Furthermore, system cell-like cancer cells (SCLCCs) are a subset of highly tumorigenic cancer cells with the ability to self-renew and escape chemotherapy [132]. Stem cell-like tumour-repopulating cells (TRCs) play a vital role in reprogramming an immunosuppressive TME [107]. For example, TRCs cultured in vitro can replace SCLCCs and exert drug resistance. However, Ma et al. [109] showed that TMPs loaded with anti-tumour drugs can reverse the drug resistance of TRCs or SCLCCs. Delivering high concentration of drugs into soft MPs can effectively facilitate drug entry into the nucleus of tumour cells. Subsequently, soft TRCs readily undergo deformation, enabling the easy uptake of the MPs [133]. These MPs not only release drugs into the cytoplasm of TRCs, but also transport drugs into the lysosomes and nucleus, causing TRC apoptosis. Research has demonstrated objective evidence for the clinical efficacy of TMPs in patients with LC, making TMPs well tolerated in clinical practice [134].
Immunomodulation effect of microparticles in lung cancer
Intricate interactions among the immune system, TME, and cancer cells are regulated by bioactive molecules and biological information. In human cancer cells, TMPs are more immunogenic than soluble antigens [135]. Rughetti et al. found that MP-mediated antigen transfer to dendritic cells (DCs) is crucial for the cross-presentation of tumour-glycosylated antigens [53, 54]. MP signalling strengthens the immunosuppressive properties of tumour cells, promoting the escape of immune surveillance and tumour metastasis. Moreover, MPs may trigger T cell-activated apoptosis by exposing the Fas ligand, which might contribute to immune suppression and indirectly promote tumour growth [136, 137]. However, MPs also mediate antigen presentation by exposing major histocompatibility complex class I and II molecules to DCs to facilitate immune surveillance [138]. Similarly, the lipid component of MPs can stimulate antigen presentation by activating toll-like receptor 4 on macrophages [139]. Further research indicates that the stage of tumour progression determines the conflicting effects of MPs in modulating the immune system [28].
Cancer immunotherapy makes use of innate immune response against tumours, proposing a paradigm shift in cancer therapy. The key point of this therapy is to present cancer-specific immunogens and initiate T cell-mediated cancer immunity. Due to the conflicting effects of TMPs, the relationship among cancer cells, the TME, and the immune system is complex. TMPs are generally more immunogenic than soluble antigens in both mouse models and human cancer cells [135, 140]. Mesenchymal stem cell-derived EMPs can be used to carry tumour RNA and provoke the strong anti-tumour immune response of cytotoxic CD8+ cells. Oral vaccination with TMPs effectively accesses and activates the mucosal epithelium, leading to anti-tumour T cell response in mouse models. The most promising therapeutic application of MPs in the field of cancer immunotherapy may be vaccines [28].
Microparticles act as potential cancer vaccines
The fundamental principle of cancer vaccines is to provide antigen-presenting cells with both tumour antigens and immune-stimulating signals, resulting in an effective T cell immune response against tumours [141]. Zhang et al. proposed TMPs as ideal candidates for the development of novel and effective tumour vaccines [141,142,143]. TMPs have several applications in tumour vaccine development [144]. Apart from being potential antigen carriers, these can also directly target cancer cells. TMPs carry repertoires of tumour antigens and present these to DCs. Moreover, TMPs derived from UV-irradiated tumour cells may contain stimulatory molecules, such as DNA fragments, which stimulate DCs to produce type I interferons, interleukin (IL)-12, and interferon (IFN)-γ [145]. Type I IFNs are essential for CD8+ T cell priming, whereas IL-12 and IFN-γ promote antitumor T cell activation [145]. Research has shown that TMPs contain excessive immunostimulatory factors, resulting in the generation of innate immune signals in DCs [144]. Herein, TMPs contain tumor antigen spectrums and carry potential innate signals, which make them ideal candidates for developing novel therapeutic cancer vaccines. We have provided a comprehensive summary of the roles of MPs in LC patients. MPs that act as remarkable biological vectors are very promising and attractive tools for developing and exploring novel and individualised therapeutic strategies.
Concluding remarks and future direction
Numerous studies on the biology and biogenesis of MPs in cancer pathophysiology have revealed the significance of MPs in cancer growth, proliferation, apoptosis, angiogenesis, coagulation, and dissemination. In the airway and LC microenvironment, MPs derived from tumour-infiltrating cells and cancer cells are likely to play key roles in intercellular communication, promoting a microenvironment conducive to tumour growth, invasion, and metastasis (Fig. 2). Due to the evidence from current research, an increasing number of studies have suggested the possible clinical application of MPs as biomarkers. The diverse biomolecular information regarding EVs provides numerous potential biomarkers for cancer risk assessment, early detection, diagnosis, prognosis, and surveillance. To date, the development of EV-based biomarkers has largely focused on exosome biomarkers, and there are a number of key questions regarding MPs that will likely receive a great deal of research attention in the future. Several studies have investigated LC-related proteins in MPs. However, key nucleic acids are yet to be elucidated by comparing patients at different stages of LC to controls by DNA or RNA sequencing and mass spectrometry. In cancer immunotherapy, cancer vaccines are the most promising therapeutic application of MPs. Accumulated studies have investigated the involvement of MPs in lung disorders and attempted to provide new insights into the development of drug delivery systems and potential cancer vaccines. Although the exact functions and mechanisms of action of MPs have been elucidated, further research in the context of LC is necessary to ultimately develop useful means for cancer diagnosis and develop novel therapeutic strategies for various types of cancers.
