Abstract
Background
Telocytes (TCs) are newly identified interstitial cells that participate in tissue protection and repair. The present study investigated the mechanisms underlying the protective effect of TCs in a mouse model of respiratory distress.
Methods
The mouse model of acute respiratory distress syndrome (ARDS) was established by intratracheal instillation of lipopolysaccharide (LPS). After instillation of TCs culture medium, lung injury was assessed, and angiogenesis markers, including CD31 and endothelial nitric oxide synthase (eNOS), were detected by immunofluorescence. Bioinformatics analysis was used to screen significantly differentially expressed microRNAs (miRNAs) in cultured TCs stimulated with LPS, and the regulation of downstream angiogenesis genes by these miRNAs was analysed and verified. PI3K subunits and pathways were evaluated by using a PI3K p110α inhibitor to study the involved mechanisms.
Results
In ARDS mice, instillation of TCs culture medium ameliorated LPS-induced inflammation and lung injury and increased the protein levels of CD31 and eNOS in the injured lungs. A total of 7 miRNAs and 1899 mRNAs were differentially regulated in TCs stimulated with LPS. Functional prediction analysis showed that the differentially expressed mRNAs were enriched in angiogenesis-related processes, which were highly correlated with miR-21a-3p. Culture medium from TCs with miR-21a-3p inhibition failed to promote angiogenesis in mouse models of LPS-induced ARDS. In cultured TCs, LPS stimulation upregulated the expression of miR-21a-3p, which further targeted the transcription factor E2F8 and decreased Notch2 protein expression. TCs culture medium enhanced hemangioendothelioma endothelial cells (EOMA cells) proliferation, which was blocked by the miR-21a-3p inhibitor. The PI3K p110α inhibitor decreased vascular endothelial growth factor levels in LPS-stimulated TCs and reversed the enhancing effect of TCs culture medium on EOMA cells proliferation.
Conclusions
TCs exerted protective effects under inflammatory conditions by promoting angiogenesis via miR-21a-3p. The PI3K p110α subunit and transcriptional factor E2F8 could be involved in this process.
Similar content being viewed by others
Introduction
Acute respiratory distress syndrome (ARDS) is a clinical syndrome characterised by acute progression of respiratory failure. According to an international multi-centre research, the prevalence of ARDS was 10.4% of ICU admissions [1]. Inflammatory responses destroy underlying vascular endothelial cells and respiratory epithelial cells and impair the lungs’ ability to exchange oxygen and carbon dioxide [2]. Therefore, decreasing inflammation and accelerating blood vessel repair are two key factors in the prevention and treatment of ARDS. Since its severity and lack of effective pharmacologic treatments [3], it is of great significance to explore novel therapeutic strategies for ARDS. Recently, cell therapy have been shown to have promising therapeutic potential. Mesenchymal stem cells ameliorated ARDS due to paracrine mechanism [4].
Telocytes (TCs) are newly identified mesenchymal cells that play a role in providing nutrition to surrounding cells by cell–cell communication and have post-injury repair and regeneration functions [5,6,7]. TCs contribute to angiogenesis within the myocardium [8]. Transplantation of cardiac TCs promotes post ischaemic myocardial repair [9]. Pulmonary TCs also assist with angiogenesis since they participate in forming the structure of the air–blood barrier [10]. Intratracheal administration of activated TCs has been reported to alleviate ventilator-induced lung injury in a mouse model by releasing angiogenic factors [11]. However, the underlying mechanism remains unclear.
Class I Phosphoinositide-3-kinases (PI3Ks) or the four subtypes of catalytic subunit—p110α, p110β, p110γ and p110δ—are expressed in all mammalian cells. The catalytic subunits bind to p85 regulatory subunits, activate receptor tyrosine kinases (RTKs), and transmit a variety of cell surface receptor signals, such as those from the epidermal growth factor receptor (EGFR) or fibroblast growth factor receptor (FGFR), to promote cell growth [12]. The PI3K subunits p110α and p110δ were demonstrated to be associated with tissue repair; however, this function is mediated by different mechanisms. The activity of PI3K p110α can be enhanced by tyrosine kinase ligands, such as vascular endothelial growth factor (VEGF) A, and can induce angiogenesis and vascular remodelling [13]. Moreover, p110α regulates endothelial cell migration through the small GTPase RhoA, mediated by PI3KCG, a gene encoding a p110γ subunit, which has a protective effect on hypoxic-reoxygenated cardiomyocytes mediated by activation of the PI3K/AKT signalling pathway and inhibition of apoptosis [14]. PI3K (p110δ)/AKT/mammalian target of rapamycin (mTOR) signalling pathway mediates interferon-γ (IFN-γ) induced airway epithelial cell growth and proliferation through interaction with CEACAM1 [15].
