Abstract
Background
The study investigated the predictive value of tumor angiogenesis observed by 18F-ALF-NOTA-PRGD2 II (denoted as 18F-Alfatide II) positron emission tomography (PET)/computed tomography (CT) before concurrent chemoradiotherapy (CCRT) for treatment response and survival among patients with locally advanced non-small cell lung cancer (LA-NSCLC).
Methods
Patients with unresectable stage IIIA or IIIB NSCLC (AJCC Cancer Staging 7th Edition) who received CCRT were included in this prospective study. All patients had undergone 18F-Alfatide PET/CT scanning before CCRT, and analyzed parameters included maximum uptake values (SUVmax) of primary tumor (SUVP) and metastatic lymph nodes (SUVLN) and mean uptake value of blood pool (SUVblood). Tumor-to-background ratios (TBRs) and changes in tumor diameter before and after CCRT (ΔD) were calculated. The ratios of SUVP to SUVblood, SUVLN to SUVblood, and SUVP to SUVLN were denoted as TBRP, TBRLN, and T/LN. Short-term treatment response, progression-free survival (PFS), and overall survival (OS) were evaluated.
Results
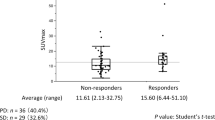
Of 38 enrolled patients, 28 completed CCRT. SUVP, SUVLN, TBRP, TBRLN and T/LN showed significant correlation with PFS (all P < 0.05). SUVP was negatively correlated with OS (P = 0.005). SUVP and TBRP were higher in non-responders than in responders (6.55 ± 2.74 vs. 4.61 ± 1.94, P = 0.039; 10.49 ± 7.58 vs. 7.73 ± 6.09, P = 0.023). ΔD was significantly greater in responders (2.78 ± 1.37) than in non-responders (-0.16 ± 1.33, P < 0.001). Exploratory receiver operating characteristic curve analysis identified TBRP (area under the curve [AUC] = 0.764, P = 0.018), with a cutoff value of 6.52, as the only parameter significantly predictive of the response to CCRT, with sensitivity, specificity, and accuracy values of 71.43%, 78.57%, and 75.00%, respectively. ROC curve analysis also identified SUVP (AUC = 0.942, P < 0.001, cutoff value 4.64) and TBRP (AUC = 0.895, P = 0.001, cutoff value 4.95) as predictive of OS with high sensitivity (84.21%, 93.75%), specificity (100.00%, 66.67%), and accuracy (89.29%, 82.14%).
Conclusions
Evaluation of tumor angiogenesis by 18F-Alfatide II at baseline may be useful in predicting the short-term response to CCRT as well as PFS and OS in patients with LA-NSCLC.
Similar content being viewed by others
Background
Locally advanced non-small cell lung cancer (LA-NSCLC) refers to stage III NSCLC, which is not suitable for treatment by surgical resection in the late stage of local foci [1]. These patients have regional lymph node invasion but no distant organ metastasis. Concurrent chemoradiotherapy (CCRT) is the standard treatment for LA-NSCLC [2, 3]. Vascular networks are derived through formation of new blood vessels via angiogenesis, which contributes to the vascular heterogeneity in and among tumors [4]. The abnormal structure of the tumor neovascularization bed is not conducive to drug delivery into solid tumors and also the main reason for the formation of the tumor hypoxic environment, which results in poor efficacy of chemotherapy as well as radiotherapy resistance [5]. Functional molecular imaging by positron emission tomography (PET) can be used to detect angiogenesis within a whole tumor.
PET/CT with a tracer based on the peptide arginine-glycine-aspartic acid peptide (Arg-Gly-Asp, commonly referred to as RGD) provides accurate imaging of integrin αvβ3 expression [6, 7] and thus is widely used to evaluate tumor angiogenesis and regarded as an ideal diagnostic tool for detecting tumors. An 18F-labeled PET imaging probe for RGD (18F-ALF-NOTA-PRGD2 II, denoted as 18F-Alfatide II) represents a potentially feasible imaging tool for assessing tumor angiogenesis [8,9,10]. Studies of 18F-Alfatide II have proven its safety and effectiveness for evaluating angiogenesis in NSCLC [11] with clarity and desirable imaging contrast, and phase III clinical trials are under way [12]. Small sample, prospective studies have suggested that RGD PET results have the ability to predict the short-term outcomes of antineoplastic therapy [13]. To date, a few studies have reported the use of RGD PET to predict short-term outcomes [14] and PFS [15] in cancer patients, but there are no related research reports on the ability to RGD PET results to predict long-term survival, especially in patients with locally advanced non-small cell lung cancer (LA-NSCLC).
