Abstract
Background
Chronic fatigue syndrome (CFS) has been shown to be associated with infections. Tuberculosis (TB) is a highly prevalent infectious disease. Patients with chronic fatigue syndrome and post-tuberculosis experience similar symptoms. Furthermore, chronic fatigue syndrome and tuberculosis share similar plasma immunosignatures. This study aimed to clarify the risk of chronic fatigue syndrome following the diagnosis of Mycobacterium tuberculosis infection (MTI), by analyzing the National Health Insurance Research Database of Taiwan.
Methods
7666 patients aged 20 years or older with newly diagnosed Mycobacterium tuberculosis infection during 2000–2011 and 30,663 participants without Mycobacterium tuberculosis infection were identified. Both groups were followed up until the diagnoses of chronic fatigue syndrome were made at the end of 2011.
Results
The relationship between Mycobacterium tuberculosis infection and the subsequent risk of chronic fatigue syndrome was estimated through Cox proportional hazards regression analysis, with the incidence density rates being 3.04 and 3.69 per 1000 person‐years among the non‐Mycobacterium tuberculosis infection and Mycobacterium tuberculosis infection populations, respectively (adjusted hazard ratio [HR] = 1.23, with 95% confidence interval [CI] 1.03–1.47). In the stratified analysis, the Mycobacterium tuberculosis infection group were consistently associated with a higher risk of chronic fatigue syndrome in the male sex (HR = 1.27, 95% CI 1.02–1.58) and age group of ≥ 65 years old (HR = 2.50, 95% CI 1.86–3.38).
Conclusions
The data from this population‐based retrospective cohort study revealed that Mycobacterium tuberculosis infection is associated with an elevated risk of subsequent chronic fatigue syndrome.
Similar content being viewed by others
Background
Chronic fatigue syndrome (CFS) is conventionally defined as the presence of unexplainable fatigue lasting > 6 months and accompanied by at least four of the following symptoms: substantial impairment in short-term memory, tender lymph nodes, sore throat, muscle pain, multiple joint pain without swelling or redness, headache, unrefreshing sleep, and postexertional malaise lasting > 24 h [1]. CFS affects not only physical but mental status with profound disability. It was considered a psychiatric disorder due to a lack of a consistent physiological marker or physical finding [2, 3]. Psychiatric conditions such as anxiety, sleep disorders, and depression are strongly related to CFS [4, 5]. It can lead to impairment in simple and complex information processing speed and tasks requiring working memory [6], and imposes huge economic costs on society [7]. The etiologies of chronic fatigue syndrome involve multiple factors. Current studies revealed the etiologies are related to infection [8], immune system differences [9], endocrine-metabolic dysfunction [10], or some specific disease such as peptic ulcer disease [11]. Multiple infectious agents have been linked to CFS, such as the varicella zoster virus [9]. Identifying potential patients with CFS from among post-infectious patients is crucial for early diagnosis and prevention. With every third person on the Earth having Mycobacterium tuberculosis infection (MTI), tuberculosis (TB) is a highly prevalent infectious disease that continues to pose a serious challenge to public health. Patients with CFS and post-TB experience similar symptoms, such as fatigue, lassitude, and articular symptoms. TB arthritis commonly presents with chronic joint pain and insidious onset of only minimal signs of inflammation. Moreover, 72% of the patients with TB have moderate-to-severe anxiety and depression according to Hospital Anxiety and Depression Scale (HADS) [12], which considers some of the somatic symptoms. In addition, patients with CFS or with TB share similar plasma immunosignatures. Cytokine alterations are correlated with duration of illness, suggesting that CFS immunopathology is “not static” [13]. Abnormal cytokine profiles such as increased production of interferon (IFN) γ were observed in patients with CFS [14] and latent MTI [15]. Other immune activation markers of CFS include higher levels of the proinflammatory cytokines, tumour necrosis factor (TNF) α, interleukin (IL) 6, and IL-1β. Thus, the chronical activation and high dysregulation of the immune system may play an essential role in CFS development [16]. TB is chronic and remains latent in the human body for a lifetime. In this study, we investigated the association of TB and CFS by using retrospective cohort data from Taiwan National Health Insurance (NHI) Research Database (NHIRD).