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate the expression of target genes via posttranscriptional degradation of mRNA and/or translational inhibition of protein expression. MiR-135a can influence cell proliferation, migration, invasion, apoptosis and tumour angiogenesis through the IGF-1/PI3K/AKT signalling pathway in non-small cell lung cancer (NSCLC) [16]. Mature miR-21a-5p was found to be secreted by lipopolysaccharide (LPS)-activated macrophages in small vesicles, which were endocytosed and internalised by renal fibroblasts, thereby promoting the expression of fibrosis and inflammation markers in a mouse model of chronic renal allograft dysfunction (CAD) in allogeneic kidney transplantation [17]. Antagonism of miR-21a-5p ameliorated CAD in mouse model following kidney transplantation [17]. In patients with renal allograft, elevation of urinary [18] and plasma [19] miR-21 level was correlated with interstitial fibrosis and tubular atrophy.
The TCs line was established by transfection with simian vacuolating virus 40 (SV40) and identified to maintain TCs morphology and immune characteristics [20]. TCs proliferation was demonstrated to be regulated by transforming growth factor-β (TGF-β) and mediated by the PI3K p110α subunit and the PI3K/AKT/mTOR signalling pathway [21]. The present study was designed to investigate the underlying protective effect of TCs in a mouse model of respiratory distress. Bioinformatics approaches were applied to analyse gene expression profiles in TCs challenged with LPS. Particular attention was devoted to the angiogenesis-related process. The protective mechanisms mediated by the PI3K subunit in TCs were further examined in hemangioendothelioma endothelial cells (EOMA cells) in vitro. The current study presents the theoretical bases of an alternative new potential therapeutic strategy for ARDS.
Methods
Animal models
Eight-week-old male C57BL/6 mice, 22 to 25 g, were purchased from Shanghai Jiesijie Company (Shanghai, China). Mice were randomly divided into four groups: Control, ARDS, ARDS with negative control (NC) TCs treatment, and ARDS with miR-21a-3p inhibited TCs treatment. Under anaesthesia (60 mg/kg sodium pentobarbital, Sinopharm Chemical Reagent Co. Shanghai, China), mice were intratracheally instilled with phosphate-buffered saline or LPS (5 mg/kg, Sigma, Germany) via 20-gauge catheters. Mice in the ARDS treatment groups were also instilled with 20 μL of TCs culture medium from TCs treated with the NC or miR-21a-3p inhibitor in the presence of LPS. Twenty-four hours later, animals were sacrificed, and the lungs were collected.
The study protocol was approved by the Animal Ethics Committee of Zhongshan Hospital, Fudan University.
TCs
Mouse primary pulmonary TCs were a kind gift from Dr. Dongli Song. TCs were cultured in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F12, Hyclone, Boston, MA) supplemented with 5% foetal bovine serum (FBS, Cellsera, Australia). Experiments with LPS (0.1 μg/mL) were performed in DMEM/F12 without FBS. TCs culture medium was collected from culture dishes after LPS stimulation for 48 h. The p110α inhibitor HS-173 (Selleck, Shanghai, China) was applied 2 h before LPS stimulation.
MiRNA transfection
Both the miR-21a-3p inhibitor and NC were purchased from China Ribobio (Ribobio, Guangzhou, China). TCs were transfected with the miR-21a-3p inhibitor and NC at a final concentration of 50 nmol/L using a lipofectamine RNAiMAX transfection system (ThermoFisher Scientific, Carlsbad, CA) according to the manufacturer’s protocol. Cells were incubated with siRNA in serum-free and antibiotic-free medium for 6 h and then in normal growth medium for another 24 h before the experiments were performed.
Gene expression profiling analysis
Gene expression profiling analysis of both miRNA and mRNA were performed with Agilent Microarray Scanner (Cat # G2565CA, Agilent technologies, Santa Clara, CA). The data were normalised with the AgiMicroRna package [22]. The gene expression files were analysed with R-3.4.1 software. Differentially expressed genes (DEGs) were defined as those with an adjusted P-value of less than 0.05. DEGs were further analysed with the limma package [23]. Heat maps were generated with the ggplot2 package [24].
The online databases miRWalk [25] and TargetScan [26] were used to screen potential miRNA target genes. Overlapping genes in the two databases were selected for further analysis. The online database STRING [27] and the Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.8 [28] were used to analyse gene function. The relationship between DEGs and miRNAs was further visualised with Cytoscape 3.7.1 [29].
