Abstract
Background
Pancreatic cancer is one of the most serious digestive malignancies. At present, there is an extreme lack of effective strategies in clinical treatment. The purpose of this study is to identify key genes and pathways in the development of pancreatic cancer and provide targets for the treatment of pancreatic cancer.
Methods
GSE15471 and GSE62165 were used to screen differentially expressed genes by GEO2R tool. Hub genes prognostic potential assessed using the GEPIA and Kaplan–Meier plotter databases. The drug susceptibility data of pan-cancer cell lines is provided by The Genomics of Drug Sensitivity in Cancer Project (GDSC). Finally, the effects of PI3K–Akt signaling pathway inhibitors on cell viability of pancreatic cancer cells were detected by cell proliferation and invasion assays.
Results
A total of 609 differentially expressed genes were screened and enriched in the focal adhesion, phagosome and PI3K–Akt signaling pathway. Of the 15 hub genes we found, four were primarily associated with the PI3K–Akt signaling pathway, including COL3A1, EGF, FN1 and ITGA2. GDSC analysis showed that mTOR inhibitors are very sensitive to pancreatic cancer cells with mutations in EWSR1.FLI1 and RNF43. Cell proliferation and invasion results showed that mTOR inhibitors (GSK2126458) can inhibit the proliferation of pancreatic cancer cells.
Conclusions
This study suggested that the PI3K–Akt signaling pathway may be a key pathway for pancreatic cancer, our study uncovered the potential therapeutic potential of GSK2126458, a specific mTOR inhibitor, for pancreatic cancer.
Similar content being viewed by others
Background
Pancreatic cancer is one of the most devastating and frequent malignancies worldwide, and its incidence rate is still nearly equal to its death rate. It’s the fourth leading cause of cancer-related death globally and is associated with the lowest 5-year survival rate of less than 5% known for human cancers [1,2,3,4]. Although great progress in the treatment of pancreatic cancer, it is still one of the leading causes of death. The main cause of high mortality in pancreatic cancer is that pancreatic cancer has the biological features that other solid tumors do not have: abnormal tumor metabolism, the high degree of cell malignancy, lack of blood supply to tumor cells and complex tumor microenvironment. Most tumor growth processes need to generate a large number of blood vessels to provide nutrition [5, 6]. However, pancreatic cancer is not sensitive to nutrient supply and pancreatic cancer can still be growing rapidly in a nutrient-deficient and sparsely-blood-driven environment [7, 8]. So far, although more and more evidence suggested that multiple genes and cellular pathways are involved in the development and progression of pancreatic cancer, the lack of knowledge about the exact molecular mechanisms underlying pancreatic cancer progression has limited the ability to treat the diseases. Therefore, identifying the hub genes (genes that play a vital role in biological processes) and key pathways of this disease is important for further studying the pathogenesis of pancreatic cancer and develop more effective treatment methods.
Gene chip, a gene detection technology, can quickly detect all the gene expression information at the same time point [9]. Nowadays, high throughput sequencing is becoming more widely used and has been used as a very effective tool in life science, such as early diagnosis of cancer, classification of tumor and prognosis prediction [10]. Therefore, a large number of gene profile data have been produced with the widespread use of gene chips, and most of the data has been stored in public databases. Integrating and re-analyzing these data can provide valuable clues for new research, which is especially suitable for the screening of differentially expressed genes (DEGs). Hence, the integrated bioinformatics methods were used to explore the pathogenesis of pancreatic cancer. Bioinformatics analysis studies have identified several potential biomarkers [11,12,13,14]. However, these results do not adequately explain how pancreatic cancer cells survive hypoxic conditions, which are often associated with inadequate nutrient supply.
In this work, we identified DEGs in pancreatic cancer using bioinformatics methods. The original data GSE15471 and GSE62165 were chosen from Gene Expression Omnibus (GEO), and DEGs were filtered by the GEO2R online tool. GSE15471 and GSE62165 have reasonable experimental design and reliable data quality, which can provide rich information for data mining [11, 13, 15,16,17]. Followed by, Gene ontology (GO) and pathway enrichment analysis were performed with DAVID [18, 19]. Moreover, we constructed protein–protein interaction (PPI) network of the DEGs and modular analysis to pick out hub genes in pancreatic cancer. Then we used GEPIA to validate the expression of hub genes between cancer patients and healthy people. Finally, the effects of PI3K–Akt signaling pathway inhibitors (mTOR inhibitor, GSK2126458) on cell viability of pancreatic cancer cells were detected by MTT, colony and invasion assays. The analysis of DEGs’ biological functions and pathways will provide better insight into molecular mechanism and potential candidate therapeutic targets for pancreatic cancer.
Materials and methods
Cell lines and reagents
The human pancreatic cancer cell lines (PANC-1) were purchased from the Chinese Academy of Sciences Cell Bank and cultured in DMEM medium with 10%FBS, 100 U/mL of penicillin, 100 μg/mL of streptomycin. The cells were cultured in a 5% CO2 incubator at 37 ℃. GSK2126458 was purchased from ApexBio (USA).
MTT assay
Logarithmic growth phase cells were seeded in 96-well plates. After 24 h, the cells cultured in different concentrations of GSK2126458 (1.0 μmol/L, 0.5 μmol/L, 0.25 μmol/L) for 24 h, 48 h or 72 h. MTT (final concentration: 5 mg/mL) were added to each well and then incubated for 4 h in the incubator. We discarded the culture solution and added 150 μL of DMSO (dimethyl sulfoxide) to each well, and measured the absorbance at 490 nm after shaking.
