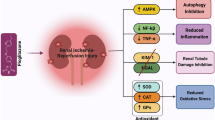
Abstract
Background
Keto-analogues administration plays an important role in clinical chronic kidney disease (CKD) adjunctive therapy, however previous studies on their reno-protective effect mainly focused on kidney pathological changes induced by nephrectomy. This study was designed to explore the currently understudied alternative mechanisms by which compound α-ketoacid tablets (KA) influenced ischemia–reperfusion (IR) induced murine renal injury, and to probe the current status of KA administration on staving CKD progression in Chinese CKD patients at different stages.
Methods
In animal experiment, IR surgery was performed to mimic progressive chronic kidney injury, while KA was administrated orally. For clinical research, a retrospective cohort study was conducted to delineate the usage and effects of KA on attenuating CKD exacerbation. End-point CKD event was defined as 50% reduction of initial estimated glomerular filtration rate (eGFR). Kaplan–Meier analysis and COX proportional hazard regression model were adopted to calculate the cumulative probability to reach the end-point and hazard ratio of renal function deterioration.
Results
In animal study, KA presented a protective effect on IR induced renal injury and fibrosis by attenuating inflammatory infiltration and apoptosis via inhibition of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways. In clinical research, after adjusting basic demographic factors, patients at stages 4 and 5 in KA group presented a much delayed and slower incidence of eGFR decrease compared to those in No-KA group (hazard ratio (HR) = 0.115, 95% confidence interval (CI) 0.021–0.639, p = 0.0134), demonstrating a positive effect of KA on staving CKD progression.
Conclusion
KA improved IR induced chronic renal injury and fibrosis, and seemed to be a prospective protective factor in end stage renal disease.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) has become a crucial public healthcare issue on account of its progressive, irreversible pathological changes and complications including anemia, dyslipidemia and hyperparathyroidism. Previous studies have demonstrated that the metabolism of protein, which mainly comes from daily diet, is involved in most of these adverse pathological changes [1]. Thus, dietary intervention with restricted protein intake has been proposed as an extremely important therapeutic strategy to delay the progressive decline of renal function and the development of CKD.
As a simple low protein diet is prone to malnutrition, it is often implemented with other nutritional interventions, such as weight control, sodium diet, keto-analogues [2,3,4]. Keto-analogues contained appropriate proportioned essential amino acids to meet the needs of human synthesis while reducing the production of metabolic waste. The study of keto-analogues combined with protein restriction started since 1967, Richards etc. suggested that a supply of keto-analogues averted the harmful effect produced by the metabolisms of Sulphur and phosphorus contained in natural foods [5]. Despite some claims that a conjunctive use of α-ketoacids was superfluous in patients with renal insufficiency, more researchers confirmed that the combined use of keto-analogues with protein restriction reduced the metabolic wastes or toxicities in CKD patients [6,7,8,9]. Despite keto-analogues administration playing an important role in clinical CKD adjunctive therapy, research focusing on its basic mechanism involved in improving renal disease were quite few. Furthermore, nephrectomy was the most used kidney disease model in animal studies. In Zhang and Wang’s research, keto-analogues supplemented with low protein diet (LPD) were proved to inhibit the intrarenal renin-angiotensin-system to attenuate proteinuria, and regulate Wnt7a/Akt/p70S6K pathway and apoptotic system to improve muscle mass [10, 11]. In Dongtao Wang’s study, keto-analogues and LPD were observed to protect 5/6 nephrectomised rats by suppression of oxidative damage and mitochondrial dysfunction [12]. Another paper mentioned that keto-analogues and LPD increased the expression of Kruppel-like factor-15 (KLF15), a transcription factor proved to reduce cardiac fibrosis, thus reduced the severity of kidney disease in a remnant model [13]. In a diabetic nephropathy model, it seemed that keto-analogues delayed the progression of diabetic nephropathy via inhibition of oxidative stress [14]. However, the limitation of animal models caused the mechanical explorations to be incomplete and imperfect. More needs to be done to improve the mechanism of keto-analogues intervention in renal protection.
The renal ischemia-reperfusion (IR) injury model confers the advantage of observing renal repair after acute kidney injury while mimicking transient renal ischemia in clinical cases such as multiple organ failure and pre-transplant kidneys, thus offering insight into allograft survival after kidney transplant [15, 16]. Keto-analogues therapy with LPD provides sufficient essential amino acid intake without protein waste and excess metabolic toxin production and accumulation. Further understanding of its mechanism of action in CKD would be beneficial for expanding its applications.
In this study, we adopted IR model to explore the molecular mechanism of compound α-ketoacid tablets (KA) in renal disease from the angle of inflammation and apoptosis. KA supplementation inhibited nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, resulting in an alleviation of inflammation and apoptosis, thus staving the progression of CKD. The animal experiment gave further evidence to support the reno-protective effects of KA in CKD as seen in our clinical research.
Methods
Animals
Male C57BL/6 mice aged 2-month-old (weighing 25 g) were purchased from Beijing Huafukang Laboratory Animal Technology Co., Ltd, Beijing, China and were acclimated for 1 week. Mice were anaesthetized with 1% sodium pentobarbital (0.01 mL/g, Sigma, USA) by intraperitoneal injection. IR surgery: bilateral kidneys of mice were clamped in 26 min (Roboz Surgical Instrument Co, Germany). Renal blood flow was restored in a few seconds after murine artery clamp was removed. Whole surgeries were performed at 36.6 °C–37.2 °C using a temperature control machine (FHC, USA). Animals were divided into three groups: Sham, IR + Nacl, IR + KA, n = 4–6/group. Mice in sham group underwent the same surgery excluding clamping renal vessels. Mice in IR + Nacl group underwent with IR surgery and were treated with Nacl. Mice in IR + KA group were operated with IR surgery and treated with KA (FreseniusKabi, Germany). Both kidneys were harvested 28 days after IR surgery. All experiments were approved by the Animal Care and Use Committee of Tongji Hospital (IACUC Number: S851) and performed in accordance with NIH guidelines.