Methods
Data source
We obtained our data from the Longitudinal Health Insurance Database 2000 (LHID2000) of NHIRD in this population-based retrospective cohort study. NHIRD contains all the reimbursement claims data from the NHI programme, including a beneficiary registry, medical records, a drug prescription registry, and other medical services. The programme is a nationwide single-payer insurance system established in March 1995 covering approximately 99% of Taiwanese residents [17]. LHID2000 contains registration and claims data of 1,000,000 insurants randomly sampled from the 2000 registry of NHIRD beneficiaries. The database renewed the claims data annually. The definition of disease in NHIRD is based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Before releasing LHID2000 for research, the original identification numbers of each insurant are removed through scrambling and linking individual claims data with random numbers.
This study was approved by the Research Ethics Committee of the China Medical University Hospital (CMUH-104-REC2-115) and the Institutional Review Board of Mackay Memorial Hospital (16MMHIS074).
Participants
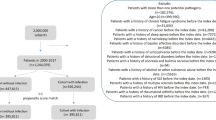
For the study cohort, we identified patients from LHID2000 aged ≥ 20 years and newly diagnosed as having a MTI (ICD-9-CM 010–018) between 2000 and 2011. The diagnosis date was defined as the index date. Patients aged below 20 years and patients with a history of CFS (ICD-9-CM 780.71) were excluded. The comparison cohort comprised individuals without MTI or CFS history. This cohort was randomly assigned the same index date as the MTI cohort. The comparison cohort was frequency-matched for age (strata of 5 years), sex, and index year at an approximately 4:1 ratio. All participants were followed from the index date until the date of CFS diagnosis, withdrawal from the programme, or the end of 2011, whichever was earliest.
Comorbidities
Baseline comorbidity history of diabetes (ICD-9-CM 250), obesity (ICD-9-CM 278.0), renal disease (ICD-9-CM 580–589), rheumatoid arthritis (RA; ICD-9-CM 714), human immunodeficiency virus infection (ICD-9-CM 042), malignancy (ICD-9-CM 140–149, 150–159, 160–165, 170–172, 174–175, 179–189, 190–199, 200–208, and 235–238), and inflammatory bowel disease (IBD; ICD-9-CM 555–556) were obtained.
Statistical analysis
The differences in demographic characteristics and comorbidities between the MTI and comparison cohorts were assessed using the chi-square test for categorical data and Student’s t test for continuous data. Cumulative incidence curves of CFS were computed using the Kaplan–Meier method, and between-cohort differences in cumulative incidence curves were assessed using the log-rank test. The incidence density of subsequent CFS for each cohort was calculated as the number of CFS events divided by the sum of follow-up duration (per 1000 person-years). Univariate and multivariate Cox proportional hazard regression models were used to examine the effect of MTI on CFS risk. The results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). The multivariate models were adjusted for age, sex, and comorbidities of diabetes, renal disease, and IBD. All analyses were generated using SAS (version 9.3; SAS Institute Inc., Cary, NC, USA), and a two-sided P value of < 0.05 was considered statistically significant.
Results
The MTI and comparison cohorts comprised 7666 and 30,663 individuals, respectively, with similar age and sex distribution (Table 1). In the MTI cohort, 47. 8% of the participants were aged ≥ 65 years and 67.9% were men. In the MTI and comparison cohorts, the mean age was 60.5 ± 18.3 and 60.0 ± 18.3 years, respectively. Compared with the comparison cohort, the MTI cohort had significantly higher percentages of comorbidities except for obesity (all P < 0.05). In the MTI and comparison cohorts, the mean follow-up duration was 5.24 and 6.08 years, respectively. Figure 1 demonstrates that the cumulative incidence of CFS was significantly higher in the MTI cohort than in the comparison cohort (P = 0.03). In the MTI and comparison cohorts, the mean CFS incidence was 3.04 and 3.69 per 1000 person-years, respectively (Table 2). The multivariable models were mutually adjusting for age, sex, and comorbidities of diabetes, renal disease, and IBD.