In this study, we analyzed the standard uptake values (SUVs) of 18F-Alfatide II on PET/computed tomography (CT) at baseline and explored the predictive value of these and related parameters for short-term treatment outcome, progression-free survival (PFS) and overall survival (OS) in LA-NSCLC patients treated with CCRT.
Methods
Patients
Between June 10, 2015, and Dec 28, 2016, a total of 38 patients with unresectable stage III NSCLC were enrolled in the study. This prospective study was approved by the local ethics committee of Shandong Cancer Hospital and Institute, and each patient gave written and informed consent before inclusion in the study. All patients were treated in Shandong Cancer Hospital and met the following criteria: (1) NSCLC diagnosed by histological and imaging examination such as CT or 18F-fluorodeoxyglucose (FDG) PET/CT; (2) Eastern Cooperative Oncology Group (ECOG) score ≤ 1; (3) clearly measurable metastatic lymph nodes and primary tumors (RECIST); and (4) age > 18 years. All patients were ready to undergo 18F-Alfatide II PET/CT before chemotherapy or radiotherapy.
Radiotracer preparation
A simple lyophilized kit for labeling with PRGD2 peptide was purchased from the Jiangsu Institute of Nuclear Medicine, and the synthesis process was carried out as described in a previous study [16]. The radiochemical purity of the 18F-alfatide II exceeded 99%, and its specific radioactivity exceeded 37 GBq (1,000 mCi)/μmol.
PET/CT scanning
Patients were given an intravenous injection of 4.81 MBq/kg (0.12 mCi/kg) 18F-alfatide II and rested for about 60 min. Patients were not asked to fast or to confirm blood glucose levels. Scanning was performed with an integrated in-line PET/CT system (GEMINI TF Big Bore; Philips Healthcare). PET images were obtained from the head to the thigh, and the spiral CT component was performed with an X-ray tube voltage peak of 120 kV, 300 mAs. A full-ring dedicated PET scan of the same axial range followed. The patients continued normal shallow respiration during image acquisition. The images were attenuation-corrected with the transmission data from CT. The attenuation-corrected PET images, CT images, and fused PET/CT images, displayed as coronal, sagittal, and trans axial slices, were viewed on a MEDEX workstation (Beijing, China).
Image analysis
Two experienced nuclear medicine physicians assessed the 18F-alfatide II PET/CT images visually, referring to PET fusion and CT images, until consensuses were reached. Acquired 18F-alfatide II PET/CT data were transferred to the workstation in the DICOM format. The radiotracer concentration in the region of interest (ROI) was normalized to the injected dose per kilogram of each patient’s body weight to derive the standardized uptake values (SUVs). The SUVs were calculated according to the following formula: [measured activity concentration (Bq/ml) × body weight (g)]/injected activity (Bq).
PET/CT parameters were generated using a vendor-provided automated contouring program. Tumor tracer uptake was quantified according to the maximum standard uptake value (SUVmax) at 1 h after injection. For calculation of the SUVmax of the primary tumor (SUVP) and metastatic lymph nodes (SUVLN), circular regions of interest (ROIs) were drawn around the tumor lesions with a focally increased uptake in transaxial slices and automatically adapted to a 3-dimensional volume with a 30% isocontour. In addition, the maximal activity of 1 cm3 within the aortic arches was measured as mean SUV for aortic arches and denoted as SUVblood. The SUV ratio for primary tumor to aortic arch was equal to SUVP/ SUVblood; that for metastatic lymph node to aortic arch was equal to SUVLN/ SUVblood; and that for primary tumor to metastatic lymph node was equal to SUVP/ SUVLN. ΔD refers to the maximum diameter of the tumor before treatment minus the diameter of the tumor within 1 month after CCRT treatment.