KA and LPD preparation and administration
Compound α-ketoacid tablets were confected by mixture of 0.375 g α-ketoacid to 6 mL 0.9% Nacl, to a final concentration of 62.5 mg/mL and were administered by gastric gavage. Administration concentration was 1000 mg per kg mouse weight per day. LPD was supplied by WQJX BIO-Technology (Wuhan, China). The diet was composed of casein (6.5%), starch (66.45%), glucose (10%), soybean oil (7%), fibrin (5%), mineral salt (3.5%), vitamin (1%), l-cystine (0.3%) and choline chloride (0.25%) in accordance with rodent diet of American Dietetic Association, with a calorie of 3.5 kcal/g to meet energy need. In IR + KA group, mice were treated with KA and LPD. In comparison, mice in the IR + Nacl group were treated with 0.9% Nacl and LPD.
BUN, TC and TG detection
Blood urea nitrogen (BUN), total cholesterol (TC) and triglyceride (TG) were tested using kits (Changchunhuili, China) according to the manufacturer’s instructions.
Histological, immunocytochemical and immunofluorescent staining
Bilateral kidneys of mice were fixed in 4% paraformaldehyde and embedded in paraffin. The paraffin-embedded kidneys were sectioned at 3 μm. Pathological staining for Periodic Acid-Schiff staining (PAS), and Sirius red were performed to evaluate renal tubular injury grade and interstitial fibrosis. For immunofluorescent (IF) staining, sections were stained with primary antibody Kim-1 (1:800; R&D), lotus tetragonolobus lectin (LTL, 1:100, Vector Lab), alpha-smooth muscle actin (α-SMA, 1:100, Abcam), Collagen I (1:100, Abcam), CD45 (1:50, Biolegend), CD3 (1:50, Guge, Wuhan) and Ly6G (1:50, Biolegend) at 4 °C overnight and fluorescent labeled secondary antibodies. Nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI). Data was analyzed by Image Pro Plus software (Media Cybemetics, Rockville, MD, USA) in a blinded manner.
Western blot
Renal tissues were lysed in RIPA lysis buffer (Promoter, Wuhan, China) containing protease inhibitor (Promoter, Wuhan, China). Cell proteins were collected in the same way. Total protein concentration was determined using a BCA protein assay kit (Promoter, Wuhan, China). Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (PVDF, Millipore, Billerica, MA, USA). The membranes were blocked with 5% skimmed milk in TBS with 0.1% Tween-20 for 1 h at 37 °C and then probed with antibodies against α-SMA (1:5000, Abcam), PDGFR-β (1:3000, Abcam), Fibronectin (1:1000, Abcam), Bcl-2 (1:1000, Abclonal), BAX (1:1000, Abclonal), Caspase 3 (1:1000, Abclonal), NFATc-1 (1:1000, Abcam), total and phosphorylated-65, -38 and -ERK (1:1000, cell signaling technology, USA) and GAPDH (1:4000, Abbkine) at 4 °C overnight. The blots were washed next day and incubated with HRP-conjugated secondary antibodies for 1 h at 37 °C. The signal intensities of targeted band were quantified using Image J (NIH, USA).
Quantitative real-time PCR
Total RNA was extracted using Trizol reagent according to the manufacturer’s instructions (Invitrogen, USA). One microgram RNA was reverse transcribed into first strand cDNA using the GoScript reverse transcription system (Promega, USA) in a 20 μL reaction system. The cycling parameters were used as follow: 40 cycle pf denaturation at 95 °C for 15 s and annealing at 60 °C for 60 s. Triplicated experiments for each sample were processed. Quantitative PCR was conducted using SYBR master mix (Qiagen, Germany) on the Roche light 480II. The mRNA expression levels of several markers, including TGF-β, fibronectin and Collagen I were conducted via the comparative cycle threshold (Ct) method and normalized to the expression levels of GAPDH. Primer sequences were listed in Table 1.
Basic information of clinical CKD patients collected from Tongji Hospital
A total number of 894 CKD outpatients and inpatients were recruited from April 2015 to September 2018 in Tongji hospital, Tongji medical college, Huazhong university of science and technology for cross-sectional and retrospective study. A total number of 452 patients were excluded, (1) hemodialysis, peritoneal dialysis or renal transplantation at the beginning of enrolled time; (2) the follow-up time was less than 2 times; (3) basic data substantially missing. Among the remaining enrolled 442 patients, 119 were at stage 1 and 2, only 9 patients were prescribed KA, which greatly influenced the result of analysis. For the remaining 323 patients at stage 3 to 5, 148 patients took KA were divided into KA group, the other 175 patients were divided into No-KA group. The clinical study was approved by Medical Ethics Committee of Tongji Hospital, Tongji medical college, Huazhong University of Science and Technology (TJ-IRB20180501). We registered in Chinese Clinical Trial Registry (ChiCTR1800016536).