After mutually adjusted for age, sex, and comorbidities of diabetes, renal disease, and IBD, the risk of CFS had a 1.23-fold greater in the MTI cohort than in the comparison cohort (95% CI = 1.03–1.47). After mutually adjusting for CFS, sex, and comorbidities of diabetes, renal disease, and IBD, compared with patients aged 34 years and younger, the risk of CFS development is 2.50-fold higher in those aged 65 and more than 65 years (95% CI = 1.86–3.38), 1.90-fold higher in those aged 50–64 years and 1.44-fold higher in those aged 35–49 years (95% CI = 1.02–2.01). Patients with renal disease and IBD had 1.41 (95% CI = 1.13–1.76) and 1.83 (95% CI = 1.06–3.17) times higher CFS risk, respectively. Compared with the participants without MTI, participants aged ≤ 49 years in the MTI cohort had 1.5 (95% CI = 1.05–2.15) times higher CFS risk (Table 3, Fig. 2). Men had 1.27 (95% CI = 1.02–1.58) times higher CFS risk in the MTI cohort than in the comparison cohort. After > 1 year of follow-up, CFS risk remained 1.22 (95% CI = 1.01–1.49) times higher in the MTI cohort than in the comparison cohort. Table 4 presents the data on the effects of CFS-associated comorbidities on CFS risk. The data showed that compared with participants without either condition, participants with MTI and diabetes disease had 1.61 (95% CI = 1.12–2.31) times increased CFS risk.
Discussion
The current results indicated that had a significantly higher CFS incidence in the MTI cohort than in the comparison cohort. The subgroup analysis demonstrated that male patients and those aged ≤ 49 years in the MTI cohort had a relatively high HR for CFS. This finding has not been reported previously. In addition, patients with MTI who had CFS-associated comorbidities such as renal disease and diabetes had increased CFS risk.
Our results suggest that men with MTI are more likely to be diagnosed as having CFS. In developed countries, MTI incidence is higher in older individuals than in younger individuals, and it is higher in men than in women [18, 19]. These findings are consistent with those in the present study (Table 1). A meta-analysis reported that older patients with pulmonary TB have lower rates of fever and sweating and lower leukocyte concentrations [20]. The current results suggest that patients with MTI aged ≤ 49 years have an increased CFS risk (Table 3), possibly because of differences in immune response between older and younger individuals [21]. However, these relevant mechanisms and immunomodulating effects of aging require further investigation.
CFS is a multifactorial disease caused by pathogens, including the Epstein–Barr virus, human herpes virus 6, and human parvovirus B19 [22, 23]. Our current findings suggest that TB is correlated with CFS. Studies have proposed possible mechanisms of disease, including immunoinflammatory pathways [14, 16], neuroimmune dysfunction [16], oxidative and nitrosative stress (O&NS) pathways [24, 25], and bacterial translocation [26].
Immunoinflammatory pathway activation is one of the most researched topics related to CFS [14, 16]. Immune activation markers in CFS include increased levels of proinflammatory cytokines such as TNF-α, IL-6, and IL-1β [27, 28]. In patients with TB, the interaction of M. tuberculosis ligands with Toll-like receptors eventually results in immune activation, including activated nuclear factor (NF) κB and TNF-α, IL-1, and IL-12 production through myeloid differentiation primary response protein 88-dependent or -independent pathways [29, 30]. Increased production of NF-κB, a major upstream molecule regulating immunoinflammatory response, is associated with fatigue and a subjective feeling of infection [31].
TNF-α, which is secreted by macrophages, dendritic cells, and T cells, plays a major protective role against MTI and transmits signals regulating immune cell migration to the infection sites [32] and the formation of microbicidal granulomas [33]. IL-1 and TNF-α levels are significantly positively correlated with fatigue, autonomic symptoms, and flu-like symptoms [34]. TNF-α inhibitors, a type of immunomodulator, has also been reported to alleviate fatigue symptoms in some autoimmune diseases [35, 36] and attenuate CFS risk in patients with psoriasis [37].