Microarray data information
The gene expression profiles of GSE15471 and GSE62165 were obtained from the GEO database (https://www.ncbi.nlm.nih.gov/geo). The microarray data of GSE15471 was based on GPL570 Platforms ([HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array) and included 39 pancreatic cancer tissues and 39 normal pancreatic tissues (Submission date: Mar 31, 2009) [20]. GSE15471 contain “pairs” of normal pancreatic tissues and pancreatic cancer tissues samples. The microarray data of GSE62165 was based on GPL13667 Platforms ([HG-U219] Affymetrix Human Genome U219 Array) and included 118 pancreatic cancer tissues and 13 normal pancreatic tissues (Submission date: Oct 08, 2014) [21]. The two gene expression profiles obtained from pancreatic ductal adenocarcinoma (PDAC). GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) will give a box plot to determine if selected samples are suitable for comparison. Viewing the distribution is important for determining if the selected samples are suitable for comparison; Generally, median-centered values are indicative that the data are normalized and cross-comparable.
DEGs identification
GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) is an interactive web tool for detecting DEGs [22]. GEO2R allows users to compare two or more groups of samples to analyze most of the GEO series with gene symbol. We used GEO2R to identify genes that are differentially expressed between pancreatic cancer samples and normal samples. GEO2R is data processed by ebayes algorithm in limma package in R language [22]. The adjust P-value < 0.05 and |logFC|> 1 were set as cut-off criteria. We used log2-fold change between two groups. Then the co-expression up-regulated and down-regulated genes of the two expression profiles were identified in the Venn diagram (http://bioinformatics.psb.ugent.be/webtools/Venn/). The heat map was plotted for samples and top 50 DEGs in Heatmao IIIustrator software (Heml 1.0.3.7) [23].
GO function and KEGG pathway enrichment analysis of DEGs
GO analysis is a useful method for annotating genes and gene products and for identifying the characteristic biological properties of high throughput genome or transcriptional data [24, 25]. KEGG is a database including biological pathway, diseases, drugs, and chemicals [26]. The Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/), a functional annotation tool, is a website which can provide GO analysis and pathway analysis. These analyses were performed using DAVID online tool to analyze the DEGs at the functional level. P-value < 0.05 was set as the cut-off criterion.
The construction of PPI network, module analysis and significant candidate genes and pathway identification
In order to study the protein–protein interaction (PPI) information, Search Tool for the Retrieval of Interacting Genes (STRING, version 10.5, https://string-db.org/) database was used and combined score > 0.4 was selected as cut-off criteria [27]. Subsequently, the PPI networks were built and visualized in Cytoscape software (version 3.6.0) [28]. The Molecular Complex Detection (MCODE) was used to analyze the modules of the PPI network with the default parameters settings such as degree cutoff = 2, node score cutoff = 0.2, k-core = 2, max. depth = 100. The cut-off criteria were set as follows: MCODE score > 4 and number of nodes > 4. Moreover, the function and pathway enrichment analysis of genes in the modules were performed by STRING.
Comparison of the hub genes expression level
The GEPIA (http://gepia.cancer-pku.cn/index.html) is a newly developed online server for interaction analysis. The standard processing pipeline is used to analyze the RNA sequence expression data of 9736 tumor samples and the 8587 normal samples from the TCGA (The Cancer Genome Atlas) and the GTEx (Genotype-Tissue Expression) projects [29]. TCGA database can compare the differential expression analysis between normal tissue and tumor tissue. However, due to the fact that its normal samples are also from cancer patients, the clustering of samples may be confused. The normal sample data of GTEx are from health people. Considering that there are too few normal samples in TCGA, the data of above two databases are merged on GEPIA website for analysis. It can provide custom functions for tumor and normal differential expression analysis. We used the boxplot to display the expression of hub genes in pancreatic cancer tissues and normal tissues. This study took the P-value less than 0.01 as the cutoff point. Furthermore, the human protein Atlas (HPA) shows the protein level of 4 hub genes in the PI3K–Akt pathway in pancreatic cancer and normal tissues [30, 31].
Survival curve analysis
Kaplan–Meier plotter was used to analyze the RNA-seq data in TCGA, EGA and geo databases (http://kmplot.com/analysis/). It can evaluate the effect of more than 54,000 biomolecules on the survival rate of various tumor tissues. Here, the Kaplan Meier plotter was used to analyze the association between key genes (COL3A1, FN1 and ITGA2) expression and survival in patients with pancreatic cancer. We searched the database with COL3A1, FN1 and ITGA2 as the input and analyzed all the samples in the database. The parameters are as previously reported [32]: cut-off value of grouping: median; hazard ratio: Yes; 95% confidence interval: Yes.
Chemotherapeutic response
We analyzed the largest open pharmacogenomics database to predict the chemotherapeutic response of PI3K–Akt–mTOR specific inhibitors (PI3K: BKM-120, Akt: MK-1102, and mTOR inhibitor: GSK2126458) to each sample [cancer drug sensitivity genomics (GDSG)], https://www.cancerrxgene.org/]. The analysis method is based on the previous reports [32].
Cell culture
PNAC-1 cells and BxPC-3 cells (purchased from Procell, China) in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin, at 37 °C, 5% Incubate in CO2 gas phase.