Statistical analysis
Data was expressed as count value (%) for categorical variables and mean ± SD for continuous variables. Comparisons between the two groups were made by nonparametric T test for continuous variables while χ2 test for categorical variables. Kaplan–Meier analysis and COX proportional hazard regression model were adopted to calculate the cumulative probability to reach the end-point and hazard ratio of renal function deterioration. The statistical analyses were performed by SAS v9.4 (SAS, Inc., 200 Cary, NC, USA), and all P-values calculated as two-sided. The association was considered significant with p-values less than 0.05.
Results
KA supplement protected mice from IR-induced renal injury and metabolic disorders
To better explore the effect of KA on mice, we adopted oral administration of KA for 28 days after bilateral IR surgery (Fig. 1a). A basic pathological PAS staining showed that IR damaged renal tubules and caused massive cast formations, expansions and necrosis (Fig. 1b). LTL, short for Lotus tetragonolobus lectin, which combines specifically with proximal tubular brush borders, is a marker used for healthy renal tubules. LTL staining showed that after IR surgery, few remaining LTL positive healthy tubules existed (Fig. 1d). Staining of the other marker Kim-1, an abbreviation for Kidney Injury Molecule-1, which is specifically dyed in injured tubules, displayed a similar image to LTL: the number of injured tubules increased as the number of healthy tubules decreased (Fig. 1f). In IR + KA group, the mice treated with KA presented an improved state of operated kidneys. The number of cast and tubular expansions were conspicuously reduced (Fig. 1b, c). Nearly twice as many healthy renal tubules were preserved after KA treatment compared to the IR + Nacl group, only sparse tubules were marked by Kim-1 (Fig. 1d–g). Next, we measured biochemical markers and found that KA can not only improve IR-induced deterioration of renal function, but also remit IR-induced lipid disorders. TG levels dropped significantly in IR + KA group. Otherwise, KA caused a slight but statistically insignificant reduction on cholesterol (Fig. 1h). The above results implied an additional positive regulation of lipid metabolism brought by KA, aside from a fundamental therapeutic effect on renal injury.
KA supplement protected mice from IR induced renal injury and metabolic disorders. a The experiment scheme in vivo. Mice were divided into 3 groups: Sham, IR + Nacl (IR surgery + 0.9% Nacl solvent), IR + KA (IR + KA dissolved in 0.9% Nacl: 1000 mg/kg/d). LPD was administrated after IR surgery, at the same time with KA for 28 days. b, c PAS staining showed a mass of tubular expansions, casts and loss of tubular brush border in IR + Nacl group. With the treatment of KA, tubular expansions were ameliorated and casts were reduced. d, e LTL staining showed IR caused more than 2.0-folds decrease of healthy tubules, KA preserved nearly 1.8 folds increased remnant healthy tubules compared to IR + Nacl group. f, g The detection of Kim-1 showed a same phenomenon as LTL. An extensive injure renal tubules were founded in IR + Nacl group. In IR + KA group, the number of kim-1 positive tubules decreased. h Serum BUN and plasma TC and TG were detected by using kits. KA reduced IR induced enhancement of BUN and TG. Otherwise, KA showed a slight decrease in IR induced TC disorder, the concentration of TC in IR + Nacl was 4.74 mmol/L while in IR + KA was 4.19 mmol/L. Scale bars: 50 μm. Data were presented as mean ± SD, *p < 0.05, **p < 0.01 (unpaired nonparametric t-test); n = 4–6/group
KA supplement improved IR-induced interstitial fibrosis
As renal fibrosis is the end-way of all chronic kidney disease, a measurement of fibrosis was necessary to evaluate the therapeutic efficiency of KA in kidney. Sirius red staining, a pathological staining to test collagen deposition showed broad collagen fibers were generated and accumulated in renal interstitium after IR. In IR + KA group, only filar collagen appeared in interstitium (Fig. 2a, b). Immunofluorescence labeling of alpha-Smooth muscle actin (α-SMA, a marker of myofibroblast) and collagen I demonstrated that IR led to a serious amount of myofibroblasts and collagen production in extracellular interstitium. With the administration of KA, the distribution of α-SMA and collagen I became sparse, the accumulation of myofibroblasts and collagen was distributed less densely (Fig. 2c–f). Next, we detected fibrosis markers in protein and RNA levels. KA significantly reduced the expressions of α-SMA, collagen I, Fibronectin and platelet-derived growth factor receptor (PDGFR-β), a marker of pericytes, which was demonstrated to be another source of fibrosis (Fig. 2g, h). A fibrosis related factor, transforming growth factor β (TGF-β), mainly participating in transforming fibroblasts into myofibroblasts and regulating fibrosis, was expressed abundantly in IR + Nacl group, with a greater than fivefold increase in RNA level compared to Sham group. The use of KA could inhibit the expression of TGF-β (Fig. 2H). The above data implied that KA supplement could improve IR induced renal injury while simultaneously slowing down progression to renal interstitial fibrosis.