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- LC:
-
Lung cancer
- EVs:
-
Extracellular vesicles
- MPs:
-
Microparticles
- EMPs:
-
Endothelial-derived microparticles
- NSCLC:
-
Non-small cell lung cancer
- TMPs:
-
Tumour-derived microparticles
- CECs:
-
Circulating endothelial cells
- IQGAP1:
-
Ras GTPase-activating-like protein 1
- BPIFA1:
-
BPI fold-containing family A member 1
- MiRNAs:
-
MicroRNAs
- PMPs:
-
Platelet-derived MPs
- EPB41L3:
-
Erythrocyte membrane protein band 4.1-like 3
- DU:
-
Differential centrifugation
- NTA:
-
Nanoparticle tracking analysis
- FCM:
-
Flow cytometry
- TRPS:
-
Tunable resistive pulse sensing
- AFM:
-
Atomic force microscopy
- DLS:
-
Dynamic light scattering
- TME:
-
Tumour microenvironment
- MMPs:
-
Macrophage-derived MPs
- LMPs:
-
Lymphocyte-derived MPs
- SPARCL1:
-
SPARC-like protein 1
- P-gp:
-
P-glycoprotein
- MDR:
-
Multidrug resistance
- MRP1:
-
MDR-associated protein 1
- ABC:
-
ATP-binding cassette
- SCLCCs:
-
System cell-like cancer cells
- TRCs:
-
Tumour-repopulating cells
- DCs:
-
Dendritic cells
- IL:
-
Interleukin
- IFN:
-
Interferon
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. Cancer J Clin. 2019;69(2019):7–34.
Chen X, Hao B, Li D, Reiter RJ, Bai Y, Abay B, Chen G, Lin S, Zheng T, Ren Y, Xu X, Li M, Fan L. Melatonin inhibits lung cancer development by reversing the Warburg effect via stimulating the SIRT3/PDH axis. J Pineal Res. 2021;71:e12755.
Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764–74.
Pirker R. Chemotherapy remains a cornerstone in the treatment of nonsmall cell lung cancer. Curr Opin Oncol. 2020;32:63–7.
Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–99.
Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163:4564–73.
Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, International Adjuvant Lung Cancer Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60.
Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–9.
Stinchcombe TE, Socinski MA. Maintenance therapy in advanced non-small cell lung cancer: current status and future implications. J Thorac Oncol. 2011;6:174–82.
Veeramachaneni NK, Feins RH, Stephenson BJ, Edwards LJ, Fernandez FG. Management of stage IIIA non-small cell lung cancer by thoracic surgeons in North America. Ann Thorac Surg. 2012;94:922–6 (discussion 926-928).
Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: prognostic and therapeutic implications. J Clin Oncol. 2005;23:3243–56.
Chanin TD, Merrick DT, Franklin WA, Hirsch FR. Recent developments in biomarkers for the early detection of lung cancer: perspectives based on publications 2003 to present. Curr Opin Pulm Med. 2004;10:242–7.
Hassanein M, Callison JC, Callaway-Lane C, Aldrich MC, Grogan EL, Massion PP. The state of molecular biomarkers for the early detection of lung cancer. Cancer Prev Res. 2012;5:992–1006.
Dolo V, D’Ascenzo S, Violini S, Pompucci L, Festuccia C, Ginestra A, Vittorelli ML, Canevari S, Pavan A. Matrix-degrading proteinases are shed in membrane vesicles by ovarian cancer cells in vivo and in vitro. Clin Exp Metastasis. 1999;17:131–40.
Morel O, Toti F, Hugel B, Freyssinet JM. Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 2004;11:156–64.
Abid Hussein MN, Nieuwland R, Hau CM, Evers LM, Meesters EW, Sturk A. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J Thromb Haemost. 2005;3:888–96.
Hamilton KK, Hattori R, Esmon CT, Sims PJ. Complement proteins C5b–9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J Biol Chem. 1990;265:3809–14.
Abid Hussein MN, Boing AN, Sturk A, Hau CM, Nieuwland R. Inhibition of microparticle release triggers endothelial cell apoptosis and detachment. Thromb Haemost. 2007;98:1096–107.
Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–10.
Vidulescu C, Clejan S, O’Connor KC. Vesicle traffic through intercellular bridges in DU 145 human prostate cancer cells. J Cell Mol Med. 2004;8:388–96.