Cell viability
PNAC-1 cells and BxPC-3 cells were seeded in a 96-well plate, cultured overnight in an incubator, and treated with or without GSK2126458 (0–10 μM) for 24 h, 48 h, and 72 h. Then, the medium was added to the 5 mg/mL (final concentration) MTT solution and incubated for 4 h. The formed formazan crystals were dissolved in 150 μL DMSO per well and measured at 490 nm by a microplate reader (Elx800 Bio-Tek, USA). Repeat the experiment at least 3 times.
Colony formation
The cells were seeded on 6-well plates, cultured overnight, and treated with different concentrations of DMSO or GSK2126458 for 48 h. Then washed with PBS and cultured in complete growth medium for another 10 days. The fresh medium was changed every 3 days. The cells were fixed with 100% methanol and stained with 0.1% crystal violet.
Invasion assay
The experimental operation was carried out according to the method previously reported [33]. PANC-1 cells in logarithmic growth phase were seeded into the upper layer of 8 μm transwell chamber. The diluted matrix glue is added to the upper layer of the chamber in advance for solidification. After 48 h of culture with different concentrations of GSK2126458 (0–1 μmol/L), PANC1 cells invading the subventricular layer were stained with 0.1% crystal violet. The invasive cells were observed and photographed under an inverted microscope (OLYMPUS, IX71).
FACS assay
The experimental methods refer to the previous literature [34]. In general, PANC-1 cells were treated with different concentrations of GSK2126458 for 48 h, and the cells were digested with trypsin to obtain cell suspension. The cells were centrifuged at 4 °C and 3000 rpm for 10 min, then completely resuspended and washed. The cells were then centrifuged at 4 °C and 2000 rpm for 10 min. We discarded the supernatant, added binding buffer, annexin V-FITC and PI working solution (BD pharmingen, USA) to each sample, mixed them evenly, and then filtered them with a 400 mesh filter to obtain single cell suspension. The number of apoptotic cells was detected by flow cytometry (BD, C6).
Quantitative PCR
Total RNA of PNAC-1 cells after treated with GSK2126458 for 48 h was extracted using TRIzol reagent (Qiagen, USA), and cDNA was synthesized from total RNA (2 μg) using the first-strand synthesis system (Vazyme, China). Dilute the cDNA to 2 ng/µL, then add 4 µL to 10 µL 2× FastStart Universal SYBR Green PCR Master (Vazyme, China). Each sample was tested in triplicate using the StepOnePlus qPCR system (Applied Biosystems 7500). The Ct value was normalized to the housekeeping gene GAPDH, which was amplified in parallel. The following qPCR primers were used: COL3A1-F, 5′-GGAGCTGGCTACTTCTCGC-3′ and COL3A1-R, 5′-GGGAACATCCTCCTTCAACAG-3′; EGF-F, 5′-TGGATGTGCTTGATAAGCGG-3′ and EGF-R, 5′-ACCATGTCCTTTCCAGTGTGT-3′; FN1-F, 5′-CGGTGGCTGTCAGTCAAAG-3′ and FN1-R, 5′-AAACCTCGGCTTCCTCCATAA-3′; ITGA2-F, 5′-CCTACAATGTTGGTCTCCCAGA-3′ and ITGA2-R, 5′-AGTAACCAGTTGCCTTTTGGATT-3′; GAPDH-F, 5′-TGGTGTCTGAGGGTTCTGTGG-3′ and GAPDH-R, 5′- TGATGACCCTTTTGGCTCCC-3′.
Statistical analysis
All data were represented by mean ± SD. Significant differences were calculated in GraphPad 5.0 (Inc., La Jolla, CA, USA) with one-way ANOVA analysis, and then Student t-test or Tukey’s multiple comparison test was conducted. P < 0.05 was significant difference.
Results
Identification of DEGs
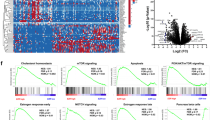
GSE15471 and GSE62165 were downloaded free from GEO database. Due to various reasons such as background and probe design, the original microarray data leads to huge differences between the microarray data. Therefore, it is necessary to check whether the data is highly standardized gene expression profile data, which can be used for subsequent DEGs analysis (Additional file 1: Figure S1). Then the GEO2R online analysis tool was used to detect differentially expressed genes. The screening criteria were adjust P-value < 0.01 and |logFC|> 1. The volcano plot (Fig. 1) showed the distribution of DEGs in GSE15471 and GSE62165. A total of 776 up-regulated DEGs were identified with the 233 down-regulated DEGs from GSE15471. And a total of 1263 up-regulated DEGs were identified with the 1374 down-regulated DEGs from GSE62165. Heat maps of the top 50 differentially expressed genes (30 up-regulated and 20 down-regulated) of GSE15471 and GSE62165 were all shown in Additional file 1: Figure S2. After a comprehensive comparative analysis, 609 differentially expressed genes were identified, including 432 up-regulated genes and 177 down-regulated genes in pancreatic cancer tissues compared with normal pancreatic tissues in two expression datasets (Additional file 1: Table S1). As shown in Fig. 1, the corresponding Venn diagrams showed the overlap region of DEGs in two gene expression profiles.
GO function enrichment analysis
We submitted the 609 DEGs to the online database DAVID for Gene ontology analysis in pancreatic cancers. In this study, the total of 253 enriched GO terms have been identified with the criteria P-value < 0.05, and the top 15 enriched GO terms of up-regulated DEGs and down-regulated DEGs were listed in Table 1, respectively. The significantly enriched go terms (top 30) of DEGs in pancreatic cancer were shown in Fig. 2A. The GO analysis results showed that DEGs were divided into three functional groups: biological processes (BP), molecular function (MF) and cell component (CC). To summarize, these results showed that most of the DEGs were mainly enriched in metabolic process, binding and extracellular part.