KA supplemented with LPD improved IR induced interstitial fibrosis. a–f Pathological staining Sirius red (red), myofibroblast marker α-SMA (red) and collagen I (red) staining presented increased interstitial myofibroblast and collagen deposition in IR + Nacl group. KA improved both myofibroblasts and collagen. Renal interstitial fibrosis was improved in IR + KA group. g, h The detections of fibrosis related factors: PDGFR-β, fibronectin, collagen I and TGF-β showed KA had an improved effect on IR induced renal fibrosis in protein and/or RNA levels. Scale bar: 50 μm. Data were presented as mean ± SD. *p < 0.05, **p < 0.01 (unpaired nonparametric t-test); n = 3–5/group
KA supplement attenuated IR-induced inflammatory infiltration and apoptosis
In IR injury, renal injury and apoptosis are regulated by some cytokines such as TGF-β, which is produced and secreted by leukocyte lineage cells, including lymphocytes, macrophages and dendritic cells. A chronic inflammatory condition promotes the increased production and activation of latent TGF-β, modulating the proliferation of fibroblasts and excessive deposition of extracellular matrix [17,18,19]. To further explore the related mechanisms of IR-induced fibrosis and a probable participating role of KA, we tested CD45, a marker presenting the whole T cells, CD3 for mature T cells and Ly6G for neutrophils. In day 28 of IR, there were still a large amount of inflammatory cells infiltration in injured kidney. The expressions of CD45 positive T cells and CD3 positive mature T cells increased widely in IR + Nacl group. With the treatment of KA, the area of inflammatory infiltration decreased more than 50% in IR + KA group (Fig. 3a-f). Later we detected the expressions of apoptotic factor BAX and Bcl-2, an anti-apoptotic marker. The ratio of Bcl-2/BAX decreased in IR + Nacl group, which meant increased apoptosis in operated kidney. Another apoptotic marker Caspase3 was also found to be increased in IR + Nacl. KA administration presented an anti-apoptotic effect on IR-induced renal injury, with an enhanced expression of Bcl-2/BAX and a reduction of Caspase3 in protein level (Fig. 4a-d).
KA supplemented with LPD attenuated IR induced inflammatory infiltration. a–f CD45 (green), CD3 (red), Ly6G (red) and DAPI (blue) staining presented a more than 8.0-folds of CD45 positive T cell, 60-folds of CD3 positive T cells and 40 folds of Ly6G positive neutrophils in IR + Nacl group after IR surgery. KA improved mature T cell and neutrophil granulocytes infiltration. Scale bar: 20 μm. Data were presented as mean ± SD. *p < 0.05, **p < 0.01 (unpaired nonparametric t-test); n = 4–5/group
KA attenuated IR induced apoptosis. a–d Western blot analysis and histograms of apoptosis markers: Bcl-2, BAX and caspase3. After IR surgery, the apoptosis related factors BAX and Caspase 3 increased. KA reduced the expressions of BAX and Caspase 3, without improved the anti-apoptotic factor Bcl-2. However, the ratio of Bcl-2/BAX increased in IR + KA group, meaning a anti-apoptotic effect of KA on IR. Data were presented as mean ± SD. *p < 0.05, **p < 0.01 (unpaired nonparametric t-test); n = 3–5/group
KA supplement might attenuate IR-induced inflammation and apoptosis by inhibiting the activation of NF-κB and MAPK pathways
NF-κB and MAPK pathways are two classic signaling pathways participating in inflammation and apoptosis [20,21,22,23,24]. In IR + Nacl group, the expression of phosphorylated-p65 increased, as well as that of its downstream molecule NFATc-1, showing that IR promoted the activation of NF-κB pathway. With the treatment of KA, the activation of phosphorylated-p65 was partly inhibited, and the expression of NFATc-1 was reduced (Fig. 5a). At the same time, we measured MAPK pathway-related molecules and observed a similar increase of phosphorylated ERK and p38 in IR + Nacl group. In the IR + KA group, the activation was inhibited with a decrease of 50% in p-p38 and 80% in p-ERK compared to IR + Nacl group (Fig. 5b). Our results suggest that KA supplement alleviate renal inflammation and apoptosis via an inhibited activation of NF-κB and MAPK pathways.
KA supplemented with LPD might attenuate IR induced inflammation and apoptosis by inhibiting the activation of NF-κB and MAPK pathways. a Western blot analysis and histograms of NF-κB pathway: p-p65 raised dramatically in IR + Nacl group compared to the Sham group. Its targeted molecule NFATc-1 increased after IR surgery too. KA inhibited the activation of NF-κB pathway, as well as NFATc-1. b MAPK pathway (p-p38 and p-ERK). KA presented a similar effect on MAPK pathway as NF-κB pathway. A nearly 2.0-folds increase of p-p38 was detected in IR + Nacl group compared to the Sham group. Half was decreased after the usage of KA. The expression of p-ERK was also reduced in protein level in KA group after IR. Data were presented as mean ± SD. *p < 0.05 (unpaired nonparametric t-test); n = 3/group
Basic demographic characteristics of clinical CKD patients at different stages and difference in renal function status between people with and without KA intervention
The above research pointed to a protective effect of KA on IR induced chronic kidney injury in mice. Next, we collected basic information of patients to further explore the characteristic difference between patients with or without KA intervention (Fig. 6). Commonly, patients prescribed with KA were mainly at CKD stages 3 to 5, with 78/201 in the stage 3 group and 70/122 in the stage 4 and 5 groups (Table 2), and only 9 among 119 patients at CKD stages 1 and 2. Basic demographic characteristics showed an interesting phenomenon that CKD patients at stages 3 to 5 mainly possessed secondary level or higher education, and were engaged in intellectual fields of work or retired. Otherwise, the age of CKD patients at stage 3 to 5 peaked between 35 to 60, displaying a characteristic chronic progression feature of CKD. Next, we analyzed the renal function indexes of patients at stages 3 to 5, including eGFR, Scr, BUN and UA. In the group of patients at stage 3, a significant worsening of baseline renal function indexes was observed in people with KA, p = 0.000 for eGFR and Scr, p = 0.068 for UA. In end-point CKD stage groups, eGFR and Scr scores in KA group were still poorer compared to No-KA group (with p = 0.000 for eGFR and p = 0.009 for Scr). BUN in the KA group was higher than No-KA group (p = 0.053) at a value of about 9.2 ± 3.9 mmol/L. Combining patients at stages 3 to 5, a similar statistical difference was analyzed between KA and No-KA group, patients with KA prescription usually had worse renal conditions. However, taking only patients at stages 4 and 5 into account, no significant difference was detected in baseline eGFR, Scr, BUN, UA and \( {\text{HCO}}_{3}^{ - } \) when comparing the KA with No-KA group (Table 3).
