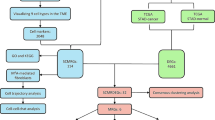
Abstract
Gastric cancer is a common type of gastrointestinal malignant tumor in China. The mechanism of the development and progression of gastric cancer remains the continuing research focus. The tumor microenvironment plays an important role in the development and progression of tumors. The present study used single-cell sequencing data to characterize the microenvironment of gastric cancer, investigate the effects of oxidative stress on gastric cancer microenvironmental cells through the comparison between cancer tissue and normal tissue, and identify the key genes associated with gastric cancer patients’ survival. The results showed that compared with normal gastric tissue, gastric cancer tissue had a decreased oxidative stress response, weaker oxidative detoxification ability, and increased oxidative stress-induced cell death. In the different types of single cells of gastric cancer microenvironment, the oxidative stress response of T cell was increased, the ability of oxidative detoxification was enhanced, and the oxidative stress-induced cell death was exacerbate. Mucous cell showed the same trend as gastric cancer cells: decreased oxidative stress response, weak oxidative detoxification ability, and weakened oxidative stress-induced cell death. Moreover, TRIM62, MET, and HBA1, which were significantly associated with oxidative stress, may be biomarkers for the prognosis of gastric cancer. High expression of TRIM62 indicated a good prognosis, while MET and HBA1 indicated a poor prognosis, which will be confirmed by further clinical studies.
Similar content being viewed by others
Introduction
Gastric cancer is the most common malignant tumor of the digestive tract and it is the fourth leading cause of cancer mortality worldwide [1]. The statistics from the International Agency for Research on Cancer in 2020 show that there were approximately 1.089 million new cases of gastric cancer and 769,000 deaths worldwide in that year, of which 43.9% of the cases and 48.6% of the deaths occurred in China [2]. At present, the overall treatment model for gastric cancer has undergone significant changes. It has evolved from anatomy-based surgical resection treatment to a comprehensive treatment model based on anatomy, tumor biology, and immunology [3]. The immune clinical treatment of gastric cancer still faces great challenges, which has a certain connection with the microenvironment of gastric cancer.
The microenvironment of gastric cancer refers to the internal environment in which gastric cancer occurs and develops. It is composed of gastric cancer cells, microvessels, microlymphatic vessels, interstitial cells, tissue fluid, immune and inflammatory cells, numerous cytokines, and a small amount of infiltrating cells, etc. [4]. The microenvironment of gastric cancer is quite complex, and it is very different from the microenvironment of normal tissues; among them, inflammation is the key initial step in the occurrence of gastric cancer, and it is the main origin of gastrointestinal cancer [5]. Many inflammatory factors, such as TNF-α, IL-6, TGF-β, and IL-10, have been confirmed to be involved in the occurrence and development of tumors. In this process, the levels of reactive oxygen and nitrogen are affected, and the oxidative stress caused by them is the main cause of deoxynucleotide damage and tumor transformation [6]. The interaction between the tumor and its microenvironment is also quite complex. Exposure of cancer stem cells to the microenvironment of reactive oxygen can stimulate the antioxidant system to enhance the antioxidant capacity and acquire the malignant phenotype [7].
Oxidative stress refers to a state of imbalance between oxidation and antioxidant effects in the body. Kruk, et al. summarized the carcinogenic effects of reactive oxygen and nitrogen. Oxidative stress is not only a major cause of gastrointestinal mucosal diseases but also relevant in the occurrence and development of gastric cancer [8]. This study will combine The Cancer Genome Atlas (TCGA) gastric cancer sequencing data and gastric cancer single-cell sequencing data to describe the gastric cancer microenvironment. Through the comparison between pathological tissues and normal tissues, analyze how oxidative stress affects microenvironmental cells, and discover the risk genes that have a key impact on the survival of gastric cancer patients.
Materials and methods
Data collection and processing
We downloaded and collected the data of gastric cancer patients in the TCGA project from the UCSC Xena (https://xenabrowser.net/datapages/) database, including RNA-sequencing analysis of gene expression profiles and patient clinical information. We also collected and downloaded all GO: BP (GO BP terms; biological process GO terms) from the Molecular Signatures Database (MsigDB; https://www.gsea-msigdb.org/gsea/msigdb) for the evaluation of oxidative activation related functions. In addition, we chose to collect and download a set of gastric cancer-related single-cell sequencing data, GSE112302, from the Gene-Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) database.
ssGSEA algorithm calculates the function score
To assess differences in oxidative stress-related functions in samples, we downloaded GO BP terms in the MsigDB database to select the required function items, then used the R package "GSVA" to score each sample with the single sample GSEA (ssGSEA) algorithm. The higher the score, the higher the relative level of gene expression related to the functional item of each sample. We use this to evaluate the activation state of each sample in the functional item [9].
