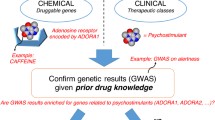
Abstract
Background
Glioblastoma is currently an incurable cancer. Genome-wide association studies have demonstrated that 41 genetic variants are associated with glioblastoma and may provide an option for drug development.
Methods
We investigated FDA-approved antipsychotics for their potential treatment of glioblastoma based on genome-wide association studies data using a ‘pathway/gene-set analysis’ approach.
Results
The in-silico screening led to the discovery of 12 candidate drugs. DepMap portal revealed that 42 glioma cell lines show higher sensitivities to 12 candidate drugs than to Temozolomide, the current standard treatment for glioblastoma.
Conclusion
In particular, cell lines showed significantly higher sensitivities to Norcyclobenzaprine and Protriptyline which were predicted to bind targets to disrupt a certain molecular function such as DNA repair, response to hormones, or DNA-templated transcription, and may lead to an effect on survival-related pathways including cell cycle arrest, response to ER stress, glucose transport, and regulation of autophagy. However, it is recommended that their mechanism of action and efficacy are further determined.
Similar content being viewed by others
Background
Glioblastoma multiforme (GBM) classified by the WHO as a grade IV glioma is the most malignant and lethal tumor to occur in the brain with rapid de novo progression and limited survival rate [1, 2]. The usual conventional therapies for GBM are surgical resection, radiation therapy, and chemotherapy. Concurrent radio- and chemotherapy will usually be enforced after surgery in case the cancer cells have invaded distal tissue or infiltrated the parenchyma. Currently, Temozolomide (TMZ) is the standard drug of choice used for the primary management against GBM. TMZ is a prodrug that is spontaneously hydrolyzed into an active form, 3-methyl-(triazen-1-yl) imidazole-4-carboxamide which alkylates DNA and triggers the death of tumor cells [3]. However, the median survival time (overall survival) of GBM patients is only extended a few months with TMZ treatment [4], and resistance to TMZ usually quickly develops through DNA repair mediated by methyl guanine methyl transferase, base excision repair or alkylpurine-DNA-N-glycosylase [3, 5]. Although an FDA-approved anti-angiogenic therapy (bevacizumab) with a combination of TMZ has been applied in GBM management, it did not result in significant improvement in overall survival but only prolonged progression-free survival [6, 7]. Therefore, a therapeutic approach for GBM still falls far short of medical needs.
Genome-wide association studies (GWASs) investigate the association between genetic variants and traits of interest, for example, the association between single nucleotide polymorphisms (SNPs) and diseases [8, 9]. In rare cases, SNPs located in the promoter or the well-known regulatory elements can be easily correlated to the regulation of genes. In most of cases (~ 88%), the SNPs of interest are located in intergenic or intronic regions, and are often associated with traits of interest with unknown reason [10]. Currently, four GWASs have reported that 41 risky SNPs are associated with GBM [11,12,13,14], only three of them are located in the untranslated regions or miRNA which is known to be implicated in oncogenesis [15,16,17]. Rs78378222 disrupts the polyadenylation of TP53 resulting in impaired mRNA processing and its reduced expression [15]. Rs10069690, providing an alternative splicing site and leading to a splice isoform of Telomerase reverse transcriptase [16], was found to have the most significant P value associated with GBM. Rs11558961 affects the secondary structure of Glial fibrillary acidic protein mRNA, which promotes the binding of miR-139, and thus decreases the susceptibility to chemotherapy [17]. The roles of other SNPs in GBM remain unclear to date.
Drug discovery and development are an expensive and highly time-consuming undertaking. It usually costs billions of dollars and takes decades to bring a compound to clinical application. Furthermore, newly developed drugs do not always confer a superior clinical benefit [18]. Scientists and pharmaceutical industries face growing demand for new drugs to fit unmet medical needs, and are under pressure to increase R&D productivity with limited resources [19]. Drug repositioning (or drug repurposing) is a process that resort to the approved drugs for new indications or answer of unmet medical needs. Clinically approved drugs have been proven to be safe, and the dosage range and formulation are already well studied. Taking advantage of abundant information about clinically approved drugs, drug development by drug repositioning may have a lower risk of failure, cost less and require less time to complete preclinical and phase I/II clinical trials [20, 21]. Furthermore, the efficacy for unidentified targets of approved drugs within other diseases remains worthwhile pursuit.
Over the past decades, GWASs have uncovered a lot of genetic variants which may provide targets for drug repositioning. Lau and So summarized several approaches for drug repositioning using GWAS data [22]. The ‘candidate gene approach’ is the most straightforward method that maps the top risky SNPs to their related genes by functional annotation tools or eQTL, and then queries these genes in the gene-drug database. This approach is a clear-cut strategy with low computational cost, but meets difficulties such as limited druggable genes [23], uncertain effect size of risky SNPs [24], and the complexity of proper annotation of risky SNPs. This approach may also miss multi-target drugs which are considered to be more effective than single target drugs [25]. The ‘pathway/gene-set analysis’ organizes multiple function or biologically-related genes and further studies their perturbational expression under interruption of drugs. Although the ‘pathway/gene-set analysis’ seems to be able to overcome the defects of the ‘candidate gene approach’, its challenge lies in the complexity of identifying the drug-mediated pathways or gene sets, because the mechanisms of many drugs are not fully understood. The computational tools Gene2drug [26] and Drug Set Enrichment Analysis (DSEA) [27] provide an opportunity to overcome the complexity of identifying drug-mediated pathways. Gene2drug analyses the genes perturbed by drug treatments and annotates those genes to biological/functional pathways; DSEA reversely annotates queried drugs to the pathways.