KEGG pathway enrichment analysis
The analysis of KEGG pathway enrichment was also using the DAVID online analysis tool. In this study, a total of 40 KEGG pathways have been identified with the criteria P value < 0.05. The significantly enriched KEGG pathways (top 30) of DEGs in pancreatic cancer were shown in Fig. 2B. Table 2 showed the top 15 enriched KEGG pathway of up-regulated DEGs and top 5 enriched KEGG pathway of down-regulated DEGs. As shown in Table 2, the KEGG pathway enrichment analysis of the DEGs showed that the up-regulated DEGs were most significantly enriched in complement and coagulation cascades; the down-regulated DEGs were most significantly enriched in pancreatic secretion glycine. Considering the count of genes, the top three enriched KEGG pathway are focal adhesion, phagosome and PI3K–Akt signaling pathway based on the count of genes (Table 2).
Hub genes and pathways screening from PPI network and modular analysis
According to the STRING database and Cytoscape software [28, 35], the total of 485 differentially expressed genes (362 up-regulated and 123 down-regulated genes) were screened out of the 609 differentially expressed genes to be showed in the PPI network map, including 485 nodes and 2202 edges (Additional file 1: Figure S3). Among the 485 nodes, the top 15 hub nodes with the higher degree of connectivity were identified and shown in Table 3. ALB had the highest node degree of connectivity, which is 120. The PPI network (15 nodes and 42 edges) map of the top 15 key genes with the higher degree were displayed by STRING (Additional file 1: Figure S4). Based on the GO function and KEGG pathway analysis using STRING, we found that COL3A1, EGF, FN1 and ITGA2 were enriched in focal adhesion and PI3K–Akt signaling pathway. Moreover, the whole PPI network was analyzed using Cytoscape MCODE to identify significant modules. Based on the criteria of MCODE score > 4 and number of nodes > 4, the top 4 modules were selected (Table 4). The KEGG pathway enrichment analysis of the genes involved in the 4 modules was calculated by STRING online software (Fig. 3A–D). Pathway enrichment analysis showed that the genes in 4 modules were mainly associated with chemokine signaling pathway, PI3K–Akt signaling pathway, phagosome, tuberculosis (Fig. 3E–H).
Comparison of the hub genes expression level
In order to validate the expression of hub genes between cancer patients and healthy people, we used GEPIA to analyze the data from TCGA normal and GTEx database. Ninety percent of Pancreatic Adenocarcinoma (PAAD) are pancreatic ductal adenocarcinomas (PDAC). Because PAAD is not classified on TCGA website and it can only be analyzed with PAAD data. The data of PAAD were used to indirectly reflect the situation of PDAC. Figure 4A reflected that compared to normal tissue, the hub genes identified in this study were also significantly abnormal expressed in the TCGA and GTEx pancreatic cancers.
Expression level of hub genes and overall survival in pancreatic cancer and normal tissues. A COL3A1, FN1 and ITGB2 level in TCGA and GTEx. PAAD Pancreatic adenocarcinoma; *P < 0.01. Red box: tumor; grey box: normal. B Overall survival of COL3A1. C Overall survival of FN1. D Overall survival of ITGB2. n = 177
We further analyzed the overall survival of COL3A1, FN1 and ITGA2 in pancreatic cancer and normal tissues. As illustrated in Fig. 4B–D, analysis based on transcriptome sequencing data showed that high FN1 expression was significantly associated with low overall survival.
Profiles of protein expression
In addition, the protein levels of COL3A1, FN1 and ITGA2 in pancreatic cancer were improved according to HPA (Fig. 5). Unfortunately, there is no EGF immunohistochemical data. Antibody HPA007583 targeting the COL3A1 protein, HPA027066 targeting the FN1 and HPA063558 targeting the ITGA2 were tested by immunohistochemistry on the normal and PAAD tissue. COL3A1 exhibited medium immunoreactivity in PAAD while staining was low in the normal pancreas; FN1 exhibited medium immunoreactivity in PAAD while staining was not detected in the normal pancreas; ITGA2 exhibited high immunoreactivity in PAAD while staining was low in the normal pancreas.
Sensitivity of the PI3K–Akt signaling pathway inhibitors to pancreatic cancer
Most of these key genes are enriched in PI3K–Akt pathway, we investigated the effect of three PI3K–Akt signaling pathway inhibitors on the cell growth of PANC-1 (human pancreatic cancer cell lines). BKM-120 is a selective PI3K inhibitor; MK2206 is a highly selective Akt1/2/3 inhibitor and the first small molecule Akt allosteric inhibitor to enter clinical research; GSK2126458 is a highly selective, potent p110α/β/γ/δ and mTORC1/2 inhibitor. These three compounds are inhibitors of three key targets in the PI3K–Akt–mTOR signaling pathway, respectively. The IC50 values on pan-cancer cell lines of BNM-120, MK2206 and GSK2126458 were predicted with the GDSC data, and our analysis indicated GSK2126458 with a significant response sensitivity against pancreatic cancer cell lines (Fig. 6A). As shown in Fig. 6B, EWSR1-FLI1 mutant pancreatic cell was the most sensitive to GSK2126458. In addition, the sensitivity of GSK2126458 to FNR43 mutant pancreatic cancer cells was significantly higher than that of wild-type cells (Fig. 6C).