Ponsaerts P, Berneman ZN. Modulation of cellular behavior by exogenous messenger RNA. Leukemia. 2006;20:767–9.
Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol. 2005;10:153–63.
Sung PS, Huang TF, Hsieh SL. Extracellular vesicles from CLEC2-activated platelets enhance dengue virus-induced lethality via CLEC5A/TLR2. Nat Commun. 2019;10:2402.
Martinez MC, Tesse A, Zobairi F, Andriantsitohaina R. Shed membrane microparticles from circulating and vascular cells in regulating vascular function. Am J Physiol Heart Circ Physiol. 2005;288:H1004-1009.
Lovren F, Verma S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin Chem. 2013;59:1166–74.
Mallat Z, Benamer H, Hugel B, Benessiano J, Steg PG, Freyssinet JM, Tedgui A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–3.
Sheu JJ, Lee FY, Wallace CG, Tsai TH, Leu S, Chen YL, Chai HT, Lu HI, Sun CK, Yip HK. Administered circulating microparticles derived from lung cancer patients markedly improved angiogenesis, blood flow and ischemic recovery in rat critical limb ischemia. J Transl Med. 2015;13:59.
Bian X, Xiao YT, Wu T, Yao M, Du L, Ren S, Wang J. Microvesicles and chemokines in tumor microenvironment: mediators of intercellular communications in tumor progression. Mol Cancer. 2019;18:50.
Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–7.
Nomura S, Kagawa H, Ozaki Y, Nagahama M, Yoshimura C, Fukuhara S. Relationship between platelet activation and cytokines in systemic inflammatory response syndrome patients with hematological malignancies. Thromb Res. 1999;95:205–13.
Janowska-Wieczorek A, Marquez-Curtis LA, Wysoczynski M, Ratajczak MZ. Enhancing effect of platelet-derived microvesicles on the invasive potential of breast cancer cells. Transfusion. 2006;46:1199–209.
Giusti I, D’Ascenzo S, Dolo V. Microvesicles as potential ovarian cancer biomarkers. Biomed Res Int. 2013;2013:703048.
Mege D, Panicot-Dubois L, Ouaissi M, Robert S, Sielezneff I, Sastre B, Dignat-George F, Dubois C. The origin and concentration of circulating microparticles differ according to cancer type and evolution: a prospective single-center study. Int J Cancer. 2016;138:939–48.
Najjar F, Alammar M, Al-Massarani G, Almalla N, Aljapawe A, Ikhtiar A. Circulating endothelial cells and microparticles for prediction of tumor progression and outcomes in advanced non-small cell lung cancer. Cancer Biomark. 2017;20:333–43.
Kanazawa S, Nomura S, Kuwana M, Muramatsu M, Yamaguchi K, Fukuhara S. Monocyte-derived microparticles may be a sign of vascular complication in patients with lung cancer. Lung Cancer. 2003;39:145–9.
Tseng CC, Wang CC, Chang HC, Tsai TH, Chang LT, Huang KT, Leu S, Yen CH, Liu SF, Chen CH, Yang CT, Yip HK, Lin MC. Levels of circulating microparticles in lung cancer patients and possible prognostic value. Dis Markers. 2013;35:301–10.
Crawford N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br J Haematol. 1971;21:53–69.
Hargett LA, Bauer NN. On the origin of microparticles: from “platelet dust” to mediators of intercellular communication. Pulm Circ. 2013;3:329–40.
Webber AJ, Johnson SA. Platelet participation in blood coagulation aspects of hemostasis. Am J Pathol. 1970;60:19–42.
Shet AS. Characterizing blood microparticles: technical aspects and challenges. Vasc Health Risk Manag. 2008;4:769–74.
Mause SF, Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–57.
VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res. 2003;59:277–87.
MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15:825–35.
Wiedmer T, Sims PJ. Participation of protein kinases in complement C5b-9-induced shedding of platelet plasma membrane vesicles. Blood. 1991;78:2880–6.
Aupeix K, Hugel B, Martin T, Bischoff P, Lill H, Pasquali JL, Freyssinet JM. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J Clin Invest. 1997;99:1546–54.
Johnson BL 3rd, Goetzman HS, Prakash PS, Caldwell CC. Mechanisms underlying mouse TNF-alpha stimulated neutrophil derived microparticle generation. Biochem Biophys Res Commun. 2013;437:591–6.
Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, Hendrix A, Mathivanan S. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47:D516-d519.
Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol. 2015;40:41–51.
Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol. 2019;37:707–29.
Sun Y, Huo C, Qiao Z, Shang Z, Uzzaman A, Liu S, Jiang X, Fan LY, Ji L, Guan X, Cao CX, Xiao H. Comparative proteomic analysis of exosomes and microvesicles in human saliva for lung cancer. J Proteome Res. 2018;17:1101–7.