Chemoradiotherapy
Patients were treated with two cycles of chemotherapy, followed by CCRT. Two additional cycles of chemotherapy were needed every 3 weeks after radiotherapy. An intensity-modulated radiotherapy technique (IMRT) or three-dimensional conformal RT (3D-CRT) was delivered to all patients with megavoltage equipment (6 MV). Radiotherapy was given as the conventionally fractionated regimen, 180–200 cGy for 5 days per week, and the total dose administered to patients ranged from 5040–6600 cGy (median dose, 6000 cGy). The chemotherapy administered was the cisplatin/docetaxel or cisplatin/pemetrexed regimen.
Response evaluation and survival assessments
Short-term outcome was assessed at 4 weeks after CCRT according to the revised RECIST criteria (v.1.1). The size change and curative effect of tumor lesions were determined by enhanced CT examination (scanning slice thickness 3 mm), and using at least one size that could be accurately measured, a tumor lesion diameter ≥ 10 mm and a metastatic lymph node short axis diameter ≥ 15 mm were taken as measurable lesions. The baseline data of the target area were recorded individually and compared with the measured data after treatment.
According to the RECIST criteria, responders included patients with an outcome of complete response (CR) or partial response (PR), and patients who had an outcome of stable disease (SD) or progressive disease (PD) were classified as the non-responders.
PFS and OS were assessed for all NSCLC patients according to the RECIST criteria. OS and PFS were estimated from initiation of chemotherapy to death (for OS) and to progression or death (for PFS). Patients were followed up by enhanced CT every 6 weeks during treatment, every 2 months in the first year after treatment, and every 6 months from the second year after treatment.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows version 20.0 (IBM, Armonk, NY, USA). Quantitative data for SUVP, SUVLN, T/LN, TBRP and TBRLN were expressed as mean ± standard deviation or median (range). Two-sample t test and Wilcoxon rank-sum tests were used to compare the PET/CT parameters between responders and non-responders. PET/CT parameters were tested by logistic regression analyses to identify the relationships between these variables and short-term outcomes or survival. Receiver-operating characteristic (ROC) curve analysis was used to determine the thresholds with the maximum Youden index as well as the predictive accuracy of 18F-Alfatide II PET/CT parameters for treatment response and survival. Both PFS and OS were assessed by Kaplan–Meier analysis. All tests were two-sided, and P < 0.05 was considered statistically significant.
Result
Patient characteristics
A total of 38 patients with pathologically proven stage III NSCLC were enrolled in the study, and of these, 28 completed CCRT and were included in the final analysis (Table 1). As of March 01, 2021, the median follow-up was 36.50 months (range, 9.00–72.50 months). The median PFS was 17 months (95% CI, 7.89 ~ 26.12 months), and the median OS was 36 months (95% CI, 11.24 ~ 60.76 months). The 1-, 3-, and 5-year survival rates were 89.29%, 53.57% and 21.43%, respectively.
Correlations between PET parameters and survival
The results of the correlation analyses for significant relationships between 18F-Alfatide II PET/CT semi-quantitative parameters and the PFS and OS of LA-NSCLC patients treated with CCRT are given in Table 2. SUVP, SUVLN, TBRP, TBRLN and T/LN were correlated significantly with PFS, and among them, SUVP, TBRLN, T/LN and ΔD were negatively correlated with PFS. Only SUVP was negatively correlated with OS (P = 0.005). Statistically significant correlations were not observed for the other parameters.
Predictive value of PET parameters for short-term treatment outcome
Several differences in SUV values were observed between responders and non-responders (Table 3; Fig. 1). SUVP and TBRP were significantly higher in non-responders than in responders (6.55 ± 2.74 vs. 4.61 ± 1.94, P = 0.039; 10.49 ± 7.58 vs. 7.73 ± 6.09, P = 0.023). ΔD was significantly greater in responders (2.78 ± 1.37) than in non-responders (-0.16 ± 1.33, P < 0.001). ROC curve analysis for the individual parameters, applying ‘responder’ as the dichotomous characteristic, revealed a significant area under the curve of 0.764 (P = 0.018) for TBRP (Fig. 2; Table 4). With a cutoff value of 6.52, derived from the Youden index, the sensitivity, specificity, and accuracy for TBRP were 71.43%, 78.57%, and 75.00%, respectively.