Previous review indicate that patients receiving drugs acting on the brain to sedate psychosis have a lower incidence of cancers than the general population [28]. Thus, antipsychotics have been considered as potential candidate agents against GBM [29,30,31]. This work aimed to discover whether there are current drugs that may be beneficial for GBM therapy using GWAS data via ‘pathway/gene-set analysis’. Gene2drug, Gene Ontology (GO) Resource [32] and the Kyoto Encyclopedia of Genes and Genomes (KEGG) [33] were used to assist in in-silico screening. The antitumor activities of the candidate drugs and TMZ were extracted from DepMap for which data were identified by PRISM viability assay on 42 glioma cell lines [34].
Materials and methods
Data source
The risky SNPs of GBM (Trait NO.: EFO_0000519) and the annotated risk genes were collected from the GWAS Catalog, EMBL-EBI [35]. Gene2drug (https://gene2drug.tigem.it/) is a state-of-the-art online tool and easily accessible technique applying the Gene Expression Profiles to Pathway Expression Profiles (gep2pep) algorithm [26] with Connectivity Map (CMap) database Molecular Signatures Database (MSigDB). The gene ontology of the annotated genes recoded by Gene Ontology Resource (GO) [32] or KEGG [33]were selected. The sensitivity of glioma cells to candidate drugs or TMZ were sorted from PRISM Repurposing Secondary Screen 19Q4 [34]. The gene mutation profiles (Mutation Public 21Q1) were extracted from DepMap portal (https://depmap.org/portal/). The gene expression profiles were downloaded from Cancer Cell Line Encyclopedia (CCLE_expression 21Q1) [36].
Gene to drug analysis
Candidate compounds were suggested by Gene2drug with the imported biological pathways in which the annotated genes were involved [26]. Briefly, the biological pathways of query genes were selected. A list of compounds will be generated by each pathway which suggests that the compounds in the list may interrupt the pathway based on data from MSigDB. Then, a merge process which selected compounds exist in all the lists and are ranked by P-value decides the output of Gene2drug.
Nine annotated genes were found to be involved in a certain biological process. The significant compounds which had a P value lower than 1E-2 were included. The compounds co-existing in more than four lists were selected (six lists at most with candidate drugs included, a median number, four, was set as cut-off), and then chosen within FDA approved drugs with known central nervous activities based on the treatment note of PRISM assay. The co-existence of compounds among the lists was analyzed with tool Venn Diagrams (Van der Peer Lab) and outputted in text format.
Prediction of pathways in which the candidate drugs are involved
The pathways which may be mediated by candidate drugs were predicted by using DSEA [27]. The downstream pathway shared by candidate drugs and the pathway based similarity were generated at the same time. The enrichment scores (E-score) from −1 to + 1 refer to down-regulation or up-regulation by drug treatments.
Validating the antitumor activity of candidate drugs
The sensitivity of candidate drugs and TMZ was extracted from DepMap for which data were identified by PRISM viability assay on more than 40 glioma cell lines. Briefly, cell lines with a barcode integrated in the genome were exposed to compounds for five days. Then, the mRNA was isolated from cells, amplified and detected. The viability of cells was calculated based on the level of barcode abundance and the sensitivity of cells to compounds was generated by comparing the treatment group and the control group [34]. The process sorted data from PRISM was performed by a python script (https://github.com/LinWZ-tw/g2d-prism).
Gene expression profile
The expression profiles of cell lines were further analyzed with data downloaded from the CCLE via DepMap portal. The cluster and heatmaps of genes were generated by using ClustVis [37].
Prediction of drug target
The targets of candidate drugs were predicted by a web-based platform, GalaxySagittarius, which combines ligand similarity-based and receptor structure-based approaches with AUC up to 0.8 [38]. Briefly, a protein target database is generated by similarity-based screening, then the target protein is ranked by protein–ligand docking. The predictions were run with a search model of similarity combined prediction and 3D structure compound obtained from ZINC: ZINC000002040609 (Norcyclobenzaprine) and ZINC000001530764 (Protriptyline). The 3D binding poses and binding structures were downloaded from the web server; the 2D binding structures were generated by using BIOVIA Discovery Studio 2020. The gene ontologies of interest were analyzed by GO Ontology Resource (http://geneontology.org/) [32].
Limitations
For in-silico screening, we collected GBM-associated SNPs and their mapping genes from the GWAS Catalog which applies Ensembl mapping pipeline for genome annotation. Although the risky SNPs are likely associated with their mapping gene, the possibility that risky SNPs have an effect on distal genes via chromatin looping structures may be omitted [10]. The in-silico screening conducted by Gene2drug was limited by the size of MSigDB while it is one of the largest and most popular databases so far [26, 39]. Validating work was relied on PRISM data which may contain divergent results in replicate tests.