Leeming GH, Kipar A, Hughes DJ, Bingle L, Bennett E, Moyo NA, Tripp RA, Bigley AL, Bingle CD, Sample JT, Stewart JP. Gammaherpesvirus infection modulates the temporal and spatial expression of SCGB1A1 (CCSP) and BPIFA1 (SPLUNC1) in the respiratory tract. Lab Investig. 2015;95:610–24.
Contzler R, Favre B, Huber M, Hohl D. Cornulin, a new member of the “fused gene” family, is expressed during epidermal differentiation. J Invest Dermatol. 2005;124:990–7.
Rughetti A, Rahimi H, Belleudi F, Napoletano C, Battisti F, Zizzari IG, Antonilli M, Bellati F, Wandall HH, Benedetti Panici P, Burchell JM, Torrisi MR, Nuti M. Microvesicle cargo of tumor-associated MUC1 to dendritic cells allows cross-presentation and specific carbohydrate processing. Cancer Immunol Res. 2014;2:177–86.
Battisti F, Napoletano C, Rahimi Koshkaki H, Belleudi F, Zizzari IG, Ruscito I, Palchetti S, Bellati F, Benedetti Panici P, Torrisi MR, Caracciolo G, Altieri F, Nuti M, Rughetti A. Tumor-derived microvesicles modulate antigen cross-processing via reactive oxygen species-mediated alkalinization of phagosomal compartment in dendritic cells. Front Immunol. 2017;8:1179.
Wu KL, Tsai YM, Lien CT, Kuo PL, Hung AJ. The roles of MicroRNA in lung cancer. Int J Mol Sci. 2019;20:1611.
Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–44.
Pan Y, Liang H, Liu H, Li D, Chen X, Li L, Zhang CY, Zen K. Platelet-secreted microRNA-223 promotes endothelial cell apoptosis induced by advanced glycation end products via targeting the insulin-like growth factor 1 receptor. J Immunol. 2014;192:437–46.
Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, Provost P. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood. 2013;122:253–61.
Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117.
Li X, Zhang Y, Zhang H, Liu X, Gong T, Li M, Sun L, Ji G, Shi Y, Han Z, Han S, Nie Y, Chen X, Zhao Q, Ding J, Wu K, Daiming F. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol Cancer Res. 2011;9:824–33.
Tran YK, Bogler O, Gorse KM, Wieland I, Green MR, Newsham IF. A novel member of the NF2/ERM/4.1 superfamily with growth suppressing properties in lung cancer. Cancer Res. 1999;59:35–43.
Laios A, O’Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D’Arcy T, McGuinness E, Sheils O, Sheppard B. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35.
Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, Sun F, Lu J, Yin Y, Cai X, Sun Q, Wang K, Ba Y, Wang Q, Wang D, Yang J, Liu P, Xu T, Yan Q, Zhang J, Zen K, Zhang CY. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–44.
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Secreted microRNAs: a new form of intercellular communication. Trends Cell Biol. 2012;22:125–32.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borras FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MA, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Gorecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzas EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekstrom K, El Andaloussi S, Elie-Caille C, Erdbrugger U, Falcon-Perez JM, Fatima F, Fish JE, Flores-Bellver M, Forsonits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gamez-Valero A, Gardiner C, Gartner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Gorgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanovic MM, Kovacs AF, Kramer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lasser C, Laurent LC, Lavieu G, Lazaro-Ibanez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Line A, Linnemannstons K, Llorente A, Lombard CA, Lorenowicz MJ, Lorincz AM, Lotvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Moller A, Moller Jorgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-’t Hoen EN, Noren Hooten N, O’Driscoll L, O’Grady T, O’Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Ostergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saa P, Sahoo S, Salas-Huenuleo E, Sanchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schoyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sodar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot E, Verweij FJ, Vestad B, Vinas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yanez-Mo M, Yin H, Yuana Y, Zappulli V, Zarubova J, Zekas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM, Nawaz M, Akbar N, Couch Y, Makin L, Cooke F, Vettore AL, Batista PX, Freezor R, Pezuk JA, Rosa-Fernandes L, Carreira ACO, Devitt A, Jacobs L, Silva IT, Coakley G, Nunes DN, Carter D, Palmisano G, Dias-Neto E. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Hu Y, Sun Y, Wan C, Dai X, Wu S, Lo PC, Huang J, Lovell JF, Jin H, Yang K. Microparticles: biogenesis, characteristics and intervention therapy for cancers in preclinical and clinical research. J Nanobiotechnol. 2022;20:189.
Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2:19861.
Cointe S, Judicone C, Robert S, Mooberry MJ, Poncelet P, Wauben M, Nieuwland R, Key NS, Dignat-George F, Lacroix R. Standardization of microparticle enumeration across different flow cytometry platforms: results of a multicenter collaborative workshop. J Thromb Haemost. 2017;15:187–93.
Krishnan SR, Luk F, Brown RD, Suen H, Kwan Y, Bebawy M. Isolation of human CD138(+) microparticles from the plasma of patients with multiple myeloma. Neoplasia. 2016;18:25–32.