The effects of KA on renal survival probability in CKD patients at different stages
We divided enrolled patients at CKD stages 3 to 5 into KA and No-KA groups according to their medications. An end-point event was defined as a > 50% reduction in the initial eGFR. In a Kaplan–Meier analysis, the cumulative probability to reach the end-point was lower in the KA group than the No-KA group (p = 0.0241) among patients at stages 4 and 5. For patients at stages 3 to 5, KA also showed a protective effect on renal function before the 30-month mark; after 30 months of treatment, patients in the KA group presented a deterioration in renal condition. Only taking patients at stage 3 into account, the incidence of end-point CKD events in the KA group was lower than the No-KA group. However, the total incidence of end-point events (n = 0.035, 7 end-point events among 201 patients) was lower than at stages 4 and 5 (n = 0.107, 13 among 122) (Fig. 7). After adjustment for basic demographic factors (including gender, age, complication, education, occupation, smoking and alcohol consumption history), people in the KA group presented a greatly delayed and slower incidence of eGFR decrease compared to the No-KA group [adjusted hazard ratio, 0.115; 95% CI 0.021 to 0.639; p = 0.0134 (Table 4)] among patients at stages 4 and 5, exhibiting a positive effect of KA on CKD progression.
The effect of KA on renal survival probability in CKD patients at different stage. The probability to reach the end-point was lower in KA group than no-KA group at CKD stages 4 and 5 when adjusted in a log rank test (p = 0.0241). For patients at stage 3 to 5, KA also showed a protective effect on renal function before 30 months, after 30 months, patients in KA group presented a deteriorate renal situation. For patients at stage 3, the incidence of end-point (n = 0.035, 7/201) in KA group was lower than no-KA group, however, the whole incidence of end-point was rather less than at stage 4 and 5 (n = 0.107, 13/12)
Discussion
In this study, we adopted IR-induced renal injury model to observe chronic progression of renal tubular injury and interstitial fibrosis. Firstly, we found that KA reduced IR-induced abnormal serum BUN concentration as well as total triglycerides with statistically significant difference, improved renal function and hyperlipidemia in mice. Kidneys play an important role in the protein reabsorption and excretion of protein metabolites. Renal injury causes abnormal protein metabolism, which can affect enzyme-related protein synthesis and metabolism, resulting in hyperlipidemia [25, 26]. KA acted as an essential protein supplement and alleviated hypoproteinemia and protein synthesis in liver, we inferred from our results that KA might increase the expression of lipoprotein lipase, hepatic lipase and very low-density lipoprotein receptor, and as a result increase triglycerides clearance and decrease their formation in liver, thereby modifying the hemostasis of triglyceride. KA also caused a slight but statistically insignificant improvement on total cholesterol compared to the IR group. This might be due to the different metabolic mechanisms involved for cholesterol and triglycerides. Cholesterol biosynthesis is dependent on the enzyme activity of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) in liver. In chronic kidney disease both the expression and activity of HMG-CoA showed a marked increase [27]. The reduction of hypercholesterolemia induced by renal injury depends on the inhibition of HMG-CoA, such as statins.
Secondly, we observed an anti-inflammatory and -apoptotic effect of KA in mice. As reported, at the early stage of renal injury, inflammatory response is a protective measure to attenuate injury and promote repair. When inflammatory infiltration is uncontrolled, abundant inflammatory cells recruited from circulation or resident cells accumulate in the tubular interstitium, producing massive amounts of proinflammatory molecules, such as IL-1β, IL-6, tumor necrosis factor-α, and dysregulated renal immunoreaction further deteriorates renal injury [21, 28]. Apoptosis is another cause accounting for renal injury. Proximal tubular epithelial cells are highly susceptible to IR injury, the necrotic or apoptotic tubular cells release harmful molecules, form cell casts, resulting in renal obstruction which further exacerbates renal injury [29]. NF-κB pathway was widely reported as transcription factors participating in renal inflammation and apoptosis by regulating the expression of some pro-inflammatory cytokines and chemokines genes [30,31,32,33,34,35]. It was shown that the MAPK signaling pathway, which includes ERK and p38 kinase, is activated by inflammatory stimuli and involved in regulating p65 NF-κB activation to promote inflammation [36]. In the present study we observed increased inflammatory T cells and neutrophils infiltration and tubular apoptosis in IR group as well as activation of NF-kB and MAPK signaling pathways. KA administration alleviated inflammatory infiltration of mature T cells and neutrophils in mice. Also, tubular apoptosis caused by IR was attenuated in KA group too, exhibiting the anti-apoptotic effect of KA treatment. Furthermore, NF-kB and MAPK signaling pathways were significantly inhibited in the IR + KA group too, as well as downstream molecule NFATc-1. We inferred that the decrease of inflammation and apoptosis brought by KA administration might have some links with an inhibited activation of NF-κB and MAPK pathways. As MAPK are catalytically inactive in base condition and their activation requires specialized enzyme to phosphorylate their activation loops. KA might inhibit MAPK pathway by the reduction of associated phosphatases. NF-κB is distributed in the cytoplasm and translocates into nucleus when stimulated, KA might inhibit the NF-κB pathway activation by reduction of stimulating factors or the receptor activator of NF-κB, or by the inhibition of MAPK pathway. Further studies to explore the detailed mechanisms of KA need to be done.