Quantification of mRNA and miRNA
Total RNA was extracted from cultured TCs with TRIzol (Takara, Shiga, Japan) according to the provided instructions. MiRNAs were reverse transcribed with a Bulge-Loop miRNA qRT-PCR Starter Kit (Ribobio, Guangzhou, China), and mRNAs were reverse transcribed to complementary DNA (cDNA) with a PrimeScript RT Reagent Kit with gDNA Eraser (Takara, Shiga, Japan). The expression levels of miR-21a-3p, miR-221-5p and mRNAs were measured by quantitative real-time polymerase chain reaction (qPCR) on a Bio-Rad IQ5 real-time PCR instrument, with U6 and GAPDH used as the housekeeping genes for miRNAs and mRNAs, respectively. MiRNA PCR was performed with the Bulge-Loop miRNA qRT-PCR Starter Kit, Bulge-Loop mmu-miR-21a-3p Primer Set and Bulge-Loop mmu-miR-221-5p Primer Set (Ribobio, Guangzhou, China). MRNA primers were synthesised by Sangon (Shanghai, China). The following mouse-specific primers were used: GAPDH sense primer: 5′-GTTCAACGGCACAGTCAAG-3′, antisense primer: 5′-GCCAGTAGACTCCACGACAT-3′; E2F8 sense primer: 5′-CTGTTT GCACGAACACTTATCAG-3′, antisense primer: 5′-GTACCGCGCTAGGAATTTGTG-3′; Acvrl1 sense primer: 5′-TGATTCCTGTTGCCGGCCT-3′, antisense primer: 5′-CAGTGTGGGCTCTCACAAGT-3′; Rbpj sense primer: 5′-TGGCGAGAGTTTGTGGAAGA-3′, antisense primer: 5′-AGCACTGTTTGATCCCCTCG-3′; Notch1 sense primer: 5′-TGTGGCTTCCTTCTACTGCG-3′, antisense primer: 5′-CTTTGCCGTTGACAGGGTTG-3′; Flt1 sense primer: 5′-GTGAGCACTGCGGCAAAAAG-3′, antisense primer: 5′-ACTCATTTTGGGAGGAGCGT -3′; EFNB2 sense primer: 5′-CGAGGTGGCAACAACAATGG-3′, antisense primer: 5′-ATAGTCCCCGCTGACCTTCT -3′; Thbs1 sense primer: 5′-CTGCCAATCATAACCAGCG-3′, antisense primer: 5′-TTCGTTAAAGGCCGAGTGCT-3′; EPAS1 sense primer: 5′-CTGAGGAAGGAGAAATCCCGT-3′, antisense primer: 5′-TGTGTCCGAAGGAAGCTGATG-3′; hypoxia inducible factor-1α (HIF-1α) sense primer: 5′-ACCTTCATCGGAAACTCCAAAG-3′, antisense primer: 5′-CTGTTGGCTGGGAAAAGTTAGG-3′; PIK3CA sense primer: 5′-CCACGACCATCTTCGGGTG-3′, antisense primer: 5′-ACGGAGGCATTCTAAAGTCACTA-3′; PIK3CB sense primer: 5′-CTATGGCAGACAACCTTGACAT-3′, antisense primer: 5′-CTTCCCGAGGTACTTCCAACT-3′; PIK3CD sense primer: 5′-GTAAACGACTTCCGCACTAAGA-3′, antisense primer: 5′-GCTGACACGCAATAAGCCG-3′; and VEGF sense primer: 5′-GTACCTCCACCATGCCAAGT-3′, antisense primer: 5′-TCCTATGTGCTGGCTTTGGT-3′.
Western blotting
Total protein was extracted from cultured TCs with lysis buffer (150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L NaF, 1 mmol/L dithiothreitol, 10 μg/μL aprotinin, 10 μg/μL leupeptin, 0.1 mmol/L Na3VO4, 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), and 0.5% NP-40). Protein extracts (20 μg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Merck Millipore, Darmstadt, Germany). After blocking with 5% non-fat milk/Tris-buffered saline containing 0.1% Tween 20 at room temperature for one hour, membranes were incubated with primary antibodies [specific for GAPDH (60004-1-Ig, Proteintech, Wuhan, China), E2F8 (ab109596, Abcam, Cambridge, UK), Delta-like 4 (DLL4)(ab7280, Abcam, Cambridge, UK), Notch1 (sc-373891, Santa Cruz, Dallas, TX), Notch2 (sc-5545, Santa Cruz, Dallas, TX), Notch4 (sc-5594, Santa Cruz, Dallas, TX), phosphatase and tensin homolog deleted on chromosome ten (PTEN)(ab32199, Abcam, Cambridge, UK), PI3K (4257T, CST, Boston, MA), p-PI3K (4228T, CST, Boston, MA), mTOR (2983T, CST, Boston, MA), p-mTOR (5536T, CST, Boston, MA), AKT (9272S, CST, Boston, MA), p-AKT (9271S, CST, Boston, MA), and p110α (4249T, CST, Boston, MA)] overnight at 4 °C. Protein expression levels were normalised to those of GAPDH with ImageJ (NIH, Bethesda, MD).