Quality control and processing of single cell data
For the GSE112302 single-cell sequencing data obtained from the GEO database, we have obtained the Transcripts Per Million (TPM) expression matrix of every single cell and performed quality control. We counted the number of genes expressed in every single cell and the number of cells expressed by each gene, determine the quality control threshold according to the overall distribution and eliminate low-quality single cells and genes. After the preliminary quality control, the R package "Seurat" was used to carry out a standardized analysis process. We first used "NormalizeData" to standardize the data, then used "RunPCA" for principal component analysis. Finally, single-cell samples were clustered using the k-nearest neighbor classification (KNN) algorithm through "FindNeighbors" and "FindClusters" [10].
Prognostic analysis
To assess the impact of genes on the prognosis of gastric cancer patients, we combined the prognostic survival clinical information of gastric cancer patients in the TCGA database, used the R package "survival" to construct a Cox proportional hazard model and performed a log-rank test, and recorded the HR (95%CI) and the P-value of the survival model.
Screening and functional annotation of differentially expressed genes
To explore the differences in the expression of cells derived from normal tissues and tumor tissues, we separately counted the average expression of each gene in normal cells and tumor cells, calculated the Fold Change value, and recorded the P-value of the Wilcoxon rank-sum test. The screening threshold was |log2FC|> 1 and P-value is < 0.01. In addition, we also use the DAVID function to perform functional annotations in response to differentially expressed genes.
Statistical analysis
The following statistical models and methods were used in this study: (1) Wilcoxon rank-sum test is used to compare the difference between two sets of data; (2) The Cox proportional hazard model is used to evaluate the impact of gene expression levels on the prognosis of gastric cancer patients; (3) Log-rank test is used to assess whether gene expression levels significantly affect patient survival.
Results
Oxidative stress is different in normal and gastric cancer tissues
The human body is always in a state of balance between the oxidation system and the antioxidant system. Oxidative stress refers to the pathological process in which the body produces too much active oxygen or the antioxidant capacity is reduced, and the oxidative system and the antioxidant system are out of balance, which leads to the pathological process of potential damage. The response to oxidative stress is the basis for ensuring the normal metabolic activity of cells. Therefore, we downloaded the gastric cancer tissue RNA-sequencing sample data from the TCGA database and compared the response of normal gastric tissue and gastric cancer tissue under oxidative stress.
According to the relevant functional items collected from the MsigDB database, we used the ssGSEA algorithm to score each TCGA gastric cancer sample for oxidative stress response, including 35 normal gastric tissue samples and 415 gastric cancer samples. First, we evaluated the "GO_RESPONSE_TO_OXIDATIVE_STRESS" of the two types of samples. We found that the response level of gastric cancer samples to oxidative stress was generally lower than that of normal gastric tissue samples (P = 0.02) (Fig. 1A). We further evaluated the “GO_CELLULAR_OXIDANT_DETOXIFICATION” cell oxidation and detoxification capabilities of the two types of samples and found that the oxidation and detoxification capabilities of gastric cancer samples were generally weaker than that of normal gastric tissue samples (P = 1.5e−8) (Fig. 1B).
A Difference of cellular oxidative stress response in TCGA gastric cancer samples. B Differences in the detoxification ability of cellular oxidants in TCGA gastric cancer samples. C Differences in the ability of oxidative stress-induced cell death in TCGA gastric cancer samples, the picture on the left shows the ssGSEA scores for specific GO BP terms in the two types of samples; The higher the score, the more active the function is. The figure on the right shows the expression of differentially expressed genes in the two types of samples in this term
Finally, we also evaluated the functional scores of "GO_CELL_DEATH_IN_RESPONSE_TO_OXIDATIVE_STRESS" for oxidative stress-induced cell death in two types of samples, and found that the ssGSEA score of tumor samples was significantly higher than that of normal gastric tissue samples (P = 0.015) (Fig. 1C).