In addition, we explored the effect of KA intervention on CKD patients. Although numerous clinical trials on the effect of KA and LPD on CKD were conducted in past years, the results were controversial [37,38,39,40]. Some studies found that the potential benefits of KA and LPD were mainly focused on reducing waste products of protein metabolism, such as modification of creatine concentration. However, their influence on renal function protection or alleviating CKD progression required more careful and adequate attention [37, 41,42,43,44]. The uncertain effect of protein restriction on CKD protection might be related to the progressive and irreversible characteristic of CKD, especially CKD patients at stages 4 and 5. Most patients recruited in KA and LPD studies were with eGFR less than 30 mL/min, or in a pre-dialysis state [8, 45, 46]. Their worse physical conditions made KA and LPD intervention only an adjunctive therapy to main therapy such as glucocorticoids and immuno-biologicals. In the meantime, different pathological types showed inconsistent progression rates, for example CKD development in patients with membranous nephropathy exacerbated more rapidly than in patients with mesangial proliferative nephritis. Different degrees or types of pathological nephropathies could also influence the results of clinical study. Furthermore, as the study on KA and LPD was conducted over a long time period, often more than 6 months, the compliance of patients recruited was especially important. An unstrict administration of LPD could influence the final result of the clinical study.
Our clinical research collected data from CKD patients from 2015 to 2018 in Tongji hospital, which partially represent the population in the area of central China along the Yangtse River. Among all the CKD patients enrolled, 119 were at CKD stages 1 and 2, only 9 patients were prescribed with KA, compared to 323 at stages 3 to 5, with 148 prescribed with KA. Thus, only CKD patients at stages 3 to 5 were taken into consideration. Our next step was to analyze the renal function between KA and No-KA group. There was a great difference in baseline renal function indexes between KA and No-KA groups in patients at CKD stage 3, patients on KA appeared to be in worse renal conditions. We speculated this may be due to the deep relationship between eGFR and the accumulation of metabolic protein waste. The lower the eGFR was, the less efficiently protein wastes were excreted from our body and the greater the accumulation of waste and toxicities, the more urgent the need for a protein intake intervention. Thus, patients at stages 3 to 5 were more often prescribed KA treatment to reduce renal load than patients at earlier stages. Using early research as reference, we defined a reduction of > 50% eGFR as an end-point event. The patients with dialysis or renal transplantation at the time of enrolment were excluded. According to Kaplan–Meier analysis, the cumulative probability to reach the end-point was lower in the KA group than the No-KA group among patients at stages 4 and 5. Otherwise, according to our data the age of patients at CKD stages 3 to 5 peaked at middle age, mainly between 34 and 60 years old (Table 2). It presented a probable protective effect of KA on CKD middle aged patients at stages 4 and 5, which meant KA was a protective factor in staving CKD progression. Our study implied that a supplement of KA could defer the progression of CKD in patients at stages 4 and 5, which was consistent to the animal experiment results and previous research [2, 47,48,49].
CKD is a chronic progressive disease which could affect the whole-body system and finally irreversibly enter end-stage kidney stage [50, 51]. Finding a way to slow down the development of chronic renal injury was important for both family and social burdens. This study demonstrated the effect of KA on IR-induced murine CKD, by inhibiting the activation of NF-κB and MAPK pathways, thereby alleviating inflammatory infiltration and apoptosis, finally attenuating renal tubular injury and interstitial fibrosis. Meanwhile, a clinical trial was conducted to demonstrate its effect on delaying CKD progression. There were still some limitations to which we are still determining the best approach: (1) In animal experiments, there was no evidence to prove that the inhibition of NF-κB and MAPK directly attenuated inflammation and apoptosis. As NF-κB and MAPK pathways participate in several physical and pathological processes, inhibitors of these two pathways might be needed to demonstrate the direct relationship between the pathways and inflammation and apoptosis caused by KA. (2) In clinical trials, apart from KA, other medications were not strictly consistent. (3) Human inflammatory and apoptotic factors need to be measured for a further demonstration of anti-inflammatory effect from KA. More longitudinal studies are required to perfectly support the anti-inflammatory and -apoptotic effects of KA and LPD on CKD progression.
Conclusions
We demonstrated experimental evidence indicating that KA played a protective role in IR induced renal injury and fibrosis, via an inhibition of NF-κB and MAPK pathway to attenuate inflammation and apoptosis. In the meantime, we observed the therapeutic effect of KA on staving CKD progression in the clinical setting, mainly in end-stage patients. Our study provided theoretical support for KA treatment in alleviating the progression of ischemic renal injury into CKD.
Abbreviations
- CKD:
-
chronic kidney disease
- LPD:
-
low protein diet
- IR:
-
ischemia–reperfusion
- KA:
-
compound α-ketoacid tablets
- eGFR:
-
estimated glomerular filtration rate
- Scr:
-
serum creatine
- BUN:
-
blood urea nitrogen
- NF-κB:
-
nuclear factor-kappa B
- MAPK:
-
mitogen-activated protein kinase
- VLPD:
-
very low protein diet
- KLF15:
-
Kruppel-like factor-15
- LTL:
-
lotus tetragonolobus lectin
- Kim-1:
-
kidney injury molecule-1
- α-SMA:
-
alpha-smooth muscle actin
- PDGFR-β:
-
platelet-derived growth factor receptor
- NFATc-1:
-
nuclear factor of activated T cells c1
- ERK:
-
extracellular regulated protein kinases
References
Franklin SS, Gordon A, Kleeman CR, Maxwell MH. Use of a balanced low-protein diet in chronic renal failure. JAMA. 1967;202:477–84.