EOMA cells proliferation assay
EOMA cells proliferation was assessed with a colorimetric assay—Cell Counting Kit-8 (CCK8, Yeasen, Shanghai, China)—following the manufacturer’s protocol. Approximately 4000 EOMA cells/well were seeded in a 96-well plate. After adhesion, EOMA cells were incubated for 24 h with culture medium from TCs transfected with the miR-21a-3p inhibitor or NC in the presence of LPS.
Dual luciferase assay
The pGL3 reporter vector (Promega, Madison, WI) was used to generate the plasmids pGL3-WT-E2F8-3′-UTR and pGL3-Mut-E2F8-3′-UTR. Human embryonic kidney cells were co-transfected with pGL3-E2F8-3′-UTR (WT or Mut) and the miR-21a-3p mimic or NC with Lipofectamine 2000 reagent (ThermoFisher Scientific, Carlsbad, CA). After incubation for 24 h, luciferase activity was assessed by the Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer’s protocol.
Enzyme-linked immunosorbent assay (ELISA)
The concentration of VEGF in the TCs culture medium was measured by a commercial VEGF ELISA kit (Westang, Shanghai, China) according to the manufacturer’s protocol.
Dynamic real-time cell observation
Live observation of EOMA cells was performed with a Cell-IQ cell culture platform (Chip-Man Technologies, Tampere, Finland) equipped with a phase contrast microscope (Nikon CFI Achromat phase contrast objective with 10 magnification) and a camera (Nikon, Fukasawa, Japan). The equipment was controlled by Imagen software (Chip-Man Technologies). Each group contained 16 replicates of visual fields. Images were acquired at 1-h intervals for 48 h.
Tissue preparation and immunofluorescence examination
Lung tissues were fixed with 10% formalin solution and embedded in paraffin. Each tissue was sectioned at 5 μm and stained with haematoxylin–eosin (HE, Beyotime, Shanghai, China) according to the manufacturer’s protocol. For immunofluorescence staining, an antigen retrieval protocol was carried out with incubation in 0.3% H2O2 for 30 min and heating to boiling in a microwave in citrate buffer for 10 min. After blocking with 5% goat serum in Tris-buffered saline, sections were incubated with diluted primary antibodies [CD31 (1:500, ab24590, Abcam, Cambridge, UK), endothelial nitric oxide synthase (eNOS) (1:500, Cat610296, BD Biotechnology, San Jose, CA)] overnight at 4 °C and then with secondary antibodies and 4′,6-diamidino-2-phenylindole (DAPI), separately.
Statistical analysis
Data are expressed as the means ± SDs and were analysed by one-way analysis of variance (ANOVA) and Tukey’s multiple comparisons test. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed with GraphPad Prism 7.04 (GraphPad, San Diego, CA).
Results
Protective effects of TCs in ARDS
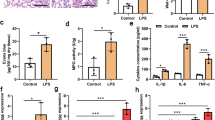
The ability for TCs protection was first estimated in ARDS mouse models. LPS stimulation caused inflammatory infiltration, alveolar wall widening, and vessel destruction (Fig. 1a). The production of inflammatory cytokines was elevated in ARDS mice (Fig. 1b). Since substances, including molecules and exosomes, released by TCs could be important factors affecting adjacent cells, the effect of TCs culture medium was assessed. Instillation of TCs culture medium reduced the inflammatory infiltration, reduced the alveolar interstitial width and decreased the levels of inflammatory cytokines. Bio-behaviours of TCs were recorded by Cell-IQ to show the typical morphology of cultured cells (Additional file 1: Figure S1).
Protective effects of TCs in ARDS mouse models. a HE staining of mice lung tissue in lungs of control mice (Control), ARDS mice (LPS), ARDS mice treated with TCs supernatant (LPS/TC), and ARDS mice treated with miR-21a-3p inhibited TCs supernatant [LPS/TC (miR-21 inhibited)]. b The mRNA expression of inflammatory cytokines in mice lungs in the above four groups. *P < 0.05 vs Control, #P < 0.05 vs LPS, **P < 0.05 vs LPS/TCs, n = 6. IL-1β interleukin-1β, IL-6 interleukin-6, TNF-α tumour necrosis factor-α
TCs promoted angiogenesis in ARDS
As angiogenesis is essential in tissue repair, the induction of angiogenic factors in TCs after stimulation with LPS was assessed. In ARDS mice, the expression of the angiogenesis-related marker CD31 and eNOS was downregulated. However, an increase in CD31 and eNOS expression was observed in the WT TCs treatment group but not in the group treated with medium from TCs with miR-21a-3p inhibition (Fig. 2).