The effects of mTOR inhibitor-GSK2126458 on pancreatic cancer cells
As illustrated in Fig. 7A, GSK2126458 decreased the cell viability of PANC-1 cells in time and dose-dependent manners after 24–72 h treatment. The IC50 of GSK216458 on PANC-1 cells were > 10 μM for 24 h, 0.87 ± 0.17 μM for 48 h, 0.23 ± 0.13 μM, respectively. An ideal therapeutic drug of pancreatic cancer is the one that can inhibit cancer cell growth but has no toxic effect to normal tissue. Therefore, we investigated the effect of GSK2126458 on HL7702 cell. We found that the GSK2126458 (1 μM) had no significant effect on the cell survival rate of HL7702 cell (Additional file 1: Figure S6). After treatment with gsk216458 for 48 h, the colony formation of PANC-1 cells was significantly inhibited in a dose-dependent manner (Fig. 7C). In addition, we also investigated the effect of GSK216458 on the invasion of PANC-1 cells. As shown in Fig. 7C, GSK216458 can significantly suppress the invasion of PANC-1 cells. FACS results showed that GSK2126458 could significantly induce the late apoptosis of PANC-1 cells (Fig. 7E, F). FACS results of BxPC-3 cells was seen in Additional file 1: Figure S7. These results showed that GSK2126458 can inhibit the proliferation and apoptosis of pancreatic cancer cells in a time- and dose-dependent manner.
The effects of mTOR inhibitor-GSK2126458 on pancreatic cancer cells. A MTT result of GSK216458 on PANC1 cell. B MTT result of GSK216458 on BxPC-3 cell. C Colony formation invasion results of GSK216458 on PANC1 cell. D FACS result of GSK216458 on PANC1 cell. E Statistical histogram of FACS results. F The mRNA expression of COL3A1, EGF, FN1 and ITGA2. The data came from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the control group
Discussion
Compared with other malignant tumors, the survival rate of advanced PDAC is the lowest, with a median overall survival of 2–8 months and a 5-year survival rate of 8.5%. This is due to the lack of effective screening tools, most of which are advanced (~ 80%) and the limited effect of FDA approved drugs [36]. Monotherapy with fluoropyrimidine, gemcitabine, irinotecan, platinum and Taxane in patients with advanced PDAC has low remission rate and poor survival benefit, and multi drug combination therapy provides slightly higher response rate and slightly higher survival benefit [4]. Therefore, our study intends to provide a new scheme for the treatment of PDAC through bioinformatics technology.
In the current study, a total of 432 up-regulated and 177 down-regulated genes were screened out by analyzing two gene expression profiles. These results suggested that the common DEGs may play a key role in the development of pancreatic cancer. We investigated the potential role of DEGs using functional enrichment analysis. Firstly, the GO functional enrichment revealed that the function of up-regulated genes enriched in areas such as extracellular matrix organization with the high significant P-value. Extracellular matrix is a dynamic microenvironment in the development of cancer [37,38,39,40]. Specifically, one of the main characteristics of pancreatic cancer is the rich extracellular matrix, which plays a vital role in the growth and metastasis of tumor cells [41]. Molecular markers such as COL11A1, SERPINE1, TGFBI, TNC and LUM were enriched in the extracellular matrix organization. Among them, the COL11A1 gene is overexpressed in pancreatic cancer and the protein encoded by the COL11A1 gene may be involved in fibrous proliferative events in pancreatic cancer [42]. LUM regulates collagen fibrillogenesis in the extracellular matrix. Recently, LUM has been reported to regulate cell behavior during embryonic development, tissue repair and tumor progression [43, 44]. These findings suggested that the microenvironment, especially the abnormality of the extracellular matrix, plays a key role in the development of pancreatic cancer.
Similarly, GO functional enrichment showed that down-regulated genes were mainly involved in reactive oxygen species metabolic process with the high significant P-value. It was reported that some factors associated with the increasing incidence of pancreatic cancer are also factors that lead to reactive oxygen species (ROS) overexpression [45]. For pancreatic cancer, ROS is a double-edged sword based on the concentration of intracellular ROS. At mild to moderate levels, ROS contributes to the proliferation, metastasis and invasion of tumor cells.
After analysis, we found that the above KEGG pathway is not related to the mechanism of tolerance to starvation in pancreatic cancer. Then we analyze the signal pathways with more enriched genes. The three KEGG pathways that enrich most genes are focal adhesion, phagosome and PI3K–Akt signaling pathway. Focal adhesion kinase, a member of the FAK subfamily, is mainly found in the focal adhesion signaling pathway. Previous studies have shown that inhibition of FAK signaling helps to suppress various types of cancer, including non-small cell lung cancer, breast cancer, ovarian cancer, etc. [46,47,48]. Phosphorylated FAK can activate or inhibit several downstream pathways, including PI3K/Akt and p53, which initiate tumorigenesis or induce apoptosis [49]. All these are signal pathways which are closely related to the occurrence and development of tumors. Tumor phagosome signaling pathway is mainly autophagy, which is a double-edged sword in cancer treatment [50]. Although these two over-expressed signaling pathways are inextricably linked to the development of pancreatic cancer, there is no better explanation for the specific mechanisms by which pancreatic cancers can be high tolerance of hypoxic and nutrient-deficient environments. Finally, we focused on the PI3K–Akt signaling pathway.