Oxidative stress response and microsatellite instability
Oxidative stress can cause damage to cell DNA or RNA. Microsatellite instability (MSI) is one of the indicators of chromosome instability. We suspect that there will be a certain relationship between the two. Therefore, we collected and sorted out the MSI of 450 gastric cancer patients in the TCGA database (Table 1). According to the MSI, gastric cancer patients are mainly divided into three categories: MSI, MSI-L and MSI-H. We also use the "ssGSEA" algorithm to calculate the "GO_RESPONSE_TO_OXIDATIVE_STRESS" oxidative stress response ssGSEA score of the three types of gastric cancer patients. We found that MSI-H gastric cancer patients had the most active oxidative stress response (MSI-H vs. MSI: P = 0.00094; MSI-H vs. MSI-L: P = 0.00018), but there is little difference between MSI-L and MSS gastric cancer patients (P = 0.15) (Fig. 2). The above results indicated that the tumor tissue of patients with MSI-H STAD has an active oxidative stress function, which causes DNA and RNA damage, and ultimately leads to chromosomal instability.
Gastric cancer microenvironment ecology
In the above analysis, we found that gastric cancer tissue is relatively inactive in response to oxidative stress, and its ability to oxidize and detoxify is relatively weak, while oxidative stress-induced cell death is strengthened. But considering that this is not consistent with the phenomenon of tumor cells evading cell death. The analysis is too consistent. The possible reason is that the above analysis is carried out in tissue samples, and the tissue samples are mixed with tumor cells and microenvironmental cells. Therefore, we also downloaded a set of single-cell sequencing data of gastric cancer from the GEO database, GSE112302, to distinguish between tumor cells and microenvironmental cells for analysis. The single cells were derived from 6 tumor tissues and 4 normal tissues. A total of 707 single cells were detected and 24,135 genes were captured.
First of all, we follow the part described in 2.3, preliminary quality control has been carried out to eliminate low-quality genes and cells. We counted the number of genes expressions in each single cell, divided the threshold according to the overall distribution to 700, and eliminated cells with less than 700 expressed genes (Fig. 3A). We also counted the number of cells expressed for each gene, divided the threshold according to the overall distribution to 25, and the genes expressed in less than 25 cells are eliminated (Fig. 3B). At this time, the expression profile we got includes 663 cells and 14,086 genes.
A Single cell quality control chart; the figure above counts the number of genes expressed in each single cell, and it is considered that cells expressing less than 700 genes are low-quality cells; the figure below counts the number of cells expressing each gene. It is considered that genes expressed in less than 25 cells are low-quality genes. B TSNE chart shows the sample source of the single cell. C TSNE chart shows the cell type of the single cell
Subsequently, we used the R package "Seurat" to standardize single cells, including data standardization, PCA dimensionality reduction, KNN, etc. In the end, we obtained 7 cell subgroups (Table 2, Fig. 3C). According to the signature expression markers of stomach cells (Fig. 4), we identified CD4 + T cells, CD8 + T cells, mucous cells (TFF1 + and MUC5AC +), intestinal mucous cells [REG4 + and SPINK4 +], Chief cells (PGC +), and the potential gastric cancer cells (ALDH1A2 + and EPCAM +) completely derived from gastric cancer tissues. Among them, ALDH1A2 is a symbolic marker of ovarian cancer cancer stem cell, and it may also be a potential marker of gastric cancer stem cells.
Single cell oxidative stress in gastric cancer
Furthermore, based on the analysis results in tissue samples, we believe that in the tumor microenvironment, tumor cells and cells in the microenvironment should have different responses to oxidative stress. Therefore, we also used the "ssGSEA" algorithm in single-cell samples to calculate the "GO_RESPONSE_TO_OXIDATIVE_STRESS" oxidative stress response ssGSEA score for each single cell.
We found that single cells in gastric cancer tissues were the same as the results in tissue samples, and the oxidative stress response ssGSEA score was significantly lower than that of single cells in normal tissues (P = 1.9e−11). Moreover, when subdivided into different cell types of single cells in gastric cancer tissue, we found that the oxidative stress response ssGSEA score of gastric cancer cells in gastric cancer tissue is much lower than that of cells in the microenvironment. Among them, the oxidative stress of T cells is the most active (vs. gastric cancer cells, P < 2.2e−16); gastric cancer cells were also significantly lower than Mucous cells in the microenvironment that had the lowest ssGSEA score in response to oxidative stress (P = 0.012) (Fig. 5A).