IFN-γ, which is produced by activated T cells and natural killer cells activated by peripheral macrophages [38], appears in patients with latent MTI and plays a critical defensive role against MTI. IFN-γ synergizes with TNF-α and activates macrophages to kill intracellular bacilli [33]. The production of Th1 cytokines such as IFN-γ in patients with CFS is associated with the extent of fatigue [39]. A recent study observed that individuals with specific genetic polymorphisms of IFN-γ experience more severe fatigue as part of an acute postinfectious sickness [40]. IFN-γ-mediated lesions in kynurenine metabolism may culminate in depression and psychomotor retardation and contribute to disability in some patients with CFS [13].
Increased numbers of reactive oxygen and nitrogen species and activated O&NS pathways may be involved in CFS pathogenesis [24, 25]. This hypothesis is based on reports of increased production of inducible nitric oxide synthase (iNOS) [31] and reduced levels of antioxidants [16]. Macrophages activated by IFN-γ and TNF-α [41] produce nitric oxide and other reactive nitrogen species through iNOS to exert toxic effects on M. tuberculosis. iNOS activity inhibition leads to latent MTI reactivation in mice [41]. Low-grade inflammation, activated O&NS pathways, and impaired oxidative defenses in CFS potentially interact to increase the magnitude of abnormality in each, constituting a vicious cycle [14]. Lactate, an antioxidant that scavenges free radicals and attenuates lipid peroxidation [42], may explain the reason that in patients with CFS, fatigue was significantly reduced and functional capacity and fitness function were significantly improved after exercise treatment compared with after flexibility treatment [43].
Pathogens commonly associated with CFS are capable of establishing prolonged infection as a result of developing sophisticated adaptations to the host immune response [16]. For example, the varicella zoster virus [44] migrates along sensory axons to establish latency in neurons within the regional ganglia and only expresses a limited number of viral proteins [45] Similarly, M. tuberculosis has numerous defensive mechanisms to circumvent host immunity, such as disrupting the maturation of bacilli-containing phagosomes into phagolysosomes through the exclusion of vH+-ATPase during phagosomal maturation to prevent their destruction by lysosomal enzymes. Although only 10% of the patients with MTI develop TB, M. tuberculosis will remain in the nonreplicating state within granuloma in the other 90%. A disturbance of the immune system (e.g., old age, malnutrition, or medical conditions [46]), can trigger TB development. Despite reactivation of latent TB, inflammation can occur when M. tuberculosis spreads to a new location through aerosols generated by inspired air because foamy macrophages phagocytose extracellular nonreplicating M. tuberculosis, drain from lung granuloma toward the bronchial tree, and return to a different region of lung parenchyma through air inspiration [47]. These new infection sites may attract immune cells, which induce all the characteristic symptoms of CFS. These reactivation-resolution and migration cycles in TB lead to the mentioned inflammatory responses that may explain the chronic and relapsing–remitting nature of CFS.
A study found administration of the antituberculosis agent, isoniazid (300 mg per day for 30 days) to alleviate CFS symptoms, as demonstrated through improved Multidimensional Fatigue Inventory and the Zung Depression Scale scores [48]. However, the effect was not long-lasting; after 6 months, the TB was reactivated. Patients with latent TB should receive antibiotic therapy with a longer treatment course to prevent TB activation [49].
Our study has several limitations. As our previous study of LHID [50, 51], data on patient history, including symptoms, occupation status, contact history, and disease severity, are unavailable in NHIRD. Furthermore, the study population was mainly composed of East Asian people living in Taiwan, which limits the generalizability of the findings to other ethnicities. Although minor database errors in diagnostic coding can affect the data analysis results, such biases result in considerable penalties for physicians who have been more meticulous when recording codes. In addition, NHIRD enrolls 99.9% of Taiwan’s population, and its reliability and validity for epidemiological investigations have been reported previously [52, 53]. Therefore, the diagnostic coding used in the present study should be reliable.
In conclusion, this is a first paper to prove the novel findings about the association of MTI and CFS. They have common immunoinflammatory pathway and cytokines such as TNF-α, IL-1, IL-6, IFN-γ and NF-κB pathway. In addition, M. tuberculosis has numerous defensive mechanisms and are capable of intracellular persistence to circumvent host Immunity [54]. Although we didn’t explore the direct causality between MTI and CFS, we provide new perceptions for future studies to evaluate the actual mechanisms.