Akt is a key regulator of cell proliferation and growth, which is often overexpressed in cancer cells [51, 52]. As we all known, the lack of blood supply in pancreatic cancer leads to the limited availability of nutrients and oxygen to the tumor cells [53, 54]. For further verification, we built the PPI network with 609 DEGs and screened the top 15 hub genes with the high degree: ALB, EGF, MMP9, CXCL10, TOP2A, FN1, CTSS, ACTB, ITGA2, ISG15, CXCR4, OAS1, COL3A1, FPR1 and ITGB2. Of these, ALB and EGF were down-regulated and the others were all up-regulated. Among them, ALB was the highest degree of connectivity gene in the hub genes. Then we used GEPIA to estimate the expression level of hub genes in cancer and normal tissues. As shown in the box plots, these results were consistent with the trend of expressions of hub genes that we identified involved in the PPI network. It further demonstrated that the results of the hub genes we have identified are reliable. It is noticeable that 4 (COL3A1, EGF, FN1 and ITGA2) of the top 15 highly expressed genes were key proteins in the PI3K–Akt signaling pathway. After the module analysis of the PPI network, 4 significant modules were selected and then the pathway enrichment analysis was carried out. Here we continue to notice the PI3K–Akt signaling pathway, which involves the largest number of genes. The PI3K–Akt signaling pathway in module 2 contains 11 genes, of which 2 (COL3A1 and FN1) belong to the hub genes. All these suggested that the PI3K–Akt signaling pathway plays a key role in the development of pancreatic cancer, which is consistent with the previous literature. They suggested that about 50% of pancreatic cancers exhibit increased activation of PI3K signaling, which is usually associated with the undifferentiated state of the tumor and poor prognosis [55,56,57].
In pre-clinical studies of pancreatic cancer, many mTOR inhibitors have shown a variety of inhibitory effects on pancreatic cancer cells and inhibit epithelial to mesenchymal transition, including the first generation of mTOR inhibitors rapamycin, the second generation of mTOR inhibitors such as KU63794 and PP242, and the new mTOR inhibitor INK-128 dual mTOR inhibition [58, 59]. In addition, there are currently 22 clinical trials using mTOR inhibitors to treat pancreatic cancer (https://clinicaltrials.gov). Therefore, the prospect of mTOR inhibitors in the treatment of pancreatic cancer is optimistic.
However, preclinical studies have shown that the combination of mTOR inhibitors and drugs targeting the emergency drug resistance pathway provides a strong theoretical basis for PDAC treatment, it is regrettable that clinical trials have failed to achieve this expected effect. Failure to obtain a meaningful response is multifactorial, due to the reactivation of upstream RTK-driven pathways, the poor vascularization caused by significant interstitial fibrosis, and the toxicity that limits the optimal biological dosing treatment [60].
This is the first report on the anti pancreatic cancer effect of GSK2126458 through bioinformatics and in vitro experiments. There are still many problems to be solved in our research, especially the essential problem in vivo research: the toxicity of limiting the optimal biological dose.
Conclusion
In summary, our study provided a comprehensive bioinformatics analysis to identify the DEGs which may be involved in the progression of pancreatic cancer. In this study, we screened 609 DEGs and then found 15 significantly changed hub genes from them which mainly related to PI3K–Akt signaling pathway. In addition, we used the GDSC database analysis to prove that the mTOR inhibitor (GSK2126458) is very sensitive to pancreatic cancer cell lines, especially for EWSR1.FLI2 and RNF43 mutant. This is the first time that we report that GSK2126458 has a potential role in the treatment of pancreatic cancer. We will explore the role of GSK2126458 against pancreatic cancer through systematic studies in vitro and in vivo, in order to provide a new strategy for the treatment of pancreatic cancer.
Data availability statement
All data generated or analysed during this study are included in this published article [and its additional information files].
Abbreviations
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differentially expressed genes
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- DAVID:
-
The Database for Annotation, Visualization and Integrated Discovery
- STRING:
-
Search Tool for the Retrieval of Interacting Genes
- PPI:
-
Protein–protein interaction
- BP:
-
Biological process
- CC:
-
Cellular component
- MF:
-
Molecular function
- MCODE:
-
Molecular Complex Detection
- TCGA:
-
The Cancer Genome Atlas
- GTEx:
-
Genotype-Tissue Expression
- GEPIA:
-
Gene Expression Profiling Interactive Analysis
- COL3A1:
-
Collagen type III alpha 1 chain
- EGF:
-
Epidermal growth factor
- FN1:
-
Fibronectin 1
- ITGA2:
-
Integrin subunit alpha 2
- ALB:
-
Albumin
- MMP9:
-
Matrix metallopeptidase 9
- CXCL10:
-
C-X-C motif chemokine ligand 10
- TOP2A:
-
Topoisomerase (DNA) II alpha
- CTSS:
-
Cathepsin S
- ACTB:
-
Actin beta
- ISG15:
-
ISG15 ubiquitin-like modifier
- CXCR4:
-
C-X-C motif chemokine receptor 4
- OAS1:
-
2ʹ-5ʹ-Oligoadenylate synthetase 1
- FPR1:
-
Formyl peptide receptor 1
- ITGB2:
-
Integrin subunit beta 2
- PI3K:
-
Phosphatidylinositol-3-OH kinase
- Akt:
-
Protein kinase B identified in the Akt virus
- GDSC:
-
The Genomics of Drug Sensitivity in Cancer Project
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- DMSO:
-
Dimethyl sulfoxide
References
Güngör C, Hofmann BT, Wolters-Eisfeld G, Bockhorn M. Pancreatic cancer. Integr Cancer Ther. 2014;171:849–58.