A Comparison of differences in oxidative stress response function scores in single cells; Note: The picture on the left is the comparison between all single cells derived from normal gastric tissue samples and single cells derived from tumor samples. On the right is a comparison between different cell populations of single cells derived from tumor tissues. B Difference of oxidative stress response function scores of microenvironmental cells in normal and tumor tissues
Due to the observation that T cells in gastric cancer tissues are highly active in response to oxidative stress, we compared cell types derived from normal tissues and gastric cancer tissues (Table 3). We found that the ssGSEA score of T cells in gastric cancer tissues in response to oxidative stress was much higher than that in normal tissues, indicating that T cells in gastric cancer tissues were greatly affected by reactive oxygen (P = 0.001082013). Similarly, Chief cells_2 in gastric cancer tissues were also much higher than normal tissues (P = 5.46E−06). The Mucous cell_1 in the tumor tissue is lower than that in the normal tissue. This scoring trend is consistent with that of the tumor cells, indicating that the mucous cell in the tumor tissue has begun to become cancerous (Fig. 5B, Table 4).
Oxidation and detoxification ability of gastric cancer single cell
We also carried out the analysis of the function score of "GO_CELLULAR_OXIDANT_DETOXIFICATION" oxidation and detoxification at the single cell level. We found that when specific to different cell types, the scores of other cell types except gastric cancer cells and mucous cells are higher, which further indicates that mucous cells may be a gradually cancerous cell (Fig. 6A).
In addition, we compared the tumor source and the normal tissue source according to the cell type, and found that the cells in the tumor microenvironment had a higher score for oxidation and detoxification of ssGSEA, especially T cells. Compared with normal tissue sources, T cells in gastric cancer tissues have significantly stronger oxidative and detoxification capabilities (P = 0.001082013) (Fig. 6B, Table 5).
Oxidative stress induced gastric cancer single cells death
Similarly, in the comparison of tissue samples, we found that the ssGSEA score of "GO_CELL_DEATH_IN_RESPONSE_TO_OXIDATIVE_STRESS" in gastric cancer tissues that oxidative stress-induced cell death was significantly higher than that of normal tissues. This phenomenon is contrary to the phenomenon of tumor cells evading cell death, so we also performed a single-cell level analysis.
The single-cell level results show that the single cells derived from gastric cancer are significantly lower (P = 3.7e−12). When specific to different cell types, other cell types except gastric cancer cells and Mucous cells have higher ssGSEA scores, which further indicates that Mucous cells may be a kind of gradually cancerous cell (Fig. 7A). In addition, we compared the levels of single cells derived from gastric cancer and normal tissues according to the cell type, and found that cells in the microenvironment of gastric cancer had higher ssGSEA scores for oxidative stress-induced cell death. It shows that cells in the microenvironment of gastric cancer tissues are all prone to cell death due to oxidative stress (Fig. 7B, Table 6).
Combined with the above overall analysis results, we found that gastric cancer cells in gastric cancer tissues are very insensitive to oxidative stress, and they will not die due to oxidative stress. However, the cells in the microenvironment are extremely sensitive to oxidative stress and easily lead to cell death, especially the T cells (P = 1.17E−08). The above results indicate that the oxidative stress on immune cells is likely to be a way for tumors to escape the attack of the immune system. In addition, we also found that mucous cells gradually become cancerous in the microenvironment of gastric cancer, suggesting that the origin of gastric cancer may start from mucous cells.
Characteristics of potential tumor stem cells in gastric cancer
In the previous related analysis, we found that there are a group of potential cancer stem cells in the single cells derived from gastric cancer tissues, which specifically express ALDH1A2 and EPCAM. Therefore, we hope to understand the characteristics of such potential gastric cancer stem cells.
We used the R package "Seurat" to obtain the differential expression markers between this cell population and the rest of the cell population. We screened low-expressed genes in gastric cancer stem cells, and explored how tumor stem cells inhibit what biological functions. Using the DAVID tool, we performed functional annotations of the top 200 low-expressed genes. We found that this group of cells inhibited "negative regulation of epithelial to mesenchymal transition" and "positive regulation of release of sequestered calcium ion into cytosol" and other functions.
Among them, epithelial-mesenchymal transition is a major feature of tumors. This group of cancer stem cells significantly inhibited the three genes TRIM62, HPN, and PBLD that negatively regulate this process. To this end, we conducted a prognostic analysis of these three genes. We obtained 450 samples from gastric cancer patients in the TCGA project that detected gene expression and recorded detailed prognostic information. Based on the expression of these three genes, we conducted prognostic analysis. We found that single cell expression of TRIM62 was increased in tumor samples (Fig. 8A), which was a protective factor for patients with gastric cancer. According to the expression of TRIM62, patients with gastric cancer are divided into high expression group (expression higher than 43.3413) and low expression group (expression lower than 43.3413). The constructed Cox proportional hazard model had HR [90% CI] = 0.7067 [0.5144–0.9709], the log-rank test P value was 0.031 (Fig. 9A). However, the expression of HPN and PBLD does not significantly affect patient survival.