Results
In-silico screening of potential drugs
Four studies from the GWAS Catalog were used [11,12,13,14], which include 41 risky SNPs and 15 annotated genes (Additional file 1: Table S1). Only 9 of the annotated genes were found to be involved in biological processes or pathways collected in GO or KEGG (Additional file 1: Table S2). Then, lists of candidate compounds from each annotated gene were generated from Gene2drug [26]. Further analysis of compounds showed that one compound co-existed in six lists, eight compounds co-existed in five lists and 20 compounds co-existed in four lists (Table S3), no compounds could be found in more than six lists, and compounds found in fewer than four lists were discarded. Given that the aim was drug repositioning for GBM therapy, compounds had been approved by FDA for safety, and compounds known to affect the central nervous system were selected. A total 12 FDA-approved drugs were suggested (Table 1, Additional file 1: Figure S1). The protocol design and overall flowchart is shown (Fig. 1).
Prediction of pathways in which the candidate drugs are involved
The downstream pathways shared by candidate drugs were predicted by DSEA (see Methods). A list of 327 downstream pathways shared by 12 candidate drugs were collected (Additional file 1: Table S4). The much commonly shared pathways of significance (lowest P value) were ranked by DSEA utility (Additional file 1: Figure S2A). Among them, several categories of pathway which may lead to cell death are summarized, including cell cycle arrest, response to ER stress, glucose transport, and regulation of autophagy (Table 2).
The sensitivities of glioma cells to candidate drugs
Among the candidate drugs output from screening protocol, Chlorprothixene was excluded because two datasets of Chlorprothixene were found in which glioma cells show disparate responses. The sensitivities of 42 glioma cells to the rest 12 candidate drugs were extracted from PRISM (Additional file 1: Table S5). The sensitivities to all the candidate drugs were higher than that to TMZ based on mean sensitivity among cell types (Fig. 2A, B).
Sensitivity of glioma cells to TMZ and candidate drugs. A The sensitivities of glioma cells to candidate drugs and TMZ ranking by mean sensitivity. (Ranking by mean sensitivity. × : mean value. CI: Confidence Interval) (B) Cells show significant higher sensitivities to two of candidate, Norcyclobenzaprine and Protriptyline (P = 5.89E−05 and 3.84E−05 respectively). C, D Heatmap of gene show significantly different expression in sensitive and non-sensitive cells to Norcyclobenzaprine and Protriptyline, respectively. The order of cell line follows the sensitivities of cells to Norcyclobenzaprine and Protriptyline from highest (left) to lowest (right)
In particular, Norcyclobenzaprine and Protriptyline were interesting in that their mean sensitivities were significantly higher than that to TMZ (P < 1 E−4) (Fig. 2A, B). The sensitivities to Protriptyline have no significant correlation with well-known oncological genes such as expression level of EGFR or mutations in TP53 and PTEN (Additional file 1: Figure S3A–C and G). The sensitivities to Norcyclobenzaprine were correlated with mutations in TP53 (0.017 by Mann–Whitney U test) but were not correlated with PTEN or expression level of EGFR (Additional file 1: Figure S3 D–F and G). It suggests that these two candidate drugs may have potential application on tumors with a wide spectrum of genetic backgrounds, while the mutation in TP53 should be avoid when Norcyclobenzaprine is applied.
To investigate the difference between sensitive and not sensitive cells, genes showing a significantly (P < 1E−3) different level of expression were selected. A group of genes with a consistent pattern in cells not sensitive to Norcyclobenzaprine was disclosed (Fig. 2C). Another group of genes showed a similar pattern in cells not sensitive to Protriptyline (Fig. 2D). Subjects with similar expression level in those groups of gene may not respond to Norcyclobenzaprine and Protriptyline.
Prediction of drug target
Several proteins were predicted by GalaxySagittarius to be targets of candidate drugs, the top ten from each prediction were selected (Additional file 1: Table S6). The top ten for Norcyclobenzaprine were: Poly(ADP-Ribose) Polymerase 1 (PARP1), Poly(ADP-Ribose) Polymerase 2 (PARP2), Orosomucoid 2 (ORM2), Retinol Binding Protein 1 (RBP1), SET domain containing lysine methyltransferase 7 (SETD7), Estrogen Related Receptor Gamma (ESRRG), Progesterone Receptor (PGR), Estrogen Receptor 1 (ESR1), Nuclear Receptor Coactivator 2 (NCOA2), Retinoic Acid Receptor Beta (RARB). The top ten for Protriptyline were: RBP1, PGR, NCOA2, PARP2, PARP1, Retinoid X Receptor Alpha (RXRA), RARB, Choline Kinase Alpha (CHKA), Retinoic Acid Receptor Gamma (RARG), ORM2. The binding poses of candidate drugs and their interaction with predicted proteins are shown (Additional file 1: Figure S4). The targets of both Norcyclobenzaprine and Protriptyline have similar gene ontology which are correlated with DNA-templated transcription, RNA synthesis, response to hormone, retinoic acid biosynthetic process, etc. (Table 3).
Direct screening protocol based on PRISM assay data
We built another screening protocol which directly ranks drugs according to PRISM assay data (Additional file 1: Figure S5A). In the field of neurology, there are 124 FDA-approved medications. Replicate tests were found in some of drugs result in a total of 140 treatments including TMZ as a control were employed. Glioma cells show higher mean sensitivities to 107 treatments (76.98%) than that to TMZ, but only 23 (16.55%) out of them show significantly higher sensitivities than TMZ (P < 1E−3). Among the candidate drugs with sensitivities higher than TMZ, the standard deviations were ranging from 0.255 to 0.805 (Additional file 1: Figure S5B). The top ten candidate drugs were Amitriptyline, Tranylcypromine, Mianserin, Triflupromazine, Doxylamine, Protriptyline, Metixene, Citalopram, Benserazide and Acepromazine (Additional file 1: Figure S5C-D). Moreover, nine of top ten candidate drugs were predicted to bind to PGR and eight were predicted to bind to Androgen Receptor (AR) (Additional file 1: Figure S5E). Both of PGR and AR have been reported to play important roles in glioma [40,41,42].