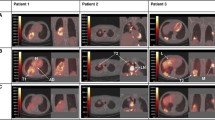
Two representatives 18F-Alfatide II PET/CT scans of responding tumors (a SUVmax = 5.18) and non-responding tumors (b SUVmax = 9.80). (Top panel) Scans from a patient with 18F-Alfatide uptake at baseline and 4 weeks after CCRT who showed a partial response to CCRT, and (bottom panel) scans from another patient with stable disease
Predictive value of PET parameters for survival
ROC curve analysis was performed to determine the predictive accuracy of the 18F-Alfatide II PET/CT parameters for survival of LA-NSCLC patients treated with CCRT. Highly significant correlations were observed between SUVP and TBRP and OS (P < 0.001 and P = 0.001; Fig. 3; Table 5). According to ROC curve analysis, the threshold values for SUVP, SUVLN, T/LN, TBRP, and TBRLN were 4.64, 3.43, 1.73, 4.92, and 3.68, respectively (Table 6). The sensitivity, specificity, and accuracy of SUVP for predicting OS were 84.21%, 100.00%, and 89.29%, respectively. The sensitivity, specificity, and accuracy of TBRP for predicting OS were 93.75%, 66.67%, and 82.14%, respectively.
Discussion
The results of this study indicate that for LA-NSCLC patients with CCRT, the baseline values of SUVP, SUVLN, TBRP, TBRLN and T/LN from pre-treatment 18F-Alfatide II PET/CT imaging correlated significantly with PFS, and SUVP was negatively correlated with OS. TBRP was an independent variable that may be useful for predicting short-term treatment outcome. From ROC curve analysis, SUVP and TBRP can predict OS with high sensitivity, specificity, and accuracy.
In clinical practice, physicians often observe differential treatment effects among different cancer patients with the same stage of disease, pathological type, and same treatment regimen [17]. The treatment of malignant tumors has entered the era of “precision medicine”. Indeed, the heterogeneity of the tumor affects the therapeutic effect and prognosis [18]. The tissues for pathological examination and gene detection are all obtained through local sampling and biopsy, and thus, it is difficult to evaluate the overall metabolism of the tumor. As new tools for the accurate diagnosis of tumors as well as prognosis prediction, new imaging methods have promoted the rapid development of individualized accurate treatment of tumors. PET is a non-invasive modality for evaluating specific molecular features and a potential tool for the prediction of treatment response. Various molecular imaging techniques have been developed for predicting tumor response to therapy, such as 18F-fluoro-D-glucose (FDG) PET [19], 18F-fluorothymidine (FLT) PET [20], 18F-fluroerythronitroimidazole (FETNIM) PET [21] and 18F-fluoromisonidazole (FMISO) PET [22].
In this study, 18F-Alfatide II PET was used to predict the short-term efficacy, PFS and OS of patients with LA-NSCLC, but not the effect of antiangiogenic therapy combined with radiotherapy and chemotherapy. Liu et al. reported that higher 18F-Alfatide II uptake on PET/CT might predict improved short-term responses and PFS after combined antiangiogenic (bevacizumab) treatments in advanced NSCLC patients [15]. Of course, at present, the standard treatment for locally advanced inoperable NSCLC is immunoconsolidation therapy after CCRT, but antiangiogenic therapy has not been recommended in the guidelines. A phase II clinical study (HELPER) explored the safety and efficacy of antiangiogenic therapy combined with CCRT for the treatment of unresectable III stage NSCLC. Although the safety was tolerable, this strategy did not prolong survival compared with radiotherapy combined with immunotherapy [23]. Ma et al. reported that continuous intravenous infusion of antiangiogenic drugs can significantly improve DFS and OS in patients with unresectable III stage NSCLC compared with intravenous administration of antiangiogenic drugs [24]. Therefore, the efficacy of antiangiogenic therapy combined with CCRT in patients with unresectable III stage NSCLC based on high RGD PET uptake is worthy of further study.