McVey MJ, Spring CM, Semple JW, Maishan M, Kuebler WM. Microparticles as biomarkers of lung disease: enumeration in biological fluids using lipid bilayer microspheres. Am J Physiol Lung Cell Mol Physiol. 2016;310:L802-814.
Atkin-Smith GK, Tixeira R, Paone S, Mathivanan S, Collins C, Liem M, Goodall KJ, Ravichandran KS, Hulett MD, Poon IK. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun. 2015;6:7439.
de Vrij J, Maas SL, van Nispen M, Sena-Esteves M, Limpens RW, Koster AJ, Leenstra S, Lamfers ML, Broekman ML. Quantification of nanosized extracellular membrane vesicles with scanning ion occlusion sensing. Nanomedicine. 2013;8:1443–58.
Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet S, Brisson AR. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12:614–27.
Obeid S, Ceroi A, Mourey G, Saas P, Elie-Caille C, Boireau W. Development of a NanoBioAnalytical platform for “on-chip” qualification and quantification of platelet-derived microparticles. Biosens Bioelectron. 2017;93:250–9.
Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, GarciaRodriguez P, Bertina RM, Osanto S. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost. 2010;8:315–23.
Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945.
Abrams CS, Ellison N, Budzynski AZ, Shattil SJ. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–38.
Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102.
Martin S, Tesse A, Hugel B, Martinez MC, Morel O, Freyssinet JM, Andriantsitohaina R. Shed membrane particles from T lymphocytes impair endothelial function and regulate endothelial protein expression. Circulation. 2004;109:1653–9.
Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8:237–41.
Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–60.
Hughes M, Hayward CP, Warkentin TE, Horsewood P, Chorneyko KA, Kelton JG. Morphological analysis of microparticle generation in heparin-induced thrombocytopenia. Blood. 2000;96:188–94.
Galli M, Grassi A, Barbui T. Platelet-derived microvesicles in thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. Thromb Haemost. 1996;75:427–31.
Ando M, Iwata A, Ozeki Y, Tsuchiya K, Akiba T, Nihei H. Circulating platelet-derived microparticles with procoagulant activity may be a potential cause of thrombosis in uremic patients. Kidney Int. 2002;62:1757–63.
Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–3.
Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–32.
Menck K, Scharf C, Bleckmann A, Dyck L, Rost U, Wenzel D, Dhople VM, Siam L, Pukrop T, Binder C, Klemm F. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J Mol Cell Biol. 2015;7:143–53.
Ismail N, Wang Y, Dakhlallah D, Moldovan L, Agarwal K, Batte K, Shah P, Wisler J, Eubank TD, Tridandapani S, Paulaitis ME, Piper MG, Marsh CB. Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121:984–95.
Liu T, Wang J, Liu Y, Wu J, Yuan Y, Wang C, Fang X, Li H. Prediction of the therapeutic effects of pembrolizumab and nivolumab in advanced non-small cell lung cancer by platelet-derived microparticles in circulating blood. Technol Cancer Res Treat. 2021;20:1533033821997817.
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, Mark MT, Steiner L, Benito-Martin A, Lucotti S, Di Giannatale A, Offer K, Nakajima M, Williams C, Nogues L, Pelissier Vatter FA, Hashimoto A, Davies AE, Freitas D, Kenific CM, Ararso Y, Buehring W, Lauritzen P, Ogitani Y, Sugiura K, Takahashi N, Aleckovic M, Bailey KA, Jolissant JS, Wang H, Harris A, Schaeffer LM, Garcia-Santos G, Posner Z, Balachandran VP, Khakoo Y, Raju GP, Scherz A, Sagi I, Scherz-Shouval R, Yarden Y, Oren M, Malladi M, Petriccione M, De Braganca KC, Donzelli M, Fischer C, Vitolano S, Wright GP, Ganshaw L, Marrano M, Ahmed A, DeStefano J, Danzer E, Roehrl MHA, Lacayo NJ, Vincent TC, Weiser MR, Brady MS, Meyers PA, Wexler LH, Ambati SR, Chou AJ, Slotkin EK, Modak S, Roberts SS, Basu EM, Diolaiti D, Krantz BA, Cardoso F, Simpson AL, Berger M, Rudin CM, Simeone DM, Jain M, Ghajar CM, Batra SK, Stanger BZ, Bui J, Brown KA, Rajasekhar VK, Healey JH, de Sousa M, Kramer K, Sheth S, Baisch J, Pascual V, Heaton TE, La Quaglia MP, Pisapia DJ, Schwartz R, Zhang H, Liu Y, Shukla A, Blavier L, DeClerck YA, LaBarge M, Bissell MJ, Caffrey TC, Grandgenett PM, Hollingsworth MA, Bromberg J, Costa-Silva B, Peinado H, Kang Y, Garcia BA, O’Reilly EM, Kelsen D, Trippett TM, Jones DR, Matei IR, Jarnagin WR, Lyden D. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–61.