Discussion
A total 12 FDA-approved drugs were suggested as drugs of possible interest for GBM in our in-silico screening: Norcyclobenzaprine, Protriptyline, Iobenguane, Haloperidol, Alimemazine, Nortriptyline, Melatonin, Trifluoperazine, Perphenazine, Spiperone, Imipramine and Levomepromazine. Protriptyline, Nortriptyline and Imipramine are used for treatment of depression and classified as tricyclic antidepressants (TCAs). Norcyclobenzaprine is one of the major metabolites of Cyclobenzaprine which is usually used for muscle spasms, while both Norcyclobenzaprine and Cyclobenzaprine can act as TCAs to block serotonin receptors. A previous case–control study indicated that long-term use of TCAs may be associated with reduced risk of glioma [43]. TCAs are known to down-regulate β-adrenergic receptors which are involved in carcinogenesis and are considered as drug targets [44]. Melatonin is commonly used for sleep disorders. Haloperidol, Trifluoperazine, Perphenazine, Spiperone and Levomepromazine are clinically used for psychosis, especially schizophrenia or bipolar disorder. Alimemazine could be used as a sedative. Perphenazine and Levomepromazine are also used to control nausea and vomiting. Alimemazine, Trifluoperazine, Perphenazine and Levomepromazine are structurally similar compounds based on a phenothiazine. One of the candidate drugs, Iobenguane, is already an FDA-approved drug for low-grade glioma, indicating the credibility of our in-silico screening protocol.
Among these candidate drugs, Norcyclobenzaprine, Iobenguane, Haloperidol, Melatonin, Trifluoperazine, Perphenazine, Spiperone and Imipramine have been studied for their effects on brain tumors. For example, Norcyclobenzaprine has been reported to inhibit the proliferation of GBM cells but with IC50 values higher than 10 µM [45]. Iobenguane has been developed and approved by the FDA as a clinical agent for treating pheochromocytoma and paraganglioma [46]. The candidate drugs identified in our study have not only been studied for use on GBM but also many types of human malignancies [47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] and many clinical trials have been completed [76,77,78] or are ongoing. Especially, there are 30 completed trials and 15 ongoing trials with intervention with Melatonin. Even though the antitumor activities of the candidate drugs have been reported, most studies exclusively report the antitumor activity of antipsychotic on a few representative cell lines. PRISM provided an overview of the sensitivity among 42 glioma cell lines to queried drugs. The cell lines which are outliers in sensitivity could be further studied as a foundation for selection of participants in future clinical trials. For example, Norcyclobenzaprine did not show significant antitumor activity on certain cell lines [45]; however, the overview of sensitivity on 42 glioma cell lines indicates promising potential. Protriptyline, Alimemazine, Nortriptyline and Levomepromazine have been studied for their effects on many types of human malignancies, but have not yet been reported to have been tested on brain cancers (Table 1).
Most of candidate drugs are similar on correlation matrix according to GO biological process except iobenguane and melatonin (Additional file 1: Figure S2B). But the candidate drugs are not very similar on correlation matrix according to sensitivity (Additional file 1: Figure S2C). Iobenguane, alimemazine, perphenazine, spiperone and imipramine show significant difference when comparing the correlation matrix according to GO biological processes and that according to sensitivities on glioma cells (Additional file 1: Figure S2D).
We further predicted the targets of candidate drugs by GalaxySagittarius. The binding poses of candidate drugs and predicted proteins show that the tricyclic structure of Norcyclobenzaprine and Protriptyline contributes to π-interactions in the inner part of the binding pocket and the amine group at the ‘tail’ structure contributes to H-bonds with residues close to the mouth of the binding pocket (Additional file 1: Figure S4). Compounds with similar structures were found to have anti-tumor activity. For example, Triflupromazine which has a similar structure to trifluoromethyl-phenothiazine in the ‘head’ and a tertiary amine in the ‘tail’ shows potential against GBM (Additional file 1: Figure S5C).
While most of the pathways participated in by each risk gene did not overlap (Additional file 1: Table S2), the candidate compounds suggested by Gene2drug co-existed in multiple lists predicted from different genes (Additional file 1: Table S3). It is common that a compound may have multiple off-target mechanisms. We hypothesize that the candidate compounds which disrupt several pathways associated with GBM may have greater potential than those with a single pathway associated with GBM. We further merged the compounds suggested by each input of a gene through ranking the candidate compounds by the number of lists in which they co-existed. Then, we selected a median number as cut-off criteria, and the candidate drugs co-existing in at least four lists of pathways were employed in further analysis. Using TMZ as a control, the sensitivity of cells to all the candidate drugs selected by this protocol was higher than that to TMZ. On the other hand, the top ten candidate drugs which were directly ranked according to mean sensitivity have lower P-value than the 12 candidate drugs correlated with multiple disease-risk genes (Fig. 2B, Additional file 1: S5D). We assume that a drug which only interrupts a few key survival-related genes still may trigger cell death and could lead to apparent antineoplastic outcome. For example, Triflupromazine was predicted to disrupt pathways which only correlate with TP53, but Triflupromazine shows an antineoplastic activity significantly higher than TMZ (P = 2.63E−05, Additional file 1: Figure S5D). While screening protocol directly based on PRISM cancer cell sensitivity data without prior search of inherent biological pathway and risk genes resulted in a parallel consistent list of candidate drugs, though more, it provided less information for further drug development other than sensitivity. Our current development regimen not only aims to work on cancer disease but also for other diseases of which potential drug targets and pathways are also involved. Furthermore, for some indications for which cell- or animal-models are difficult to construct, the approach that selects candidates correlated with several disease-related genes may reduce the number of candidates to a small figure, reducing the resources required for bench work.