TCs promoted angiogenesis in ARDS mouse models. The expression of CD31 and eNOS in lungs of control mice (Control), ARDS mice (LPS), ARDS mice treated with TCs supernatant (LPS/TC), and ARDS mice treated with miR-21a-3p inhibited TCs supernatant [LPS/TC (miR-21 inhibited)] was shown in green fluorescence, dapi was blue. *P < 0.05 vs Control, #P < 0.05 vs LPS, **P < 0.05 vs LPS/TCs, n = 6. Original magnification: 200x
MiRNA and mRNA profiles in LPS-stimulated TCs
To identify the critical miRNAs in the regulation of angiogenesis by TCs, miRNA and mRNA profiles were generated, and the relationship of differentially expressed miRNAs with downstream angiogenesis factor-associated mRNAs were analysed in LPS-stimulated TCs. In LPS-stimulated TCs, six miRNAs, including miR-155-5p, miR-21a-3p, miR-5100, miR-221-5p, miR-7a-3p and miR-146a-5p, were upregulated, and one miRNA (miR-188-5p) was downregulated with an absolute fold change > 2 (Fig. 3a and Table 1). By referring these results to two online databases (miRWalk and TargetScan), 4368 target genes were predicted to be downstream targets of the differentially expressed miRNAs.
Differentially expressed genes in TCs with LPS treatment. a Heat map of differential expressed miRNAs in cultured TCs stimulated with LPS. b Heat map of differential expressed genes in cultured TCs stimulated with LPS. c Relationship between differential expressed miRNAs and their differential expressed target mRNAs. Yellow indicated miRNAs, green indicated downregulated mRNAs, and red indicated upregulated mRNAs. d Interaction of the angiogenesis-related proteins in STRING. Different colours indicated the involvement of proteins in different processes. Red indicated angiogenesis, pink indicated blood vessel morphogenesis, purple indicated vasculature development, brown indicated blood vessel remodelling, blue indicated sprouting angiogenesis, cyan indicated venous blood vessel sprouting, orange indicated venous blood vessel morphogenesis, green indicated regulation of angiogenesis, and yellow indicated positive regulation of angiogenesis. e Angiogenesis related miRNAs and their downstream genes. Red indicated upregulated miRNAs, green indicated downregulated miRNAs, yellow indicated genes that were enriched in more than three processes in STRING, blue indicated other mRNAs

In total, 1899 mRNAs—901 upregulated and 998 downregulated—were differentially expressed in TCs after LPS stimulation (Fig. 3b and Table 2). A total of 519 genes overlapped with those from the online prediction (Fig. 3c).

Pulmonary TCs were reported to promote angiogenesis in a mouse model of ARDS [11]; thus, particular attention was devoted to angiogenesis in the gene ontology (GO) functional analysis. According to the DAVID online database, 28 DEGs were enriched in the processes of blood vessel formation, angiogenesis, blood vessel morphogenesis, blood vessel remodelling, and sprouting angiogenesis. For further analysis, the DEGs were enriched in the STRING database. According to the STRING database, the DEGs were enriched in 9 angiogenesis-related processes: angiogenesis, blood vessel morphogenesis, vasculature development, blood vessel remodelling, sprouting angiogenesis, venous blood vessel sprouting, venous blood vessel morphogenesis, regulation of angiogenesis, and positive regulation of angiogenesis (Fig. 3d). As most genes participated in at least 3 biological processes, those involved in more than three processes—i.e. E2F8, Notch1, EPAS1, Rbpj, Flt1, ACVRL1, EFNB2 and Thbs1—were selected for further research. MiR-21a-3p, miR-221-5p, miR-146a-5p and miR-188-5p regulated these 8 genes (Fig. 3e).