Features of cancerous mucous cell
In addition to cancer stem cells, we also found in the previous analysis that mucous cells derived from tumor tissues gradually began to become cancerous, getting closer and closer to cancer stem cells, and exposed to a high degree of reactive oxygen stress. However, the response to oxidative stress was significantly reduced.
Therefore, we performed differential expression analysis of mucous cells derived from gastric cancer and normal gastric tissue, and extracted genes related to oxidative stress. According to the "GO_RESPONSE_TO_OXIDATIVE_STRESS" oxidative stress response related genes recorded in MsigDB, we obtained a total of 366 related genes, of which 141 genes were differentially expressed, including 22 up-regulated genes and 119 down-regulated genes (Additional file 1: Table S1).
Among them, we found that MET was highly expressed in single cells of tumor samples (Fig. 8B) and cancerous mucous cells, and it has been reported in the literature that it is a proto-oncogene, but there are few reports in gastric cancer. According to the expression value of MET, patients were divided into high expression group (expression higher than 84.25) and low expression group (expression lower than 84.25). The constructed Cox proportional hazard model had HR [95% CI] = 1.512[1.061–2.155], log-rank test P value was 0.021 (Fig. 9B).
In addition, we also found that HBA1 was highly expressed in single cells of tumor samples (Fig. 8C) and cancerous mucous cells, and the results of survival analysis show that it is indeed a risk factor. According to the expression value of HBA1, patients were divided into high expression group (expression higher than 68.7978) and low expression group (expression lower than 68.7978), and the constructed Cox proportional hazard model had HR (95% CI) = 1.808(1.196–2.733), log-rank test P-value was 0.0044 (Fig. 9C).
Disccusion
This study systematically clarified the difference in the ssGSEA scores of gastric cancer tissue levels and the microenvironment of gastric cancer in single-cell oxidative stress response, oxidative detoxification, and oxidative stress-induced cell death. The results showed that compared with normal gastric tissue, gastric cancer tissue has a lower oxidative stress response, weaker oxidative detoxification ability, and increased oxidative stress-induced cell death; in the microenvironment of gastric cancer, specific to different types of single cells, we found that the level of T cell oxidative stress response is increased, the ability of oxidative detoxification is enhanced, and the oxidative stress-induced cell death is enhanced. It indicates that the oxidative stress on immune cells is likely to be a way for gastric cancer to evade the immune system. At the same time, in the comparative analysis results of gastric cancer microenvironmental single cells and normal gastric tissues, we can see that Mucous cells show the same trend as gastric cancer cells: poor oxidative stress response, weak oxidative detoxification ability, and weakened oxidative stress-induced cell death, shows that Mucous cell may be a gradually cancerous cell.
Oxidative stress refers to a state in which the oxidation and antioxidant effects in the body are out of balance. Oxidative stress and the resulting oxidative damage are important factors in the occurrence and development of tumors [11, 12]. Studies have compared the oxidative stress levels of the tumor center, tumor margins and healthy tissues of head and neck cancer, and found that the oxidative stress level at the tumor margins was significantly higher than that in the tumor center and healthy tissues, indicating that oxidative stress is related to the occurrence of head and neck cancer [13]. Reuter, er al. summarized the correlation between oxidative stress, inflammation and cancer and points out that oxidative stress can activate transcription factors such as NF-κB, AP-1, p53, HIF-1α, PPAR-γ, β-catenin/Wnt and Nrf2. The activation of these transcription factors can trigger the expression of more than 500 different genes including inflammatory factors. This is closely related to the transformation, survival and proliferation of normal cells to tumor cells [14]. Reactive oxygen species (ROS) is a metabolic by-product produced in the gastrointestinal tract. It is the main attribution of oxidative stress. The oxidative stress caused by it plays an important role in the pathogenesis of gastrointestinal mucosal diseases including gastrointestinal cancer [15]. So far, the pathogenesis of ROS mediated including the occurrence and development of gastrointestinal mucosal diseases is not clear. Huang et al. concluded that ROS participates in the occurrence of gastric cancer by regulating the expression of microRNAs [16]. ROS can not only participate in the process of many diseases by regulating the expression of microRNAs, but it can also participate in the process of long non-coding RNAs (lncRNAs) [17].