Tumor angiogenesis as evaluated by 18F-Alfatide II PET/CT before CCRT has been previously shown to predict the short-term treatment outcome in patients with advanced NSCLC [25], with the previous results showing that the maximum SUVs of tumor (SUVmax) and the SUVmax to SUVblood ratio (T/NTblood) were higher in non-responders (P = 0.0024, P = 0.003). The corresponding AUCs determined by ROC curve analysis were 0.815 (P = 0.079) and 0.889 (P = 0.005), respectively. These results are consistent with the results of the present study, in which we instead analyzed pre-treatment baseline data from 18F-Alfatide II PET/CT imaging and enrolled LA-NSCLC patients. Zhang et al. [26] also reported the predictive sensitivity of 18F-RGD imaging in glioblastoma patients treated with CCRT and found that both pre-treatment SUVs and intra-treatment SUVs were predictive parameters from 18F-RGD PET/CT images. They also found that glioblastoma patients with higher SUVs were less likely to respond to CCRT. Li Li et al. [27] reported that 18F-RGD uptake on PET/CT imaging pre-treatment can predict the response to antiangiogenic therapy in patients who receive apatinib therapy. They enrolled patients with lung cancer, esophageal cancer, breast cancer, cervical cancer, ovarian cancer, stomach cancer, and other cancer types who were scheduled for second- or third-line apatinib therapy and found that higher SUVs on PET/CT predicted a better response to apatinib. From these findings, we can hypothesize that the addition of anti-angiogenesis therapy in cases with a poor response to radiotherapy and chemotherapy may improve the therapeutic effect.
18F-FDG PET is the most widely used examination method in tumor diagnosis and staging at present, and its imaging principle is based on the characteristics of glucose metabolism within tumor cells. Its ability to predict the efficacy of NSCLC treatment has been established [28, 29]. This present study is based on the evaluation of tumor angiogenesis via 18F-Alfatide II PET, given that angiogenesis is closely related to the delivery of chemotherapeutic drugs and radiotherapy resistance [5]. Invasive gene detection can reveal the characteristics of gene mutation, the tumor mutation burden, programmed death ligand 1 expression, and microsatellite instability of lung cancer, which are related to the prognosis of the tumor [30]. A disadvantage is that it is difficult to obtain tumor tissue dynamically, and some lesions are too small or too close to important organs for biopsy tissue to be effectively and safely obtained. Another disadvantage is that the tissue obtained by point puncture does not represent the tumor as a whole. Through RGD PET functional imaging, dynamic, non-invasive, and three-dimensional data regarding angiogenesis within tumors can be obtained. The results of the present study demonstrate that high 18F-Alfatide II uptake is associated with LA-NSCLC non-response to CCRT, while previous studies have shown that high 18F-Alfatide II uptake is related to response to anti-angiogenic therapy for advanced NSCLC as well as PFS [15, 27]. Therefore, RGD PET may be an ideal examination method for screening the population most likely to respond to anti-angiogenic therapy.
This was a single-center study that has several limitations. First, the sample size was relatively small, and the credibility of the results needs to be further verified. Therefore, larger studies are needed, not only to verify the results but also to screen the population of LA-NSCLC patients most likely to benefit from anti-angiogenesis therapy. Additionally, the 18F-Alfatide II PET/CT characteristics that could predict short-term efficacy and survival were inconsistent. This may have led to inconsistent predictions, and studies with a larger sample size are needed. In this study, 18F-Alfatide II PET/CT imaging was performed only once in NSCLC patients before treatment, and not during or after treatment. Further research to determine whether changes in the SUVs on 18F-Alfatide II PET/CT correlate with prognosis is warranted. Nevertheless, these shortcomings diminish neither the potential of our findings nor the importance of dedicated prospective investigations to corroborate these findings.