Validation of miRNAs and their target mRNAs in TCs
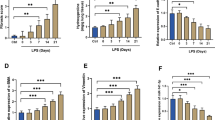
We next assessed the mRNA levels of angiogenesis factors. The mRNA expression of E2F8, Notch1, EPAS1, Rbpj, Flt1, ACVRL1, EFNB2 and Thbs1 was measured in TCs after LPS stimulation. After LPS stimulation, E2F8, EFNB2, and EPAS1 were significantly downregulated, while Flt1 was upregulated. Given that miRNAs usually negatively regulate downstream genes, E2F8, EFNB2, and EPAS1 were further studied. LPS stimulation significantly increased miR-21a-3p and miR-221-5p expression in TCs compared with that in cells under control conditions. To clarify the relationship between miRNAs and mRNAs, miRNA inhibitors were applied. MiR-221-5p inhibition restored the expression of EPAS1 but not EFNB2, and miR-21a-3p inhibition restored the expression of E2F8 but not EPAS1. MiR-21 had been reported to increase proliferation, migration and tube formation of Human Umbilical Vein Endothelial Cells (HUVECs) and induce angiogenesis by directly targeting PTEN [30, 31]. Moreover, miR-21a-3p and its downstream target E2F8 were further studied. After 24 h, the protein expression of E2F8 was decreased in TCs challenged with LPS and was restored by inhibition of miR-21a-3p. The dual luciferase reporter assay indicated that E2F8 was the direct target of miR-21a-3p (Fig. 4).
Expression of angiogenesis-related genes in TCs with LPS treatment. a mRNA levels of E2F8, Notch1, EPAS1, Rbpj, Flt1, ACVRL1, EFNB2, Thbs1, FLT1, miR-21a-3p and miR-221-5p in TCs treated with LPS and/or miR-21a-3p inhibitor or miR-221-5p inhibitor. *P < 0.05 vs Control, #P < 0.05 vs LPS. b Protein levels of E2F8 in TCs treated with LPS and/or miR-21a-3p inhibitor. *P < 0.05 vs Control, #P < 0.05 vs LPS. c MiR-21a-3p led to a significant reduction of the luciferase activity of reporter with the wildtype 3′ UTR but not that of the mutant reporter. *P < 0.05 vs E2F8-WT/miRNA miR-21a-3p mimic. n = 6
MiR-21a-3p regulated angiogenesis under inflammatory conditions
The transcription factors E2F7/8 were reported to regulate vessel branching via DLL4-Notch approaches [32] or HIF-1α/VEGFA signalling [33]. In the present study, LPS stimulation reduced the protein expression of Notch 2 but not Notch 1, Notch 4 or DLL4. Inhibition of miR-21a-3p restored Notch 2 protein expression in TCs in the presence of LPS. LPS did not affect HIF-1α expression. However, LPS increased the expression of VEGFA at the mRNA level, and this increase was reversed by miR-21a-3p inhibition in cultured TCs (Fig. 5).
PI3K signalling might participate in angiogenesis
PI3K, especially the Class I catalytic isoforms, plays an important role in angiogenesis. To study the mechanisms underlying the effect of miR-21a-3p in TCs on angiogenesis induction, PI3K subunit expression was first examined. The mRNA levels of the Class I PI3K isoforms PIK3CA, PIK3CB, and PIK3CD did not significantly change with LPS stimulation. However, the protein level of p110α in TCs was significantly increased with LPS stimulation and decreased with miR-21a-3p inhibitor co-treatment. The PI3K signalling molecules AKT, mTOR, and PTEN were unaffected by either LPS or miR-21a-3p. These results indicated that PI3K signalling might participate in angiogenesis via the p110α isoform (Fig. 6).
Expression of PI3K signalling in TCs induced with LPS. a mRNA levels of PIK3CA, PIK3CB, and PIK3CD in TCs treated with LPS and/or miR-21a-3p inhibitor. b, c Protein levels of p110α, p-PI3K, PI3K, p-mTOR, mTOR, PTEN, p-AKT, AKT in TCs treated with LPS and/or miR-21a-3p inhibitor. *P < 0.05 vs Control, #P < 0.05 vs LPS, n = 6
MiR-21a-3p and p110α in TCs promoted the proliferation of EOMA cells
The proliferation of EOMA cells was then estimated after co-culture with TCs pre-treated with the miR-21a-3p or PI3K p110α inhibitor. Culture medium from TCs stimulated with LPS promoted EOMA cells proliferation, as determined by the CCK8 assay. Compared with medium from NC TCs, culture medium from TCs with miR-21a-3p inhibition significantly reduced EOMA cells proliferation (Fig. 7a). The effect of p110α was examined by dynamic real-time cell observation. The proliferation assay indicated that EOMA cells proliferation decreased with LPS stimulation but was restored by co-culture with TCs. Inhibition of miR-21a-3p or p110α (with its inhibitor HS-173) weakened the protective effect of TCs (Fig. 7b, d). The scratch assay showed similar results (Fig. 7c, e). VEGF protein expression was significantly elevated with LPS stimulation, and this increase was reversed by inhibition of either miR-21a-3p or p110α (Fig. 7f). The results above indicated that VEGF is regulated by both miR-21a-3p and p110α.