Based the target prediction of candidate drugs by GalaxySagittarius and disrupted pathway predicted by DSEA, we suspect that Norcyclobenzaprine and Protriptyline may bind to targets of the “shell”, such as PARP1, PARP2 or PGR, to interrupt a certain molecular function, for example, DNA-template transcription, response to hormone stimulation, etc. Then the influence of treatment is further extended to survival-related “core” pathways including cell cycle arrest, response to ER stress, glucose transport, and regulation of autophagy. However, the link between “shell” and “core” remains to be further investigated.
Conclusion
The current study presents a screening protocol following selection of candidates using Gene2drug. Selection from candidate compounds which correlate with multiple disease-risk genes or variants may reduce the number of candidates and decrease the burden of bench work to validate their therapeutic efficacy.
Our in-silico screening led to ten antipsychotics which show anti-tumor activity which is higher than TMZ in 42 cell lines. In particular, Norcyclobenzaprine and Protriptyline show significant potential against GBM; they are predicted to bind targets such as PARP1, PARP2, PRG, RBP1 to disrupt DNA repair pathways, respond to hormone and DNA-templated transcription and the retinoic acid signaling pathway, further effect survival-related pathways including cell cycle arrest, response to ER stress, glucose transport, and regulation of autophagy. Of these, the activity of Protriptyline against GBM has not yet been reported. The mechanism of action and therapeutic efficacy of Norcyclobenzaprine and Protriptyline are worth pursuing further.
Data availability
The datasets generated and/or analysed during the current study are available in the GWAS Catalog, https://www.ebi.ac.uk/gwas/; PRISM, https://www.theprismlab.org/; DepMap, https://depmap.org/portal/.
Abbreviations
- AR:
-
Androgen receptor
- CHKA:
-
Choline Kinase Alpha
- CMap:
-
Connectivity Map
- DSEA:
-
Drug Set Enrichment Analysis
- ESR1:
-
Estrogen Receptor 1
- ESRRG:
-
Estrogen Related Receptor Gamma
- gep2pep:
-
Gene Expression Profiles to Pathway Expression Profiles
- GO:
-
Gene Ontology
- GBM:
-
Glioblastoma multiforme
- GWASs:
-
GWASs: Genome-wide association studies
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MSigDB:
-
Molecular Signatures Database
- NCOA2:
-
Nuclear Receptor Coactivator 2
- ORM2:
-
Orosomucoid 2
- PARP1:
-
Poly(ADP-Ribose) Polymerase 1
- PARP2:
-
Poly(ADP-Ribose) Polymerase 2
- PGR:
-
Progesterone Receptor
- RARB:
-
Retinoic Acid Receptor Beta
- RARG:
-
Retinoic Acid Receptor Gamma
- RXRA:
-
Retinoid X Receptor Alpha
- RBP1:
-
Retinol Binding Protein 1
- SETD7:
-
SET domain containing lysine methyltransferase 7
- SNPs:
-
Single nucleotide polymorphisms
- TMZ:
-
Temozolomide
- TCAs:
-
Tricyclic antidepressants
References
Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11–27.
Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–72.
Ramirez YP, Weatherbee JL, Wheelhouse RT, Ross AH. Glioblastoma multiforme therapy and mechanisms of resistance. Pharmaceuticals (Basel, Switzerland). 2013;6:1475–506.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96.
Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3:198–210.
Ameratunga M, Pavlakis N, Wheeler H, Grant R, Simes J, Khasraw M. Anti-angiogenic therapy for high-grade glioma. Cochrane Database Syst Rev. 2018;11:008218.
Batchelor TT, Reardon DA, de Groot JF, Wick W, Weller M. Antiangiogenic therapy for glioblastoma: current status and future prospects. Clin Cancer Res. 2014;20:5612–9.
Pearson TA, Manolio TA. How to interpret a genome-wide association study. JAMA. 2008;299:1335–44.
Manolio TA. Genomewide association studies and assessment of the risk of disease. N Engl J Med. 2010;363:166–76.
Edwards SL, Beesley J, French JD, Dunning AM. Beyond GWASs: illuminating the dark road from association to function. Am J Hum Genet. 2013;93:779–97.
Xiao Y, Decker PA, Rice T, McCoy LS, Smirnov I, Patoka JS, Hansen HM, Wiemels JL, Tihan T, Prados MD, et al. SSBP2 variants are associated with survival in glioblastoma patients. Clin Cancer Res. 2012;18:3154–62.
Kinnersley B, Labussière M, Holroyd A, Di Stefano AL, Broderick P, Vijayakrishnan J, Mokhtari K, Delattre JY, Gousias K, Schramm J, et al. Genome-wide association study identifies multiple susceptibility loci for glioma. Nat Commun. 2015;6:8559.