Conclusions
The results of this prospectively study confirm that pre-treatment tumor angiogenesis as evaluated by 18F-Alfatide II PET/CT imaging may be useful in predicting the short-term outcome of CCRT as well as the PFS and OS in patients with advanced NSCLC. For LA-NSCLC patients with higher SUVs on baseline 18F-Alfatide II PET/CT images, the addition of anti-vascular therapy may improve the efficacy of CCRT and prolong survival.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 18F-Alfatide:
-
18F-ALF-NOTA-PRGD2
- PET/CT:
-
Positron emission tomography /computed tomography
- CCRT:
-
Concurrent chemoradiotherapy
- LA-NSCLC:
-
Locally advanced non-small cell lung cancer
- SUVmax :
-
Maximum uptake values
- SUVP :
-
Maximum uptake values of primary tumor
- SUVLN :
-
Maximum uptake values of metastatic lymph nodes
- SUVblood :
-
Mean uptake value of blood pool
- TBRs:
-
Tumor-to-background ratios
- TBRP :
-
Ratios of SUVP to SUVblood, TBRLN, ratios of SUVLN to SUVblood, T/LN: ratios of SUVP to SUVLN
- △D:
-
Changes in tumor diameter before and after treatment
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- CR:
-
Complete response
- PR:
-
Partial response
- SD:
-
Stable disease
- PD:
-
Progressive disease
References
Auperin A, Le PC, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28(13):2181–90.
Pignon JP, Stewart LA. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer: a meta-analysis. Cancer. 1996;77(11):2413–4.
Marino P, Preatoni A, Cantoni A. Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer. 1995;76(4):593–601.
Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–74.
Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62.
Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100.
Liu Z, Wang F, Chen X. Integrin alpha(v)beta(3)-targeted cancer therapy. Drug Dev Res. 2008;69(6):329–39.
Kumar CC. Integrin alpha v beta 3 as a therapeutic target for blocking tumor-induced angiogenesis. Curr Drug Targets. 2003;4(2):123–31.
Yu YP, Wang Q, Liu YC, Xie Y. Molecular basis for the targeted binding of RGD-containing peptide to integrin alphaVbeta3. Biomaterials. 2014;35(5):1667–75.
Iagaru A, Gambhir SS. Imaging tumor angiogenesis: the road to clinical utility. AJR Am J Roentgenol. 2013;201(2):W183–91.
Gao S, Wu H, Li W, Zhao S, Teng X, Lu H, et al. A pilot study imaging integrin alphavbeta3 with RGD PET/CT in suspected lung cancer patients. Eur J Nucl Med Mol Imaging. 2015;42(13):2029–37.
Wu C, Yue X, Lang L, Kiesewetter DO, Li F, Zhu Z, et al. Longitudinal PET imaging of muscular inflammation using 18F-DPA-714 and 18F-Alfatide II and differentiation with tumors. Theranostics. 2014;4(5):546–55.
Liu J, Yuan S, Wang L, Sun X, Hu X, et al. Diagnostic and predictive value of using RGD PET/CT in patients with cancer: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:8534761.
Tonnelet D, Bohn MP, Becker S, Decazes P, Camus V, et al. Angiogenesis imaging study using interim [(18)F] RGD-K5 PET/CT in patients with lymphoma undergoing chemotherapy: preliminary evidence. EJNMMI Res. 2021;11(1):37.
Liu J, Wu L, Liu Z, Seery S, Li J, Gao Z, et al. (18)F-RGD PET/CT and systemic inflammatory biomarkers predict outcomes of patients with advanced NSCLC receiving combined antiangiogenic treatment. Front Oncol. 2021;11:671912.
Wan W, Guo N, Pan D, Yu C, Weng Y, Luo S, et al. First experience of 18F-alfatide in lung cancer patients using a new lyophilized kit for rapid radiofluorination. J Nucl Med. 2013;54(5):691–8.
Nakajima N, Kataoka M, Sugawara Y, Ochi T, Kiyoto S, Ohsumi S, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve disease recurrence prediction in postmastectomy breast cancer patients with 1 to 3 positive axillary lymph nodes. Int J Radiat Oncol Biol Phys. 2013;87(4):738–46.
Wolf Y, Bartok O, Patkar S, Eli GB, Cohen S, Litchfield K, et al. UVB-induced tumor heterogeneity diminishes immune response in melanoma. Cell. 2019;179(1):219-35.e21.
Huang W, Zhou T, Ma L, Sun H, Gong H, Wang J, et al. Standard uptake value and metabolic tumor volume of (1)(8)F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38(9):1628–35.