Rhee CM, Ahmadi SF, Kovesdy CP, Kalantar-Zadeh K. Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cachexia Sarcopenia Muscle. 2018;9:235–45.
Fouque D, Mitch WE. Low-protein diets in chronic kidney disease: are we finally reaching a consensus? Nephrol Dial Transplant. 2015;30:6–8.
Cianciaruso B, Bellizzi V, Capuano A, Bovi G, Nastasi A, Conte G, De Nicola L. Short-term effects of low protein-normal sodium diet on renal function in chronic renal failure. Kidney Int. 1994;45:852–60.
Richards P, Metcalfe-Gibson A, Ward EE, Wrong O, Houghton BJ. Utilisation of ammonia nitrogen for protein synthesis in man, and the effect of protein restriction and uraemia. Lancet. 1967;2:845–9.
Chang JH, Kim DK, Park JT, Kang EW, Yoo TH, Kim BS, Choi KH, Lee HY, Han DS, Shin SK. Influence of ketoanalogs supplementation on the progression in chronic kidney disease patients who had training on low-protein diet. Nephrology (Carlton). 2009;14:750–7.
Mou S, Li J, Yu Z, Wang Q, Ni Z. Keto acid-supplemented low-protein diet for treatment of adult patients with hepatitis B virus infection and chronic glomerulonephritis. J Int Med Res. 2013;41:129–37.
Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol. 2016;27:2164–76.
Frohling PT, Schmicker R, Vetter K, Kaschube I, Gotz KH, Jacopian M, Klinkman H. Conservative treatment with ketoacid and amino acid supplemented low-protein diets in chronic renal failure. Am J Clin Nutr. 1980;33:1667–72.
Zhang JY, Yin Y, Ni L, Long Q, You L, Zhang Q, Lin SY, Chen J. Low-protein diet supplemented with ketoacids ameliorates proteinuria in 3/4 nephrectomised rats by directly inhibiting the intrarenal renin-angiotensin system. Br J Nutr. 2016;116:1491–501.
Wang DT, Lu L, Shi Y, Geng ZB, Yin Y, Wang M, Wei LB. Supplementation of ketoacids contributes to the up-regulation of the Wnt7a/Akt/p70S6K pathway and the down-regulation of apoptotic and ubiquitin-proteasome systems in the muscle of 5/6 nephrectomised rats. Br J Nutr. 2014;111:1536–48.
Wang D, Wei L, Yang Y, Liu H. Dietary supplementation with ketoacids protects against CKD-induced oxidative damage and mitochondrial dysfunction in skeletal muscle of 5/6 nephrectomised rats. Skelet Muscle. 2018;8:18.
Gao X, Huang L, Grosjean F, Esposito V, Wu J, Fu L, Hu H, Tan J, He C, Gray S, et al. Low-protein diet supplemented with ketoacids reduces the severity of renal disease in 5/6 nephrectomized rats: a role for KLF15. Kidney Int. 2011;79:987–96.
Liu D, Wu M, Li L, Gao X, Yang B, Mei S, Fu L, Mei C. Low-protein diet supplemented with ketoacids delays the progression of diabetic nephropathy by inhibiting oxidative stress in the KKAy mice model. Br J Nutr. 2018;119:22–9.
Whalen H, Shiels P, Littlejohn M, Clancy M. A novel rodent model of severe renal ischemia reperfusion injury. Ren Fail. 2016;38:1694–701.
Smith SF, Hosgood SA, Nicholson ML. Ischemia-reperfusion injury in renal transplantation: 3 key signaling pathways in tubular epithelial cells. Kidney Int. 2019;95:50–6.
Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(535–543):1p–143p.
Qi W, Chen X, Poronnik P, Pollock CA. Transforming growth factor-beta/connective tissue growth factor axis in the kidney. Int J Biochem Cell Biol. 2008;40:9–13.
Wilson HM, Chettibi S, Jobin C, Walbaum D, Rees AJ, Kluth DC. Inhibition of macrophage nuclear factor-kappaB leads to a dominant anti-inflammatory phenotype that attenuates glomerular inflammation in vivo. Am J Pathol. 2005;167:27–37.
Meng XM, Nikolic-Paterson DJ, Lan HY. Inflammatory processes in renal fibrosis. Nat Rev Nephrol. 2014;10:493–503.
Gomez-Garre D, Largo R, Tejera N, Fortes J, Manzarbeitia F, Egido J. Activation of NF-kappaB in tubular epithelial cells of rats with intense proteinuria: role of angiotensin II and endothelin-1. Hypertension. 2001;37:1171–8.
Stambe C, Atkins RC, Hill PA, Nikolic-Paterson DJ. Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int. 2003;64:2121–32.
Stambe C, Nikolic-Paterson DJ, Hill PA, Dowling J, Atkins RC. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J Am Soc Nephrol. 2004;15:326–36.
Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290:F262–72.
Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol. 2016;310:F433–45.
Vaziri ND, Liang KH. Hepatic HMG-CoA reductase gene expression during the course of puromycin-induced nephrosis. Kidney Int. 1995;48:1979–85.
Rodriguez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001;59:1626–40.