Asuthkar S, Rao JS, Gondi CS. Drugs in preclinical and early-stage clinical development for pancreatic cancer. Expert Opin Investig Drugs. 2012;21:143–52.
Izuishi K, Katou K, Imamura K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation and its blockade. Clin Cancer Res. 2000;6:6201–7.
Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, Benson AB, Binder E, Cardin DB, Cha C, et al. Pancreatic adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2017;15:1028–61.
Passos PJ, Vagner B, Silva CNH, Damasceno RO, Adriana CB, Lucia PV. Microvascular density of regenerative nodule to small hepatocellular carcinoma by automated analysis using CD105 and CD34 immunoexpression. BMC Cancer. 2014;14:1–7.
Sun H, Tang Z, Li XM, Zhou Y, Sun B, Ma Z. Microvessel density of hepatocellular carcinoma: its relationship with prognosis. J Cancer Res Clin Oncol. 1999;125:419–26.
Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–48.
Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res. 2000;60:6201–7.
Ting C, Jun A, Shun Z. Detection of the common resistance genes in Gram-negative bacteria using gene chip technology. Indian J Med Microbiol. 2013;31:142–7.
Kulasingam V, Diamandis EP. Strategies for discovering novel cancer biomarkers through utilization of emerging technologies. Nat Clin Pract Oncol. 2008;5:588–99.
Sun H, Han B, Cheng X, Ma K. Transcriptional regulatory network and protein–protein interaction to reveal the mechanism of pancreatic cancer. Mol Biol Rep. 2014;41:387–95.
Olsen PA, Lund K, Krauss S. Expression profiling of wild type and Î2-catenin gene disrupted human BxPC-3 pancreatic adenocarcinoma cells. Genom Data. 2015;4:150–2.
Wang B, Sun S, Liu Z. Analysis of dysregulation of immune system in pancreatic cancer based on gene expression profile. Mol Biol Rep. 2014;41:4361–7.
He C, Jiang H, Geng S, Sheng H, Shen X, Zhang X, Zhu S, Chen X, Yang C, Gao HJ. Analysis of whole genomic expression profiles and screening of the key signaling pathways associated with pancreatic cancer. Int J Clin Exp Pathol. 2012;5:537–46.
Li X, Chen H, Liu Z, Ye Z, Gou S, Wang C. Overexpression of MIST1 reverses the epithelial-mesenchymal transition and reduces the tumorigenicity of pancreatic cancer cells via the Snail/E-cadherin pathway. Cancer Lett. 2018;431:96–104.
Lu Y, Li C, Chen H, Zhong W. Identification of hub genes and analysis of prognostic values in pancreatic ductal adenocarcinoma by integrated bioinformatics methods. Mol Biol Rep. 2018;144:1–9.
Tang Y, Zhang Z, Tang Y, Chen X, Zhou J. Identification of potential target genes in pancreatic ductal adenocarcinoma by bioinformatics analysis. Oncol Lett. 2018;16:2453–61.
Thomas PD. The Gene Ontology and the Meaning of Biological Function. Methods Mol Biol (Clifton, NJ). 2017;1446:15–24.
Xing Z, Chen C, Chen L, Kong X. The use of Gene Ontology terms and KEGG pathways for analysis and prediction of oncogenes ☆. Biochem Biophys Acta. 2016;1860:2725–34.
Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology. 2008;55:2016–27.
Janky RS, Binda MM, Allemeersch J, Broeck AVD, Govaere O, Swinnen JV, Roskams T, Aerts S, Topal B. Prognostic relevance of molecular subtypes and master regulators in pancreatic ductal adenocarcinoma. BMC Cancer. 2016;16:632.
Davis S, Meltzer PS. GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–7.
Deng W, Wang Y, Liu Z, Cheng H, Xue Y. HemI: a toolkit for illustrating heatmaps. PLoS ONE. 2014;9:e111988.
Consortium GO. The Gene Ontology (GO) project in 2006. Nucleic Acids Res. 2006;34:D322.
Sherlock G. Gene Ontology: tool for the unification of biology. Can Inst Food Sci Technol J. 2009;22:415.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;27:29–34.
Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huertacepas J, Simonovic M, Roth A, Santos A, Tsafou KP. STRING v10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102.
Uhlén M, Björling E, Agaton C, Szigyarto CA, Amini B, Andersen E, Andersson AC, Angelidou P, Asplund A, Asplund C. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics MCP. 2005;4:1920–32.
Mathias U, Linn F, Hallström BRM, Cecilia L, Per O, Adil Mardinoglu S, Caroline K, Evelina SS, Anna A. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Luo Z, Chen X, Zhang Y, Huang Z, Zhao H, Zhao J, Li Z, Zhou J, Liu J, Cai J, Bi X. Development of a metastasis-related immune prognostic model of metastatic colorectal cancer and its usefulness to immunotherapy. Front Cell Dev Biol. 2021;8:577125.
Lu H-Y, Zu Y-X, Jiang X-W, Sun X-T, Liu T-Y, Li R-L, Wu Q, Zhang Y-S, Zhao Q-C. Novel ADAM-17 inhibitor ZLDI-8 inhibits the proliferation and metastasis of chemo-resistant non-small-cell lung cancer by reversing Notch and epithelial mesenchymal transition in vitro and in vivo. Pharmacol Res. 2019;148:104406.