Herrmann K, Buck AK, Schuster T, Junger A, Wieder HA, Graf N, et al. Predictive value of initial 18F-FLT uptake in patients with aggressive non-Hodgkin lymphoma receiving R-CHOP treatment. J Nucl Med. 2011;52(5):690–6.
Lehtio K, Eskola O, Viljanen T, Oikonen V, Gronroos T, Sillanmaki L, et al. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59(4):971–82.
Askoxylakis V, Dinkel J, Eichinger M, Stieltjes B, Sommer G, Strauss LG, et al. Multimodal hypoxia imaging and intensity modulated radiation therapy for unresectable non-small-cell lung cancer: the HIL trial. Radiat Oncol. 2012;7:157.
Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang J, et al. HELPER study: A phase II trial of continuous infusion of endostar combined with concurrent etoposide plus cisplatin and radiotherapy for treatment of unresectable stage III non-small-cell lung cancer. Radiother Oncol. 2019;131:27–34.
Honglian M, Zhouguang H, Fang P, Lujun Z, Dongming L, et al. Different administration routes of recombinant human endostatin combined with concurrent chemoradiotherapy might lead to different efficacy and safety profile in unresectable stage III non-small cell lung cancer: updated follow-up results from two phase II trials. Thorac Cancer. 2020;11(4):898–906.
Luan X, Huang Y, Gao S, Sun X, Wang S, Ma L, et al. (18)F-alfatide PET/CT may predict short-term outcome of concurrent chemoradiotherapy in patients with advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2016;43(13):2336–42.
Zhang H, Liu N, Gao S, Hu X, Zhao W, Tao R, et al. Can an (1)(8)F-ALF-NOTA-PRGD2 PET/CT scan predict treatment sensitivity to concurrent chemoradiotherapy in patients with newly diagnosed glioblastoma. J Nucl Med. 2016;57(4):524–9.
Li L, Ma L, Shang D, Liu Z, Yu Q, Wang S, et al. Pretreatment PET/CT imaging of angiogenesis based on (18)F-RGD tracer uptake may predict antiangiogenic response. Eur J Nucl Med Mol Imaging. 2019;46(4):940–7.
Greenspan BS. Role of PET/CT for precision medicine in lung cancer: perspective of the Society of Nuclear Medicine and Molecular Imaging. Transl Lung Cancer Res. 2017;6(6):617–20.
Sharma A, Mohan A, Bhalla AS, Sharma MC, Vishnubhatla S, Das CJ, et al. Role of various metabolic parameters derived from baseline 18F-FDG PET/CT as prognostic markers in non-small cell lung cancer patients undergoing platinum-based chemotherapy. Clin Nucl Med. 2018;43(1):e8-8e17.
Sutic M, Vukic A, Baranasic J, Forsti A, Dzubur F, Samarzija M, et al. Diagnostic, predictive, and prognostic biomarkers in non-small cell lung cancer (NSCLC) management. J Pers Med. 2021;11(11):1102.
Acknowledgements
The authors thank Laney Webber for English language editing services.
Funding
The study was supported by funds from the Major Scientific and Technological Innovation Projects of Shandong (2018YFJH0502), the Academic Promotion Program of Shandong First Medical University (2019ZL002) and the foundation of National Natural Science Foundation of China (81872475, 81372413, 81627901 and 82030082).
Author information
Authors and Affiliations
Contributions
SY and JY conceived of the study and participated in its designed. YW participated in the experiments and drafted the manuscript. QT, XL and YZ contributed to the sample collection and interpretation the data. XL performed the statistical analysis. JZ carried out the nuclear medicine. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This prospective study was approved by the local ethics committee of Shandong Cancer Hospital and Institute, and each patient gave written and informed consent before inclusion in the study.
Consent for publication
All the personal data involved in this article have been signed with informed consent.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, Y., Qin, X., Liu, X. et al. Tumor angiogenesis at baseline identified by 18F-Alfatide II PET/CT may predict survival among patients with locally advanced non-small cell lung cancer treated with concurrent chemoradiotherapy. J Transl Med 20, 63 (2022). https://doi.org/10.1186/s12967-022-03256-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-022-03256-3