MiR-21a-3p and p110α mediated the promotion of TCs on EOMA proliferation induced by LPS. a Cells proliferation rate of EOMA treated with LPS and/or TCs and miR-21a-3p inhibitor measured by CCK8 assay. *P < 0.05 vs Control, #P < 0.05 vs LPS/TC. b, d Cell proliferation of EOMA treated with LPS and/or TCs and miR-21a-3p inhibitor or p110α inhibitor measured by Cell-IQ. *P < 0.05 vs Control, #P < 0.05 vs LPS, **P < 0.05 vs LPS/TC, n = 6. c, e Cell movement of EOMA treated with LPS and/or TCs and miR-21a-3p inhibitor or p110α inhibitor measured by Cell-IQ. f VEGFA levels secreted by TCs treated with LPS and/or miR-21a-3p inhibitor or p110α inhibitor measured by ELISA. *P < 0.05 vs Control, #P < 0.05 vs LPS, n = 6
Discussion
This study reports that TCs culture medium can alleviate ARDS in mice probably via angiogenesis-associated factors regulated by miR-21a-3p. TCs exposed to LPS exhibited increased miR-21a-3p expression and VEGF production, which further promoted vascular endothelial cell proliferation. The protective effects of TCs mediated by miR-21a-3p might be regulated through PI3K (p110α)/AKT/mTOR signalling and the expression levels of the downstream targets E2F8 and Notch 2 (Fig. 8).
Endotoxin-induced ARDS has been reported to affect both respiratory epithelial cells and the underlying vascular endothelial cells [34]. In the present study, LPS stimulation induced severe vascular damage in the lungs, as shown by the reduced levels of CD31 and eNOS. TCs are distinct from mesenchymal stem cells and fibroblasts and have been reported to have specific roles in cell signalling, tissue remodelling and angiogenesis [35]. In the present study, TCs culture medium exhibited great potential to reverse the angiogenic signalling that was reduced by LPS-induced inflammation, supporting the observation that TCs alleviate LPS-induced lung injury in mice by releasing angiogenic factors [11].
Non-coding miRNAs are involved in several pathological processes, including angiogenesis [36, 37]. MiR-221-5p [38], miR-146a-5p [39], and miR-21a-3p [40,41,42] are reported to be associated with the angiogenesis process. MiR-21a-3p and miR-221-5p were demonstrated to be involved in the promotion of angiogenesis in TCs. As miR-21a-3p was more frequently reported on angiogenesis, it was further studied. MiR-21a-3p knockdown in TCs reduced CD31 and eNOS expression in the lungs of ARDS mice in vivo. MiR-21a-3p exerts its protective effects on injury repair by inducing angiogenesis-associated signalling pathways. For instance, miR-21a-3p activates the AKT pathway and increases matrix metalloproteinase-2 (MMP-2) expression to reduce the extent of the infarcted region in heart ischaemia/reperfusion injury [41], inhibits PTEN and sprouty homolog 1 (SPRY1) to heal soft tissue wounds [40], and upregulates VEGF and activates the Ang-1/Tie-2 axis in traumatic brain injury [42]. In the current study, the p110α isoform in PI3K/AKT/mTOR signalling pathway was demonstrated to be involved in miR-21a-3p-mediated angiogenic factor induction in TCs. However, the alteration of other protein levels and HIF-1α in TCs treated with LPS and the miR-21a-3p inhibitor indicated that more complex signalling pathways were involved in regulating the angiogenic function of TCs. Culture medium from LPS-induced TCs promoted EOMA cells proliferation in vitro, accompanied by elevated levels of VEGF mRNA and secretion, which further demonstrated that the functional miR-21a-3p was generated by TCs. These data support the hypothesis that miR-21a-3p plays a role in angiogenesis and profoundly demonstrate the mechanisms mediated by PI3K p110α.
The E2F family was first reported to induce cell proliferation [43], and E2F family members are essential transcriptional regulators of cell cycle progression [44], as well as apoptosis, metabolism and angiogenesis [45]. E2F8 is an atypical transcriptional repressor in the E2F family since it contains domains that differ from the canonical domains [46]. By forming homodimers or heterodimers with E2F7, E2F8 reduces the excessive and destructive activation of E2F1 [47]. However, reports of E2F8 in angiogenesis in the literature are controversial. E2F7/8 has been reported to positively regulate the formation of blood vessels during embryonic development via HIF-1α/VEGFA signalling [33]. On the other hand, E2F7/8 suppresses tumour angiogenesis via the induction of DLL4 [32]. In the present study, E2F8 expression was reduced after LPS stimulation in TCs and restored with miR-21a-3p inhibition, indicating that E2F8 plays a negative role in angiogenesis under inflammatory conditions.