The regulation of oxidative stress plays an important role in tumor development and the response to anti-cancer treatments. Gorrini et al. believed that targeted regulation of the antioxidant capacity of tumor cells can produce positive therapeutic effects [18]. In this study, it was found that the ssGSEA score of oxidative stress-induced cell death in gastric cancer tissue was higher than that of normal gastric tissue. Interesting results were found in the microenvironment of gastric cancer. The ssGSEA scores of gastric cancer cells and mucous cells were lower than those of other cell subgroups. Therefore, regulating gastric cancer cells to oxidative stress-induced cell death may have a positive therapeutic effect. In addition, the regulation of oxidative stress response and oxidative detoxification of gastric cancer cells may also produce positive therapeutic effects. For cancer treatment, targeting the tumor microenvironment may become a new strategy for cancer treatment in the future in response to changes in the level of oxidative stress caused by genetic alterations in tumor cells [19].
The microenvironment of gastric cancer is very complex, it contains a variety of cell types: inflammatory cells, fibroblasts, nerve cells and vascular endothelial cells, etc. [5]. Zeng, et al. comprehensively described all the characteristics of the gastric cancer microenvironment and made a detailed analysis of immune infiltration [20]. This study revealed seven cell subgroups in the microenvironmental ecology of gastric cancer through single-cell sequencing data. They are intestinal mucous cells, T cells, Chief cells_1, Chief cells_2, Mucous cells_1, Mucous cells_2 and Tumor cells. It contains a potential gastric cancer cell (ALDH1A2 + and EPCAM +) completely derived from gastric cancer tissue, and ALDH1A2 is a marker of ovarian cancer cancer stem cell [21, 22]. This group of cells inhibited functions such as "negative regulation of epithelial to mesenchymal transition" and inhibited the three genes TRIM62, HPN, and PBLD in the process. After prognostic analysis of the three, TRIM62 was found to be a protective factor for the development of gastric cancer. TRIM62 is a plasmosin, which refers to the E3 ubiquitin ligase related to the RING finger domain. It can catalyze autoubiquitination in vivo and in vitro, and inhibit the proliferation, migration, invasion and Warburg Effect of lung cancer cells in a hypoxic environment [23, 24].
We analyzed the oxidative stress response of different cell subgroups in the microenvironment of gastric cancer and found: increased T cell oxidative stress response level, enhanced oxidative detoxification ability and enhanced oxidative stress-induced cell death, which indicate that the oxidative stress on immune cells is likely to be a way for gastric cancer to escape the immune system. “How can tumors evade immune surveillance and resist immune attacks” has attracted widespread attention [25]. Studies have shown that regulatory T cells are recruited into the human tumor microenvironment and inhibit TAA-specific T cell immunity [26]. It accelerates apoptosis due to its high vulnerability to free oxygen species and weak NRF2-related antioxidant system in the metabolically abnormal tumor microenvironment [27]. This study reveals the possible mechanism of gastric cancer cells evading immune surveillance and resisting immune attack from the single-cell level, which is related to the difference in the oxidative stress level of single cells in the microenvironment of gastric cancer.
In addition, we also found that Mucuous cells and tumor cells in the microenvironment of gastric cancer have the same ssGSEA score trend in the immune stress response, oxidative detoxification ability and oxidative stress-induced cell death. It may be a gradually cancerous cell. Similar results were found in previous single-cell sequencing studies. Glandular mucous cells tend to acquire intestinal stem cell-like phenotypes during metaplasia [28]. The development of intestinal gastric cancer precedes the appearance of the metaplastic cell lineage in the gastric mucosa. The metaplastic cell lineage is characterized by the secretion of mucus. At first, it provides a protective barrier for epithelial cells, but persistent damage and chronic inflammation can make cell reprogramming and metaplasia pattern cycle permanent, which promotes the occurrence of gastric cancer [29]. The genes MET and HBA1, which were significantly related to oxidative stress, were extracted from mucous cells, and their expression significantly affected the survival and prognosis of gastric cancer patients. The results of previous studies have shown that high expression of MET gene predicts a poor prognosis of gastric cancer, which is consistent with our findings [30, 31]. Hemoglobin Subunit Alpha 1 (HBA1) has not been reported much in previous clinical studies on tumor prognosis. Previous bioinformatics analysis showed that it is a key gene in castration-resistant prostate cancer [32]. Our analysis showed that it was also a key gene for predicting the survival and prognosis of gastric cancer.