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Zhou X, Xie F, Wang L, Zhang L, Zhang S, Fang M, Zhou F. The function and clinical application of extracellular vesicles in innate immune regulation. Cell Mol Immunol. 2020;17:323–34.
Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–8.
Isler SG, Schenk S, Bendik I, Schraml P, Novotna H, Moch H, Sauter G, Ludwig CU. Genomic organization and chromosomal mapping of SPARC-like 1, a gene down regulated in cancers. Int J Oncol. 2001;18:521–6.
Najjar F, Alammar M, Al-Massarani G, Almalla N, Japawe A, Ikhtiar A. Circulating endothelial cells and microparticles as diagnostic and prognostic biomarkers in small-cell lung cancer. Lung Cancer. 2018;124:23–30.
Wang CC, Tseng CC. Circulating endothelial-derived activated microparticle: a useful biomarker for predicting one-year mortality in patients with advanced non-small cell lung cancer. Biomed Res Int. 2014;2014: 173401.
Wang CC, Tseng CC, Chang HC, Huang KT, Fang WF, Chen YM, Yang CT, Hsiao CC, Lin MC, Ho CK, Yip HK. Circulating microparticles are prognostic biomarkers in advanced non-small cell lung cancer patients. Oncotarget. 2017;8:75952–67.
Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33.
Collins LG, Haines C, Perkel R, Enck RE. Lung cancer: diagnosis and management. Am Fam Physician. 2007;75:56–63.
Shen L, Zhou Y, He H, Chen W, Lenahan C, Li X, Deng Y, Shao A, Huang J. Crosstalk between macrophages T cells, and iron metabolism in tumor microenvironment. Oxid Med Cell Longev. 2021;2021:8865791.
Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50.
Liu Z, Han C, Fu YX. Targeting innate sensing in the tumor microenvironment to improve immunotherapy. Cell Mol Immunol. 2020;17:13–26.
Saari H, Lazaro-Ibanez E, Viitala T, Vuorimaa-Laukkanen E, Siljander P, Yliperttula M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J Control Release. 2015;220:727–37.
Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, Zhang G, Feng ZH, Ye D, Huang B. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282.
Zhang Z, Xiao C, Yong T, Yang X, Gan L, Li Z. Cellular microparticles for tumor targeting delivery: from bench to bedside. Chem Commun. 2020;56:6171–88.
Sun Y, Zheng Z, Zhang H, Yu Y, Ma J, Tang K, Xu P, Ji T, Liang X, Chen D, Jin X, Zhang T, Long Z, Liu Y, Huang B. Chemotherapeutic tumor microparticles combining low-dose irradiation reprogram tumor-promoting macrophages through a tumor-repopulating cell-curtailing pathway. Oncoimmunology. 2017;6: e1309487.
Xu P, Tang K, Ma J, Zhang H, Wang D, Zhu L, Chen J, Wei K, Liu J, Fang H, Tang L, Zhang Y, Xie J, Liu Y, Meng R, Liu L, Dong X, Yang K, Wu G, Ma F, Huang B. Chemotherapeutic tumor microparticles elicit a neutrophil response targeting malignant pleural effusions, cancer. Immunol Res. 2020;8:1193–205.
Ma J, Zhang Y, Tang K, Zhang H, Yin X, Li Y, Xu P, Sun Y, Ma R, Ji T, Chen J, Zhang S, Zhang T, Luo S, Jin Y, Luo X, Li C, Gong H, Long Z, Lu J, Hu Z, Cao X, Wang N, Yang X, Huang B. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713–27.
Guo M, Wu F, Hu G, Chen L, Xu J, Xu P, Wang X, Li Y, Liu S, Zhang S, Huang Q, Fan J, Lv Z, Zhou M, Duan L, Liao T, Yang G, Tang K, Liu B, Liao X, Tao X, Jin Y. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Sci Transl Med. 2019;11:e5690.
Ran L, Tan X, Li Y, Zhang H, Ma R, Ji T, Dong W, Tong T, Liu Y, Chen D, Yin X, Liang X, Tang K, Ma J, Zhang Y, Cao X, Hu Z, Qin X, Huang B. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56–66.
Chen G, Zhu JY, Zhang ZL, Zhang W, Ren JG, Wu M, Hong ZY, Lv C, Pang DW, Zhao YF. Transformation of cell-derived microparticles into quantum-dot-labeled nanovectors for antitumor siRNA delivery. Angew Chem Int Ed Engl. 2015;54:1036–40.
Wendler F, Favicchio R, Simon T, Alifrangis C, Stebbing J, Giamas G. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene. 2017;36:877–84.
Liebhardt S, Ditsch N, Nieuwland R, Rank A, Jeschke U, Von Koch F, Friese K, Toth B. CEA-, Her2/neu-, BCRP- and Hsp27-positive microparticles in breast cancer patients. Anticancer Res. 2010;30:1707–12.