Conclusion
This study is the first population-based study to investigate the risk of CFS in patients with MTI, and its pilot finding is sufficient to provide perceptions for recognizing high-risk people likely to suffer from CFS. Future studies could examine the mechanisms underlying CFS risk following tuberculosis and discover the preventive and personalised medicine to improve the patient’s quality of life.
Availability of data and materials
The data underlying this study is from the National Health Insurance Research database (NHIRD). Interested researchers can obtain the data through formal application to the Ministry of Health and Welfare, Taiwan.
Abbreviations
- CFS:
-
Chronic fatigue syndrome
- TB:
-
Tuberculosis
- MTI:
-
Mycobacterium tuberculosis infection
- HADS:
-
Hospital Anxiety and Depression Scale
- IFN:
-
Interferon
- TNF:
-
Tumour necrosis factor
- IL:
-
Interleukin
- NHIRD:
-
National Health Insurance Research Database
- NF:
-
Nuclear factor
References
Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–9.
Stewart DE. Emotional disorders misdiagnosed as physical illness: Environmental hypersensitivity, candidiasis hypersensitivity, and chronic fatigue syndrome. Int J Ment Health. 1990;19:56–68.
Manu P, Lane TJ, Matthews DA. Chronic fatigue and chronic fatigue syndrome: clinical epidemiology and aetiological classification. In: Chronic Fatigue Syndrome, Ciba Foundation Symposium. 1993. p. 23–30.
Lievesley K, Rimes KA, Chalder T. A review of the predisposing, precipitating and perpetuating factors in Chronic Fatigue Syndrome in children and adolescents. Clin Psychol Rev. 2014;34:233–48.
Jacob L, Haro JM, Kostev K. Associations of physical and psychiatric conditions with chronic fatigue syndrome in Germany: an exploratory case-control study. Psychol Med. 2020;8:1–7.
Cockshell S, Mathias J. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40:1253–67.
McCrone P, Darbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med. 2003;33:253–61.
Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575.
Hornig M, Gottschalk G, Peterson D, Knox K, Schultz A, Eddy M, Che X, Lipkin W. Cytokine network analysis of cerebrospinal fluid in myalgic encephalomyelitis/chronic fatigue syndrome. Mol Psychiatry. 2016;21:261–9.
Naviaux RK, Naviaux JC, Li K, Bright AT, Alaynick WA, Wang L, Baxter A, Nathan N, Anderson W, Gordon E. Metabolic features of chronic fatigue syndrome. Proc Natl Acad Sci. 2016;113:E5472–80.
Kuo C-F, Shi L, Lin C-L, Yao W-C, Chen H-T, Lio C-F. How peptic ulcer disease could potentially lead to the lifelong, debilitating effects of chronic fatigue syndrome: an insight. Sci Rep. 2021;11:1–11.
Aamir S. Co-morbid anxiety and depression among pulmonary tuberculosis patients. J Coll Physicians Surg Pak. 2010;20:703–4.
Hornig M, Montoya JG, Klimas NG, Levine S, Felsenstein D, Bateman L, Peterson DL, Gottschalk CG, Schultz AF, Che X, et al. Distinct plasma immune signatures in ME/CFS are present early in the course of illness. Sci Adv. 2015;1:56.
Maes M, Twisk FN. Chronic fatigue syndrome: Harvey and Wessely’s (bio)psychosocial model versus a bio (psychosocial) model based on inflammatory and oxidative and nitrosative stress pathways. BMC Med. 2010;8:35.
Sauzullo I, Mengoni F, Mascia C, Rossi R, Lichtner M, Vullo V, Mastroianni CM. Treatment of latent tuberculosis infection induces changes in multifunctional Mycobacterium tuberculosis-specific CD4 T cells. Med Microbiol Immunol. 2015;9:89.
Morris G, Maes M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab Brain Dis. 2013;28:523–40.
Tsai SY, Lin CL, Shih SC, Hsu CW, Leong KH, Kuo CF, Lio CF, Chen YT, Hung YJ, Shi L. Increased risk of chronic fatigue syndrome following burn injuries. J Transl Med. 2018;16:342.
Hochberg NS, Horsburgh CR Jr. Prevention of tuberculosis in older adults in the United States: obstacles and opportunities. Clin Infect Dis. 2013;56:1240–7.
Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15.
Perez-Guzman C, Vargas MH, Torres-Cruz A, Villarreal-Velarde H. Does aging modify pulmonary tuberculosis?: A meta-analytical review. Chest. 1999;116:961–7.
Sadighi Akha AA. Aging and the immune system: An overview. J Immunol Methods. 2018;463:21–6.
Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, Staines D, Powles AC, Speight N, Vallings R, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270:327–38.
Sotzny F, Blanco J, Capelli E, Castro-Marrero J, Steiner S, Murovska M, Scheibenbogen C. European Network on MC: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome - Evidence for an autoimmune disease. Autoimmun Rev. 2018;17:601–9.
Maes M, Mihaylova I, De Ruyter M. Lower serum zinc in Chronic Fatigue Syndrome (CFS): relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. J Affect Disord. 2006;90:141–7.
Maes M, Mihaylova I, Leunis JC. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuro Endocrinol Lett. 2006;27:615–21.
Maes M, Twisk FN, Kubera M, Ringel K, Leunis JC, Geffard M. Increased IgA responses to the LPS of commensal bacteria is associated with inflammation and activation of cell-mediated immunity in chronic fatigue syndrome. J Affect Disord. 2012;136:909–17.
Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209–17.
Maes M, Twisk FN, Johnson C. Myalgic Encephalomyelitis (ME), Chronic Fatigue Syndrome (CFS), and Chronic Fatigue (CF) are distinguished accurately: results of supervised learning techniques applied on clinical and inflammatory data. Psychiatry Res. 2012;200:754–60.
Jo EK. Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis. 2008;21:279–86.
Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–44.
Maes M, Mihaylova I, Bosmans E. Not in the mind of neurasthenic lazybones but in the cell nucleus: patients with chronic fatigue syndrome have increased production of nuclear factor kappa beta. Neuro Endocrinol Lett. 2007;28:456–62.
Algood HM, Chan J, Flynn JL. Chemokines and tuberculosis. Cytokine Growth Factor Rev. 2003;14:467–77.
Chan J, Flynn J. The immunological aspects of latency in tuberculosis. Clin Immunol. 2004;110:2–12.
Maes M, Twisk FN, Kubera M, Ringel K. Evidence for inflammation and activation of cell-mediated immunity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): increased interleukin-1, tumor necrosis factor-alpha, PMN-elastase, lysozyme and neopterin. J Affect Disord. 2012;136:933–9.
Wu Q, Inman RD, Davis KD. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain. 2015;156:297–304.
Skoie IM, Dalen I, Omdal R. Effect of biological treatment on fatigue in psoriasis: a systematic review and meta-analysis. Am J Clin Dermatol. 2019;20:493–502.
Tsai SY, Chen HJ, Chen C, Lio CF, Kuo CF, Leong KH, Wang YT, Yang TY, You CH, Wang WS. Increased risk of chronic fatigue syndrome following psoriasis: a nationwide population-based cohort study. J Transl Med. 2019;17:154.
Darwich L, Coma G, Pena R, Bellido R, Blanco EJ, Este JA, Borras FE, Clotet B, Ruiz L, Rosell A, et al. Secretion of interferon-gamma by human macrophages demonstrated at the single-cell level after costimulation with interleukin (IL)-12 plus IL-18. Immunology. 2009;126:386–93.
Pokryszko-Dragan A, Frydecka I, Kosmaczewska A, Ciszak L, Bilinska M, Gruszka E, Podemski R, Frydecka D. Stimulated peripheral production of interferon-gamma is related to fatigue and depression in multiple sclerosis. Clin Neurol Neurosurg. 2012;114:1153–8.
Piraino B, Vollmer-Conna U, Lloyd AR. Genetic associations of fatigue and other symptom domains of the acute sickness response to infection. Brain Behav Immun. 2012;26:552–8.
Scanga CA, Mohan VP, Tanaka K, Alland D, Flynn JL, Chan J. The inducible nitric oxide synthase locus confers protection against aerogenic challenge of both clinical and laboratory strains of Mycobacterium tuberculosis in mice. Infect Immun. 2001;69:7711–7.