Havasi A, Borkan SC. Apoptosis and acute kidney injury. Kidney Int. 2011;80:29–40.
Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NF-kappaB in renal inflammation. J Am Soc Nephrol. 2010;21:1254–62.
Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71.
Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–24.
Meng XM, Huang XR, Xiao J, Chen HY, Zhong X, Chung AC, Lan HY. Diverse roles of TGF-beta receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol. 2012;227:175–88.
Rodriguez-Iturbe B, Sindhu RK, Quiroz Y, Vaziri ND. Chronic exposure to low doses of lead results in renal infiltration of immune cells, NF-kappaB activation, and overexpression of tubulointerstitial angiotensin II. Antioxid Redox Signal. 2005;7:1269–74.
Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13.
Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–67.
Levey AS, Greene T, Sarnak MJ, Wang X, Beck GJ, Kusek JW, Collins AJ, Kopple JD. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease (MDRD) study. Am J Kidney Dis. 2006;48:879–88.
Cirillo M, Lombardi C, Chiricone D, De Santo NG, Zanchetti A, Bilancio G. Protein intake and kidney function in the middle-age population: contrast between cross-sectional and longitudinal data. Nephrol Dial Transplant. 2014;29:1733–40.
Lew QJ, Jafar TH, Koh HW, Jin A, Chow KY, Yuan JM, Koh WP. Red meat intake and risk of ESRD. J Am Soc Nephrol. 2017;28:304–12.
Metzger M, Yuan WL, Haymann JP, Flamant M, Houillier P, Thervet E, Boffa JJ, Vrtovsnik F, Froissart M, Bankir L, et al. Association of a low-protein diet with slower progression of CKD. Kidney Int Rep. 2018;3:105–14.
Shah AP, Kalantar-Zadeh K, Kopple JD. Is there a role for ketoacid supplements in the management of CKD? Am J Kidney Dis. 2015;65:659–73.
Cianciaruso B, Pota A, Bellizzi V, Di Giuseppe D, Di Micco L, Minutolo R, Pisani A, Sabbatini M, Ravani P. Effect of a low- versus moderate-protein diet on progression of CKD: follow-up of a randomized controlled trial. Am J Kidney Dis. 2009;54:1052–61.
Piccoli GB, Ferraresi M, Deagostini MC, Vigotti FN, Consiglio V, Scognamiglio S, Moro I, Clari R, Fassio F, Biolcati M, Porpiglia F. Vegetarian low-protein diets supplemented with keto analogues: a niche for the few or an option for many? Nephrol Dial Transplant. 2013;28:2295–305.
Piccoli GB, Deagostini MC, Vigotti FN, Ferraresi M, Moro I, Consiglio V, Scognamiglio S, Mongilardi E, Clari R, Aroasio E, et al. Which low-protein diet for which CKD patient? An observational, personalized approach. Nutrition. 2014;30:992–9.
Chauveau P, Couzi L, Vendrely B, de Precigout V, Combe C, Fouque D, Aparicio M. Long-term outcome on renal replacement therapy in patients who previously received a keto acid-supplemented very-low-protein diet. Am J Clin Nutr. 2009;90:969–74.
Piccoli GB, Ventrella F, Capizzi I, Vigotti FN, Mongilardi E, Grassi G, Loi V, Cabiddu G, Avagnina P, Versino E. Low-protein diets in diabetic chronic kidney disease (CKD) patients: are they feasible and worth the effort? Nutrients. 2016;8:649.
Fouque D, Wang P, Laville M, Boissel JP. Low protein diets delay end-stage renal disease in non-diabetic adults with chronic renal failure. Nephrol Dial Transplant. 2000;15:1986–92.
Piccoli GB, Nazha M, Capizzi I, Vigotti FN, Mongilardi E, Bilocati M, Avagnina P, Versino E. Patient survival and costs on moderately restricted low-protein diets in advanced CKD: equivalent survival at lower costs? Nutrients. 2016;8:758.
Yen CL, Tu KH, Lin MS, Chang SW, Fan PC, Hsiao CC, Chen CY, Hsu HH, Tian YC, Chang CH. Does a supplemental low-protein diet decrease mortality and adverse events after commencing dialysis? A nationwide cohort study. Nutrients. 2018;10:1035.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FD. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS ONE. 2016;11:e158765.
Allinovi M, De Chiara L, Angelotti ML, Becherucci F, Romagnani P. Anti-fibrotic treatments: a review of clinical evidence. Matrix Biol. 2018;68–69:333–54.
Zhu F, Chong LSO, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, et al. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8:70707–26.
Authors’ contributions
RZ and YY designed the study; HZ, MW, HX and HZ carried out experiments. Data analysis was carried out by MW, Li Li and FZ. Reagents were contributed by OLCLS and HZ, and the mice were managed by HX, and ZZ. Clinical patient information was collected by WL, KQ and CZ and the paper was written by MW, OLCLS and HG. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the department of nephrology of Tongji Hospital for their help on the clinical aspects of our project.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All relevant data and materials are included in this published article.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All experiments were approved by the Animal Care and Use Committee of Tongji Hospital.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81570669, 81770684, 81570615, and 81770681) and the Wuhan health and family planning commission (Grant 2016whzqnyxggrc2).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, M., Xu, H., Chong Lee Shin, O.LS. et al. Compound α-keto acid tablet supplementation alleviates chronic kidney disease progression via inhibition of the NF-kB and MAPK pathways. J Transl Med 17, 122 (2019). https://doi.org/10.1186/s12967-019-1856-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-019-1856-9