The present results had pointed out three key genes for the prognosis of gastric cancer. The results of previous bioinformatics analysis identified many key genes associated with the pathogenesis and prognosis of gastric cancer: COL1A1, CXCL8, COL3A1, SPP1, COL1A2, TIMP1, CXCL1, BGN, MMP3 and SERPINE1 [33]. These results are useful for providing a promising therapeutic target in the treatment of cancers [34,35,36].
Conclusion
In summary, we analyzed the oxidative stress response of the gastric cancer microenvironment through single-cell sequencing. In the gastric cancer microenvironment, we identified mucous cells with metaplastic characteristics, and speculated that gastric cancer cells evade immune system attacks might be associated with aggravation of T cells death through oxidative stress in the gastric cancer microenvironment. Our analysis also provides potential biomarkers for prognostic survival analysis of gastric cancer related to oxidative stress: TRIM62, MET, and HBA1.
Data availability
Raw data of this study are available from the corresponding author ZLH upon request.
References
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21114012].
Cao M, Li H, Sun D, He S, Lei L, Peng J, Chen W. Epidemiological trend analysis of gastric cancer in China from 2000 to 2019. Chin J Dig Surg. 2021;20:102–9.
Ji JF, Shan F. Comprehensive therapy of gastric carcinoma. Zhonghua Wai Ke Za Zhi. 2011;49:193–7.
Arneth B. Tumor Microenvironment. Medicina (Kaunas). 2019. https://doi.org/10.3390/medicina56010015].
Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111:2696–707. https://doi.org/10.1111/cas.14521].
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014: 149185. https://doi.org/10.1155/2014/149185].
Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Cancer Biol. 2013;23:522–32. https://doi.org/10.1016/j.semcancer.2013.08.007].
Kruk J, Aboul-Enein HY. Reactive oxygen and nitrogen species in carcinogenesis: implications of oxidative stress on the progression and development of several cancer types. Mini Rev Med Chem. 2017;17:904–19. https://doi.org/10.2174/1389557517666170228115324].
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. J Proc Natl Acad Sci. 2005;102:15545–50. https://doi.org/10.1073/pnas.0506580102.
Slovin S, Carissimo A, Panariello F, Grimaldi A, Bouché V, Gambardella G, Cacchiarelli D. Single-cell RNA sequencing analysis: a step-by-step overview. Methods Mol Biol (Clifton, NJ). 2021;2284:343–65. https://doi.org/10.1007/978-1-0716-1307-8_19].
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, ME LL. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12:376–90. https://doi.org/10.1016/j.arr.2012.10.004.
Klaunig JE. Oxidative stress and cancer. Curr Pharm Des. 2018;24:4771–8. https://doi.org/10.2174/1381612825666190215121712].
Dogan R, Guler EM, Kocyigit A, Çelik İ, Senturk E, Yenigun A, Tugrul S, Ozturan O. Are the oxidative stress levels in the tumor center and tumor boundary different from those in healthy tissue? Eur Arch Otorhinolaryngol. 2021. https://doi.org/10.1007/s00405-021-06749-x].
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49:1603–16. https://doi.org/10.1016/j.freeradbiomed.2010.09.006].
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–54. https://doi.org/10.1152/physrev.00040.2012].
Huang T, Wang-Johanning F, Zhou F, Kallon H, Wei Y. MicroRNAs serve as a bridge between oxidative stress and gastric cancer (review). Int J Oncol. 2016;49:1791–800. https://doi.org/10.3892/ijo.2016.3686].
Ghafouri-Fard S, Shoorei H, Taheri M. Non-coding RNAs are involved in the response to oxidative stress. Biomed Pharmacother. 2020;127: 110228. https://doi.org/10.1016/j.biopha.2020.110228].
Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–47. https://doi.org/10.1038/nrd4002.
Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20040840].
Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, Bin J, Liao Y, Rao J, Liao W. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7:737–50. https://doi.org/10.1158/2326-6066.Cir-18-0436].
Choi JA, Kwon H, Cho H, Chung JY, Hewitt SM, Kim JH. ALDH1A2 is a candidate tumor suppressor gene in ovarian cancer. Cancers. 2019. https://doi.org/10.3390/cancers11101553.
Auersperg N. The stem-cell profile of ovarian surface epithelium is reproduced in the oviductal fimbriae, with increased stem-cell marker density in distal parts of the fimbriae. Int J Gynecol Pathol. 2013;32:444–53. https://doi.org/10.1097/PGP.0b013e3182800ad5].