Lu JF, Luk F, Gong J, Jaiswal R, Grau GE, Bebawy M. Microparticles mediate MRP1 intercellular transfer and the re-templating of intrinsic resistance pathways. Pharmacol Res. 2013;76:77–83.
Jaiswal R, Luk F, Dalla PV, Grau GE, Bebawy M. Breast cancer-derived microparticles display tissue selectivity in the transfer of resistance proteins to cells. PLoS ONE. 2013;8: e61515.
Konieczna A, Erdosova B, Lichnovska R, Jandl M, Cizkova K, Ehrmann J. Differential expression of ABC transporters (MDR1, MRP1, BCRP) in developing human embryos. J Mol Histol. 2011;42:567–74.
Ziemann C, Burkle A, Kahl GF, Hirsch-Ernst KI. Reactive oxygen species participate in mdr1b mRNA and P-glycoprotein overexpression in primary rat hepatocyte cultures. Carcinogenesis. 1999;20:407–14.
Bebawy M, Morris MB, Roufogalis BD. Selective modulation of P-glycoprotein-mediated drug resistance. Br J Cancer. 2001;85:1998–2003.
Gong J, Jaiswal R, Mathys JM, Combes V, Grau GE, Bebawy M. Microparticles and their emerging role in cancer multidrug resistance. Cancer Treat Rev. 2012;38:226–34.
Munoz M, Henderson M, Haber M, Norris M. Role of the MRP1/ABCC1 multidrug transporter protein in cancer. IUBMB Life. 2007;59:752–7.
Bebawy M, Combes V, Lee E, Jaiswal R, Gong J, Bonhoure A, Grau GE. Membrane microparticles mediate transfer of P-glycoprotein to drug sensitive cancer cells. Leukemia. 2009;23:1643–9.
Jaiswal R, Luk F, Gong J, Mathys JM, Grau GE, Bebawy M. Microparticle conferred microRNA profiles–implications in the transfer and dominance of cancer traits. Mol Cancer. 2012;11:37.
Jaiswal R, Gong J, Sambasivam S, Combes V, Mathys JM, Davey R, Grau GE, Bebawy M. Microparticle-associated nucleic acids mediate trait dominance in cancer. FASEB J. 2012;26:420–9.
Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–9.
Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–8.
Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–7.
Bourzac K. Biology: Three known unknowns. Nature. 2014;509:S69-71.
Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84.
Liu Y, Ao X, Ji G, Zhang Y, Yu W, Wang J. Mechanisms of action and clinical implications of MicroRNAs in the drug resistance of gastric cancer. Front Oncol. 2021;11: 768918.
Baylin SB. Resistance, epigenetics and the cancer ecosystem. Nat Med. 2011;17:288–9.
Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells—what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512.
Chowdhury F, Na S, Li D, Poh YC, Tanaka TS, Wang F, Wang N. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 2010;9:82–8.
Zhu S, Li S, Yi M, Li N, Wu K. Roles of microvesicles in tumor progression and clinical applications. Int J Nanomed. 2021;16:7071–90.
Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, Delcayre A, Le Pecq JB, Combadiere B, Amigorena S, Thery C. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008;68:1228–35.
Martinez-Lorenzo MJ, Anel A, Gamen S, Monle I, Lasierra P, Larrad L, Pineiro A, Alava MA, Naval J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J Immunol. 1999;163:1274–81.
Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–20.
Van Niel G, Mallegol J, Bevilacqua C, Candalh C, Brugiere S, Tomaskovic-Crook E, Heath JK, Cerf-Bensussan N, Heyman M. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690–7.
Thomas LM, Salter RD. Activation of macrophages by P2X7-induced microvesicles from myeloid cells is mediated by phospholipids and is partially dependent on TLR4. J Immunol. 1950;185(2010):3740–9.
Napoletano C, Rughetti A, Landi R, Pinto D, Bellati F, Rahimi H, Spinelli GP, Pauselli S, Sale P, Dolo V, De Lorenzo F, Tomao F, Benedetti-Panici P, Frati L, Nuti M. Immunogenicity of allo-vesicle carrying ERBB2 tumor antigen for dendritic cell-based anti-tumor immunotherapy. Int J Immunopathol Pharmacol. 2009;22:647–58.
Zhang H, Tang K, Zhang Y, Ma R, Ma J, Li Y, Luo S, Liang X, Ji T, Gu Z, Lu J, He W, Cao X, Wan Y, Huang B. Cell-free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2015;3:196–205.
Zhang H, Huang B. Tumor cell-derived microparticles: a new form of cancer vaccine. Oncoimmunology. 2015;4: e1017704.
Goldberg SR, Prada JA, Katz JL. Stereoselective behavioral effects of N6-phenylisopropyl-adenosine and antagonism by caffeine. Psychopharmacology. 1985;87:272–7.
Ma J, Zhang H, Tang K, Huang B. Tumor-derived microparticles in tumor immunology and immunotherapy. Eur J Immunol. 2020;50:1653–62.