Groussard C, Morel I, Chevanne M, Monnier M, Cillard J, Delamarche A. Free radical scavenging and antioxidant effects of lactate ion: an in vitro study. J Appl Physiol. 1985;2000(89):169–75.
Fulcher KY, White PD. Randomised controlled trial of graded exercise in patients with the chronic fatigue syndrome. BMJ. 1997;314:1647–52.
Tsai SY, Yang TY, Chen HJ, Chen CS, Lin WM, Shen WC, Kuo CN, Kao CH. Increased risk of chronic fatigue syndrome following herpes zoster: a population-based study. Eur J Clin Microbiol Infect Dis. 2014;33:1653–9.
Debrus S, Sadzot-Delvaux C, Nikkels AF, Piette J, Rentier B. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J Virol. 1995;69:3240–5.
Ahmad S. Pathogenesis, immunology, and diagnosis of latent Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:814943.
Cardona PJ. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection. 2009;37:80–6.
Svirshchevskaya EV. Chronic immune response hypothesis for chronic fatigue syndrome: experimental results and literature overview. In: Immunosuppression - Role in Health and Diseases. 2012.
Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D, Den Boon S, Borroto Gutierrez SM, Bruchfeld J, Burhan E, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563–76.
Shi L, Lin C-L, Su C-H, Lin K-C, Leong K-H. Wang Y-TT, Kuo C-F, Tsai S-Y. The risk of developing osteoporosis in hemolytic anemia—what aggravates the bone loss? J Clin Med. 2021;10:3364.
Yao W-C, Chen H-J, Leong K-H, Chang K-L. Wang Y-TT, Wu L-C, Tung P-Y, Kuo C-F, Lin C-C, Tsai S-Y. The risk of fibromyalgia in patients with iron deficiency anemia: a nationwide population-based cohort study. Sci Rep. 2021;11:1–8.
Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, Lai EC. Taiwan’s National Health Insurance Research Database: past and future. Clin Epidemiol. 2019;11:349–58.
Tsai SY, Chen HJ, Lio CF, Kuo CF, Kao AC, Wang WS, Yao WC, Chen C, Yang TY. Increased risk of chronic fatigue syndrome in patients with inflammatory bowel disease: a population-based retrospective cohort study. J Transl Med. 2019;17:55.
Ercoli G, Fernandes VE, Chung WY, Wanford JJ, Thomson S, Bayliss CD, Straatman K, Crocker PR, Dennison A, Martinez-Pomares L, et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat Microbiol. 2018;3:600–10.
Acknowledgements
We would like to extend acknowledgment to Yu-Tien Chen's and Kam‐Hang Leong's, Cheng-Han Chou's material support, and the Department of Medical Research at Mackay Memorial Hospital, and Mackay Medical College, Taiwan for funding support.
Funding
This work was supported by the Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW109-TDU-B-212-114004), MOST Clinical Trial Consortium for Stroke (MOST 109-2321-B-039-002), China Medical University Hospital (DMR-109-231), Tseng-Lien Lin Foundation, Taichung, Taiwan, Mackay Medical College (1082A03), Department of Medical Research at Mackay Memorial Hospital (MMH-107-135; MMH-109-103) and Min-Sheng Hospital (2021003).
Author information
Authors and Affiliations
Contributions
S-YT and C-LL had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: S-YT. Acquisition, analysis, or interpretation of data: C-LL, C-FK, and S-YT. Drafting of the manuscript: All authors. Critical revision of the manuscript for important: S-YT. Intellectual content: S-YT; Statistical analysis: and C-LL. Obtained funding: S-YT, Y-WC and C-LL. Administrative, technical, or material supports: S-YT, and C-LL. Study supervision: S-YT. Submission: S-YT, W-PC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the Research Ethics Committee of the China Medical University Hospital (CMUH-104-REC2-115) and the Institutional Review Board of Mackay Memorial Hospital (16MMHIS074).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, TY., Lin, CL., Yao, WC. et al. How mycobacterium tuberculosis infection could lead to the increasing risks of chronic fatigue syndrome and the potential immunological effects: a population-based retrospective cohort study. J Transl Med 20, 99 (2022). https://doi.org/10.1186/s12967-022-03301-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03301-1