Melin BS, Barnholtz-Sloan JS, Wrensch MR, Johansen C, Il’yasova D, Kinnersley B, Ostrom QT, Labreche K, Chen Y, Armstrong G, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49:789–94.
Ostrom QT, Kinnersley B, Armstrong G, Rice T, Chen Y, Wiencke JK, McCoy LS, Hansen HM, Amos CI, Bernstein JL, et al. Age-specific genome-wide association study in glioblastoma identifies increased proportion of ’lower grade glioma’-like features associated with younger age. Int J Cancer. 2018;143:2359–66.
Diskin SJ, Capasso M, Diamond M, Oldridge DA, Conkrite K, Bosse KR, Russell MR, Iolascon A, Hakonarson H, Devoto M, Maris JM. Rare variants in TP53 and susceptibility to neuroblastoma. J Natl Cancer Inst. 2014;106:dju047.
Killedar A, Stutz MD, Sobinoff AP, Tomlinson CG, Bryan TM, Beesley J, Chenevix-Trench G, Reddel RR, Pickett HA. A Common Cancer Risk-Associated Allele in the hTERT Locus Encodes a Dominant Negative Inhibitor of Telomerase. PLoS Genet. 2015;11:e1005286.
Wang J, Wang ML, Wang CH, Sun SY, Zhang HB, Jiang YY, Xu QW, Wang Y, Gu SX. A novel functional polymorphism of GFAP decrease glioblastoma susceptibility through inhibiting the binding of miR-139. Aging (Albany NY). 2018;10:988–99.
Goldstein DA, Stemmer SM, Gordon N. The cost and value of cancer drugs - are new innovations outpacing our ability to pay? Isr J Health Policy Res. 2016;5:40.
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL. How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov. 2010;9:203–14.
Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18:41–58.
Breckenridge A, Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat Rev Drug Discov. 2019;18:1–2.
Lau A, So HC. Turning genome-wide association study findings into opportunities for drug repositioning. Comput Struct Biotechnol J. 2020;18:1639–50.
Finan C, Gaulton A, Kruger FA, Lumbers RT, Shah T, Engmann J, Galver L, Kelley R, Karlsson A, Santos R, et al. The druggable genome and support for target identification and validation in drug development. Sci Transl Med. 2017;9:1.
White MJ, Yaspan BL, Veatch OJ, Goddard P, Risse-Adams OS, Contreras MG. Strategies for Pathway Analysis Using GWAS and WGS Data. Curr Protoc Hum Genet. 2019;100:e79.
Talevi A. Multi-target pharmacology: possibilities and limitations of the “skeleton key approach” from a medicinal chemist perspective. Front Pharmacol. 2015;6:205.
Napolitano F, Carrella D, Mandriani B, Pisonero-Vaquero S, Sirci F, Medina DL, Brunetti-Pierri N, di Bernardo D. gene2drug: a computational tool for pathway-based rational drug repositioning. Bioinformatics. 2018;34:1498–505.
Napolitano F, Sirci F, Carrella D, di Bernardo D. Drug-set enrichment analysis: a novel tool to investigate drug mode of action. Bioinformatics. 2016;32:235–41.
Fond G, Macgregor A, Attal J, Larue A, Brittner M, Ducasse D, Capdevielle D. Antipsychotic drugs: pro-cancer or anti-cancer? A systematic review. Med Hypotheses. 2012;79:38–42.
Lee JK, Nam DH, Lee J. Repurposing antipsychotics as glioblastoma therapeutics: potentials and challenges. Oncol Lett. 2016;11:1281–6.
Huang J, Zhao D, Liu Z, Liu F. Repurposing psychiatric drugs as anti-cancer agents. Cancer Lett. 2018;419:257–65.
Zhuo C, Xun Z, Hou W, Ji F, Lin X, Tian H, Zheng W, Chen M, Liu C, Wang W, Chen C. Surprising anticancer activities of psychiatric medications: old drugs offer new hope for patients with brain cancer. Front Pharmacol. 2019;10:1262.
Gene Ontology C. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49:D325–34.
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49:D545–51.
Corsello SM, Nagari RT, Spangler RD, Rossen J, Kocak M, Bryan JG, Humeidi R, Peck D, Wu X, Tang AA, et al. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer. 2020;1:235–48.
Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, McMahon A, Morales J, Mountjoy E, Sollis E, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47:D1005–12.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehar J, Kryukov GV, Sonkin D, et al. The Cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7.
Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566-570.
Yang J, Kwon S, Bae SH, Park KM, Yoon C, Lee JH, Seok C. GalaxySagittarius: structure- and similarity-based prediction of protein targets for druglike compounds. J Chem Inf Model. 2020;60:3246–54.
Lin X, Li X, Lin X. A review on applications of computational methods in drug screening and design. Molecules. 2020;25:1.
Tavares CB, Gomes-Braga F, Costa-Silva DR, Escorcio-Dourado CS, Borges US, Conde-Junior AM, Barros-Oliveira Mda C, Sousa EB, Barros Lda R, Martins LM, et al. Expression of estrogen and progesterone receptors in astrocytomas: a literature review. Clinics (Sao Paulo). 2016;71:481–6.