Ma J, Wei K, Zhang H, Tang K, Li F, Zhang T, Liu J, Xu P, Yu Y, Sun W, Zhu L, Chen J, Zhou L, Liang X, Lv J, Fiskesund R, Liu Y, Huang B. Mechanisms by which dendritic cells present tumor microparticle antigens to CD8(+) T cells, cancer. Immunol Res. 2018;6:1057–68.
Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–8.
Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–39.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–7.
Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, Thomas PC, Raposo G, Marks MS. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10:343–54.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–21.
Fullard JF. The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr Pharm Des. 2004;10:1567–76.
Li R, Emsley J. The organizing principle of the platelet glycoprotein Ib-IX-V complex. J Thromb Haemost. 2013;11:605–14.
Koedam JA, Cramer EM, Briend E, Furie B, Furie BC, Wagner DD. P-selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J Cell Biol. 1992;116:617–25.
Tokes-Fuzesi M, Ruzsics I, Rideg O, Kustan P, Kovacs GL, Molnar T. Role of microparticles derived from monocytes, endothelial cells and platelets in the exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3749–57.
Aloui C, Prigent A, Sut C, Tariket S, Hamzeh-Cognasse H, Pozzetto B, Richard Y, Cognasse F, Laradi S, Garraud O. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci. 2014;15:22342–64.
Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–88.
Deng F, Wang S, Zhang L. Endothelial microparticles act as novel diagnostic and therapeutic biomarkers of circulatory hypoxia-related diseases: a literature review. J Cell Mol Med. 2017;21:1698–710.
Barbara NP, Wrana JL, Letarte M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J Biol Chem. 1999;274:584–94.
Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18.
Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–30.
Lastres P, Letamendia A, Zhang H, Rius C, Almendro N, Raab U, Lopez LA, Langa C, Fabra A, Letarte M, Bernabeu C. Endoglin modulates cellular responses to TGF-beta 1. J Cell Biol. 1996;133:1109–21.
Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003;98:257–65.
Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–32.
Woodfin A, Voisin MB, Nourshargh S. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol. 2007;27:2514–23.
Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11.
Ley K, Huo Y. VCAM-1 is critical in atherosclerosis. J Clin Invest. 2001;107:1209–10.
Kraft M, Striz I, Georges G, Umino T, Takigawa K, Rennard S, Martin RJ. Expression of epithelial markers in nocturnal asthma. J Allergy Clin Immunol. 1998;102:376–81.
Cabrales P. RRx-001 Acts as a dual small molecule checkpoint inhibitor by downregulating CD47 on cancer cells and SIRP-alpha on monocytes/macrophages. Transl Oncol. 2019;12:626–32.
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin R, Cohen JD, Lovelace P, Scheeren FA, Chao MP, Weiskopf K, Tang C, Volkmer AK, Naik TJ, Storm TA, Mosley AR, Edris B, Schmid SM, Sun CK, Chua MS, Murillo O, Rajendran P, Cha AC, Chin RK, Kim D, Adorno M, Raveh T, Tseng D, Jaiswal S, Enger PO, Steinberg GK, Li G, So SK, Majeti R, Harsh GR, van de Rijn M, Teng NN, Sunwoo JB, Alizadeh AA, Clarke MF, Weissman IL. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–7.
Taylor-Papadimitriou J, Burchell JM, Plunkett T, Graham R, Correa I, Miles D, Smith M. MUC1 and the immunobiology of cancer. J Mammary Gland Biol Neoplasia. 2002;7:209–21.
Chen S, Yang Q, Liu J, Huang H, Shi M, Feng X. Tsc1 controls the development and function of alveolar macrophages. Biochem Biophys Res Commun. 2018;498:592–6.
Chowdhury IH, Koo SJ, Gupta S, Liang LY, Bahar B, Silla L, Nunez-Burgos J, Barrientos N, Zago MP, Garg NJ. Gene expression profiling and functional characterization of macrophages in response to circulatory microparticles produced during trypanosoma cruzi infection and chagas disease. J Innate Immun. 2017;9:203–16.
Paul P, Pedini P, Lyonnet L, Di Cristofaro J, Loundou A, Pelardy M, Basire A, Dignat-George F, Chiaroni J, Thomas P, Reynaud-Gaubert M, Picard C. FCGR3A and FCGR2A genotypes differentially impact allograft rejection and patients’ survival after lung transplant. Front Immunol. 2019;10:1208.
Acknowledgements
We would like to thank BioRender.com for illustration drawing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82070099; No. 82041018; No. 82102496).
Author information
Authors and Affiliations
Contributions
YJ designed the study. LY and SW wrote the draft. XH and SS made the table and figures. YJ, XT reviewed and edited the manuscript before submission. SZ, DM and QC, commented and added extra information. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Wang, S., Xia, H. et al. The potential applications of microparticles in the diagnosis, treatment, and prognosis of lung cancer. J Transl Med 20, 404 (2022). https://doi.org/10.1186/s12967-022-03599-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03599-x