Huang F, Xiao H, Sun BL, Yang RG. Characterization of TRIM62 as a RING finger E3 ubiquitin ligase and its subcellular localization. Biochem Biophy Res Commun. 2013;432:208–13. https://doi.org/10.1016/j.bbrc.2013.02.012.
Chen M, Huang X, Li L, Huang M, Cai R, Liao X. A regulatory axis of circ_0008193/miR-1180–3p/TRIM62 suppresses proliferation, migration, invasion, and warburg effect in lung adenocarcinoma cells under hypoxia. Med Sci Monit. 2020;26: e922900. https://doi.org/10.12659/msm.922900].
Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, Chouaib S. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11:824–46. https://doi.org/10.1002/1878-0261.12093].
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. https://doi.org/10.1038/nm1093].
Maj T, Wang W, Crespo J, Zhang H, Wang W, Wei S, Zhao L, Vatan L, Shao I, Szeliga W, Lyssiotis C, Liu JR, Kryczek I, Zou W. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat Immunol. 2017;18:1332–41. https://doi.org/10.1038/ni.3868].
Zhang P, Yang M, Zhang Y, Xiao S, Lai X, Tan A, Du S, Li S. Dissecting the single-cell transcriptome network underlying gastric premalignant lesions and early gastric cancer. Cell Rep. 2019;27:1934-1947.e1935. https://doi.org/10.1016/j.celrep.2019.04.052].
Meyer AR, Goldenring JR. Injury, repair, inflammation and metaplasia in the stomach. J Physiol. 2018;596:3861–7. https://doi.org/10.1113/jp275512].
Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Li Y, Ge S, Shen L. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS ONE. 2014;9: e84502. https://doi.org/10.1371/journal.pone.0084502].
Moosavi F, Giovannetti E, Saso L, Firuzi O. HGF/MET pathway aberrations as diagnostic, prognostic, and predictive biomarkers in human cancers. Crit Rev Clin Lab Sci. 2019;56:533–66. https://doi.org/10.1080/10408363.2019.1653821].
Wu YP, Ke ZB, Lin F, Wen YA, Chen S, Li XD, Chen SH, Sun XL, Huang JB, Zheng QS, Xue XY, Wei Y, Xu N. Identification of key genes and pathways in castrate-resistant prostate cancer by integrated bioinformatics analysis. Pathol Res Pract. 2020;216: 153109. https://doi.org/10.1016/j.prp.2020.153109].
Nie K, Shi L, Wen Y, Pan J, Li P, Zheng Z, Liu F. Identification of hub genes correlated with the pathogenesis and prognosis of gastric cancer via bioinformatics methods. Miner Med. 2020;111:213–25. https://doi.org/10.23736/s0026-4806.19.06166-4].
Pandey P, Siddiqui MH, Behari A, Kapoor VK, Mishra K, Sayyed U, Tiwari RK, Shekh R, Bajpai P. Jab1-siRNA induces cell growth inhibition and cell cycle arrest in gall bladder cancer cells via targeting Jab1 signalosome. Anti Cancer Agents Med Chem. 2019;19:2019–33. https://doi.org/10.2174/1871520619666190725122400].
Pandey P, Khan F. Jab1 inhibition by methanolic extract of Moringa Oleifera leaves in cervical cancer cells: a potent targeted therapeutic approach. Nutr Cancer. 2021;73:2411–9. https://doi.org/10.1080/01635581.2020.1826989].
Teng L, Lu J. cMET as a potential therapeutic target in gastric cancer (review). Int J Mol Med. 2013;32:1247–54. https://doi.org/10.3892/ijmm.2013.1531].
Acknowledgements
Not applicable.
Author information
Authors and Affiliations
Contributions
ZLH designed this study. YWH and CGJ performed the research, analyzed the data and wrote the paper. Yan JF, Wang XF and Zhu YP helped to search and collect the data. Weihua Yu and Guojun Chen contributed equally to this work. All the authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Showed the differentially expressed oxidative stress response-related genes. It obtained a total of 366 related genes, of which 141 genes were differentially expressed, including 22 up-regulated genes and 119 down-regulated genes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, W., Chen, G., Yan, J. et al. Single-cell sequencing analysis reveals gastric cancer microenvironment cells respond vastly different to oxidative stress. J Transl Med 20, 250 (2022). https://doi.org/10.1186/s12967-022-03411-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03411-w