Altinoz MA, Ozpinar A, Elmaci I. Reproductive epidemiology of glial tumors may reveal novel treatments: high-dose progestins or progesterone antagonists as endocrino-immune modifiers against glioma. Neurosurg Rev. 2019;42:351–69.
Carrano A, Juarez JJ, Incontri D, Ibarra A, Guerrero Cazares H. Sex-specific differences in glioblastoma. Cells. 2021;10:1.
Pottegård A, García Rodríguez LA, Rasmussen L, Damkier P, Friis S, Gaist D. Use of tricyclic antidepressants and risk of glioma: a nationwide case-control study. Br J Cancer. 2016;114:1265–8.
Peixoto R, Pereira ML, Oliveira M. Beta-blockers and cancer: where are we? Pharmaceuticals (Basel). 2020;13:1.
Cheng HW, Liang YH, Kuo YL, Chuu CP, Lin CY, Lee MH, Wu AT, Yeh CT, Chen EI, Whang-Peng J, et al. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6:e1753.
Jimenez C. Treatment for patients with malignant pheochromocytomas and paragangliomas: a perspective from the hallmarks of cancer. Front Endocrinol (Lausanne). 2018;9:277.
Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A, Zmoos AF, Vaka D, Tran KQ, Zhou M, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013;3:1364–77.
Alburquerque-González B, Bernabé-García M, Montoro-García S, Bernabé-García Á, Rodrigues PC, Ruiz Sanz J, López-Calderón FF, Luque I, Nicolas FJ, Cayuela ML, et al. New role of the antidepressant imipramine as a Fascin1 inhibitor in colorectal cancer cells. Exp Mol Med. 2020;52:281–92.
Ford JM, Prozialeck WC, Hait WN. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol Pharmacol. 1989;35:105–15.
Kuzu OF, Gowda R, Noory MA, Robertson GP. Modulating cancer cell survival by targeting intracellular cholesterol transport. Br J Cancer. 2017;117:513–24.
Chen Y, Cui Y, Sun X, Wu H, Ou M, Tang Y, Ni S, Li X, Zhu J, Mao F, et al. Repurposing of antipsychotics perphenazine for the treatment of endometrial cancer. Bioorg Med Chem Lett. 2020;30:127239.
Gutierrez A, Pan L, Groen RW, Baleydier F, Kentsis A, Marineau J, Grebliunaite R, Kozakewich E, Reed C, Pflumio F, et al. Phenothiazines induce PP2A-mediated apoptosis in T cell acute lymphoblastic leukemia. J Clin Invest. 2014;124:644–55.
Gil-Ad I, Shtaif B, Levkovitz Y, Nordenberg J, Taler M, Korov I, Weizman A. Phenothiazines induce apoptosis in a B16 mouse melanoma cell line and attenuate in vivo melanoma tumor growth. Oncol Rep. 2006;15:107–12.
Otręba M, Pajor M, Warncke JD. Antimelanoma activity of perphenazine and prochlorperazine in human COLO829 and C32 cell lines. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1257–64.
Wei JW, Hickie RA, Klaassen DJ. Inhibition of human breast cancer colony formation by anticalmodulin agents: trifluoperazine, W-7, and W-13. Cancer Chemother Pharmacol. 1983;11:86–90.
Gulino A, Barrera G, Vacca A, Farina A, Ferretti C, Screpanti I, Dianzani MU, Frati L. Calmodulin antagonism and growth-inhibiting activity of triphenylethylene antiestrogens in MCF-7 human breast cancer cells. Cancer Res. 1986;46:6274–8.
Park SH, Chung YM, Ma J, Yang Q, Berek JS, Hu MC. Pharmacological activation of FOXO3 suppresses triple-negative breast cancer in vitro and in vivo. Oncotarget. 2016;7:42110–25.
Fancy RM, Kim H, Zhou T, Zinn KR, Buchsbaum DJ, Song Y. Calmodulin binding to death receptor 5-mediated death-inducing signaling complex in breast cancer cells. J Cell Biochem. 2017;118:2285–94.
Feng Z, Xia Y, Gao T, Xu F, Lei Q, Peng C, Yang Y, Xue Q, Hu X, Wang Q, et al. The antipsychotic agent trifluoperazine hydrochloride suppresses triple-negative breast cancer tumor growth and brain metastasis by inducing G0/G1 arrest and apoptosis. Cell Death Dis. 2018;9:1006.
Gao Y, Sun TY, Bai WF, Bai CG. Design, synthesis and evaluation of novel phenothiazine derivatives as inhibitors of breast cancer stem cells. Eur J Med Chem. 2019;183:111692.
Qian K, Sun L, Zhou G, Ge H, Meng Y, Li J, Li X, Fang X. Trifluoperazine as an alternative strategy for the inhibition of tumor growth of colorectal cancer. J Cell Biochem. 2019;120:15756–65.
Xia Y, Jia C, Xue Q, Jiang J, Xie Y, Wang R, Ran Z, Xu F, Zhang Y, Ye T. Antipsychotic drug trifluoperazine suppresses colorectal cancer by inducing G0/G1 arrest and apoptosis. Front Pharmacol. 2019;10:1029.
Polischouk AG, Holgersson A, Zong D, Stenerlöw B, Karlsson HL, Möller L, Viktorsson K, Lewensohn R. The antipsychotic drug trifluoperazine inhibits DNA repair and sensitizes non small cell lung carcinoma cells to DNA double-strand break induced cell death. Mol Cancer Ther. 2007;6:2303–9.