Feng Y, Hao F. Hansenia weberbaueriana (Fedde ex H. Wolff) Pimenov & Kljuykov extract suppresses proliferation of HepG2 cells via the PTEN-PI3K–Akt pathway uncovered by integrating network pharmacology and in vitro experiments. Front Pharmacol. 2021;12:620897.
Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, Von MC. STRING vol 91: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–15.
Kamarajah SK, Burns WR, Frankel TL, Cho CS, Nathan H. Validation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with pancreatic adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol. 2017;24:2023–30.
Dorn DC, Dorn A. Stem cell autotomy and niche interaction in different systems. World J Stem Cells. 2015;7:922–44.
Hellewell AL, Adams JC. Insider trading: extracellular matrix proteins and their non-canonical intracellular roles. Bioessays News Rev Mol Cell Dev Biol. 2016;38:77–88.
Hynes RO, Naba A. Overview of the matrisome—an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspect Biol. 2012;4:a004903.
Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:1750–4.
Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut. 2012;61:172–8.
Carmen GP, Galván JA, Natalia GC, Lorena SG, Eva GP, Marcos GO, Jokin AI, Primitiva MR, Juan GG, De Los Toyos JR. Overexpression of COL11A1 by cancer-associated fibroblasts: clinical relevance of a stromal marker in pancreatic cancer. PLoS ONE. 2013;8:e78327.
Baba H, Ishiwata T, Takashi E, Xu G, Asano G. Expression and localization of lumican in the ischemic and reperfused rat heart. Jpn Circ J. 2001;65:445–50.
Lu YP, Toshiyuki I, Kiyoko K, Masanori W, Zenya N, Yukichi M, Yuichi S, Goro A. Expression of lumican in human colorectal cancer cells. Pathol Int. 2002;52:519–26.
Martinezuseros J, Li W, Cabezamorales M, Garciafoncillas J. Oxidative stress: a new target for pancreatic cancer prognosis and treatment. J Clin Med. 2017;6:29.
Zhou X, Gu R, Han X, Wu G, Liu J. Cyclin-dependent kinase 5 controls vasculogenic mimicry formation in non-small cell lung cancer via the FAK-AKT signaling pathway. Biochem Biophys Res Commun. 2017;492:447–52.
Porter PL. Global trends in breast cancer incidence and mortality. Salud Publica Mex. 2009;51:141–6.
Xu B, Lefringhouse J, Liu Z, West D, Baldwin LA, Ou C, Chen L, Napier D, Chaiswing L, Brewer LD. Inhibition of the integrin/FAK signaling axis and c-Myc synergistically disrupts ovarian cancer malignancy. Oncogenesis. 2017;6:e295.
Wang ZG, Fukazawa T, Nishikawa T, Watanabe N, Sakurama K, Motoki T, Takaoka M, Hatakeyama S, Omori O, Ohara T. TAE226, a dual inhibitor for FAK and IGF-IR, has inhibitory effects on mTOR signaling in esophageal cancer cells. Oncol Rep. 2008;20:1473–7.
Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42.
Blum R, Kloog Y. Metabolism addiction in pancreatic cancer. Cell Death Dis. 2014;5:e1065.
Parsons CM, Muilenburg D, Bowles TL, Virudachalam S, Bold RJ. The role of Akt activation in the response to chemotherapy in pancreatic cancer. Anticancer Res. 2010;30:3279–89.
Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34:525–32.
Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266.
Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br J Cancer. 2003;89:2110–5.
Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846–50.
Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, Hieber M, Arbeiter A, Klein S, Kong B. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406–20.
Soares HP, Ming M, Mellon M, Young SH, Han L, Sinnet-Smith J, Rozengurt E. Dual PI3K/mTOR inhibitors induce rapid overactivation of the MEK/ERK pathway in human pancreatic cancer cells through suppression of mTORC2. Mol Cancer Ther. 2015;14:1014–23.
Iriana S, Ahmed S, Gong J, Annamalai AA, Tuli R, Hendifar AE. Targeting mTOR in pancreatic ductal adenocarcinoma. Front Oncol. 2016;6:99.
Babiker HM, Karass M, Recio-Boiles A, Chandana SR, McBride A, Mahadevan D. Everolimus for the treatment of advanced pancreatic ductal adenocarcinoma (PDAC). Expert Opin Investig Drugs. 2019;28:583–92.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
YF designed experiments and wrote the manuscript, performed experiments. JY and FH analyzed data. All authors contributed to the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
None.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. Normalized expression value data box plots. Black line in each box represents the median of each sample. All the black lines are almost in the same position, which indicates high degree of standardization (“the black lines” refers to “median-centered values”). Figure S2. Heat maps of the top 50 differentially expressed genes (30 up-regulated and 20 down-regulated) of GSE15471 (A) and GSE62165 (B). Gray, case group; yellow, control group. Red: high expression level; blue: low expression level. Figure S3. The PPI network of 485 DEGs (Pink: 362 up-regulated genes; blue: 123 down-regulated genes) which were screened out by STRING. Figure S4. The PPI network of top 15 hub genes. Figure S5. Expression level of hub genes and overall survival in pancreatic cancer and normal tissues. Figure S6. MTT result of GSK216458 on HL7702 cells. Figure S7. FACS results of GSK216458 on GxPC-3 cells. Table S1. 609 DEGs were identified from two profile datasets.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Feng, Y., Jiang, Y. & Hao, F. GSK2126458 has the potential to inhibit the proliferation of pancreatic cancer uncovered by bioinformatics analysis and pharmacological experiments. J Transl Med 19, 373 (2021). https://doi.org/10.1186/s12967-021-03050-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-021-03050-7