Chen QY, Wu LJ, Wu YQ, Lu GH, Jiang ZY, Zhan JW, Jie Y, Zhou JY. Molecular mechanism of trifluoperazine induces apoptosis in human A549 lung adenocarcinoma cell lines. Mol Med Rep. 2009;2:811–7.
Yeh CT, Wu AT, Chang PM, Chen KY, Yang CN, Yang SC, Ho CC, Chen CC, Kuo YL, Lee PY, et al. Trifluoperazine, an antipsychotic agent, inhibits cancer stem cell growth and overcomes drug resistance of lung cancer. Am J Respir Crit Care Med. 2012;186:1180–8.
Abdülrezzak Ü, Erdoğan Z, Silov G, Özdal A, Turhal Ö. Effect of trifluoperazine on Tc-99m sestamibi uptake in patients with advanced nonsmall cell lung cancer. Indian J Nucl Med. 2016;31:103–7.
Li Y, Yin Y, Ma J, Sun Y, Zhou R, Cui B, Zhang Y, Yang J, Yan X, Liu Z, Ma Z. Combination of AAV-mediated NUPR1 knockdown and trifluoperazine induces premature senescence in human lung adenocarcinoma A549 cells in nude mice. Oncol Rep. 2020;43:681–8.
Hu L, Zhang X, Wang J, Wang S, Amin HM, Shi P. Involvement of oncogenic tyrosine kinase NPM-ALK in trifluoperazine-induced cell cycle arrest and apoptosis in ALK(+) anaplastic large cell lymphoma. Hematology. 2018;23:284–90.
Zhu FX, He YC, Zhang JY, Wang HF, Zhong C, Wang XT. Using prognosis-related gene expression signature and connectivity map for personalized drug repositioning in multiple myeloma. Med Sci Monit. 2019;25:3247–55.
Perez RP, Handel LM, Hamilton TC. Potentiation of cisplatin cytotoxicity in human ovarian carcinoma cell lines by trifluoperazine, a calmodulin inhibitor. Gynecol Oncol. 1992;46:82–7.
Molins EAG, Jusko WJ. Assessment of three-drug combination pharmacodynamic interactions in pancreatic cancer cells. Aaps j. 2018;20:80.
Huang C, Lan W, Fraunhoffer N, Meilerman A, Iovanna J, Santofimia-Castaño P. Dissecting the Anticancer Mechanism of Trifluoperazine on Pancreatic Ductal Adenocarcinoma. Cancers (Basel). 2019;11:1.
Pajak B, Molnar J, Engi H, Orzechowski A. Preliminary studies on phenothiazine-mediated reversal of multidrug resistance in mouse lymphoma and COLO 320 cells. In Vivo. 2005;19:1101–4.
Wang Z, Liu Y, Musa AE: Regulation of cell death mechanisms by melatonin: implications to cancer therapy. Anticancer Agents Med Chem 2021.
Wan H, Xie R, Xu J, He J, Tang B, Liu Q, Wang S, Guo Y, Yang X, Dong TX, et al. Anti-proliferative Effects of Nucleotides on Gastric Cancer via a Novel P2Y6/SOCE/Ca(2+)/beta-catenin Pathway. Sci Rep. 2017;7:2459.
Miller RL, Bukowski RM, Budd GT, Purvis J, Weick JK, Shepard K, Midha KK, Ganapathi R. Clinical modulation of doxorubicin resistance by the calmodulin-inhibitor, trifluoperazine: a phase I/II trial. J Clin Oncol. 1988;6:880–8.
Budd GT, Bukowski RM, Lichtin A, Bauer L, Van Kirk P, Ganapathi R. Phase II trial of doxorubicin and trifluoperazine in metastatic breast cancer. Invest New Drugs. 1993;11:75–9.
Murren JR, Durivage HJ, Buzaid AC, Reiss M, Flynn SD, Carter D, Hait WN. Trifluoperazine as a modulator of multidrug resistance in refractory breast cancer. Cancer Chemother Pharmacol. 1996;38:65–70.
Acknowledgements
We gratefully thank Ms. Miranda Loney, US Board of Editor in the Life Sciences (BELS) certified editor, for English editing.
Funding
This work is partially supported by Grants (NO.: MAB-108-085 and MAB-109-033) from the Medical Affairs Bureau, Ministry of National Defense, R.O.C. (Taiwan) and grant (No.: TSGH-C108-114) from Tri-sever General Hospital, R.O.C (Taiwan). W-Z L, was supported by a two-year studentship from the PhD program at the National Defense Medical Center, and also sponsored by Fidelity Regulation Therapeutics Inc. through a one year stipend.
Author information
Authors and Affiliations
Contributions
Conceptualization, W-Z L, Y–T C, C-M C, C-Y S.; data collection: W-Z L, Y-C L, Y–T C; data analysis: W-Z L, Y-C L; validation: Y–T C, M-C L; data interpretation: W-Z L, C-T T, Y–T C; writing—original draft preparation, W-Z L; writing—review and editing, C-T T, G-J W, Y–T C, C-M C, C-Y S; supervision, G-J W, C-M C, C-Y S. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Additional figures and tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lin, WZ., Liu, YC., Lee, MC. et al. From GWAS to drug screening: repurposing antipsychotics for glioblastoma. J Transl Med 20, 70 (2022). https://doi.org/10.1186/s12967-021-03209-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-021-03209-2