The Notch family, which contains several receptors and ligands, is fundamental in the regulation of blood vessel branching [48]. DLL4, a Notch ligand, has an inhibitory function in blood vessel branching [49] that is compromised by Jagged 1 activation [50]. Notch1 positively regulates angiogenesis [51], while Notch2 negatively regulates cell proliferation [52] and angiogenesis [53]. In the initial stage of angiogenesis, inhibition of Notch 2 promotes vascular endothelial cell proliferation, while activation of Notch 2 reduces endothelial cell responses to VEGF [54, 55]. In the present study, Notch2 expression was mediated by miR-21a-3p. However, the relationship between the transcription factor E2F8 and Notch2 was not illustrated. Further experiments should be conducted to confirm the signalling pathway of E2F8/Notch2 in angiogenesis.
Conclusion
TCs have been reported to be important in tissue repair and healing processes. Via mouse models, bioinformatics approaches and molecular biological methods, the present study shows that activated TCs promote endothelial regeneration and angiogenesis through miR-21a-3p-PI3K (p110α)/AKT/mTOR signalling and further demonstrates the key roles of VEGF in TCs. The E2F8/Notch2 signalling might also participates in this process. These findings shed light on miR-21a-3p in TCs as a new therapeutic target for vessel protection.
Availability of data and materials
Not applicable.
Abbreviations
- ARDS:
-
acute respiratory distress syndrome
- TC:
-
telocyte
- PI3K:
-
phosphoinositide-3-kinase
- RTK:
-
receptor tyrosine kinase
- EGFR:
-
epidermal growth factor receptor
- FGFR:
-
fibroblast growth factor receptor
- VEGF:
-
vascular endothelial growth factor
- mTOR:
-
mammalian target of rapamycin
- IFN-γ:
-
interferon-γ
- miRNAs:
-
microRNA
- NSCLC:
-
non-small cell lung cancer
- LPS:
-
lipopolysaccharide
- CAD:
-
chronic renal allograft dysfunction
- SV40:
-
simian vacuolating virus 40
- TGF-β:
-
transforming growth factor-β
- EOMA cells:
-
hemangioendothelioma endothelial cells
- NC:
-
negative control
- DMEM/F12:
-
Dulbecco’s modified Eagle’s medium/F12
- FBS:
-
foetal bovine serum
- DAVID:
-
Database for Annotation, Visualization and Integrated Discovery
- cDNA:
-
complementary DNA
- qPCR:
-
quantitative real-time polymerase chain reaction
- HIF-1α:
-
hypoxia inducible factor-1α
- PMSF:
-
phenylmethylsulfonyl fluoride
- DLL4:
-
Delta-like 4
- PTEN:
-
phosphatase and tension homolog on chromosome ten
- ELISA:
-
enzyme-linked immunosorbent assay
- HE:
-
hematoxylin–eosin
- eNOS:
-
endothelial nitric oxide synthase
- DAPI:
-
4′,6-diamidino-2-phenylindole
- ANOVA:
-
analysis of variance
- GO:
-
gene ontology
- SPRY1:
-
sprouty homolog 1
References
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–49.
Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698–710.
Abraham A, Krasnodembskaya A. Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome: concise review. Stem Cells Transl Med. 2019. https://doi.org/10.1002/sctm.19-0205.
Song D, Yang D, Powell CA, Wang X. Cell-cell communication: old mystery and new opportunity. Cell Biol Toxicol. 2019;35(2):89–93.
Ibba-Manneschi L, Rosa I, Manetti M. Telocyte implications in human pathology: an overview. Semin Cell Dev Biol. 2016;55:62–9.
Popescu LM, Faussone-Pellegrini MS. TELOCYTES—a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14(4):729–40.
Manole CG, Cismasiu V, Gherghiceanu M, Popescu LM. Experimental acute myocardial infarction: telocytes involvement in neo-angiogenesis. J Cell Mol Med. 2011;15(11):2284–96.
Zhao B, Chen S, Liu J, Yuan Z, Qi X, Qin J, et al. Cardiac telocytes were decreased during myocardial infarction and their therapeutic effects for ischaemic heart in rat. J Cell Mol Med. 2013;17(1):123–33.
Zheng Y, Bai C, Wang X. Potential significance of telocytes in the pathogenesis of lung diseases. Expert Rev Respir Med. 2012;6(1):45–9.
Ma R, Wu P, Shi Q, Song D, Fang H. Telocytes promote VEGF expression and alleviate ventilator-induced lung injury in mice. Acta Biochim Biophys Sin (Shanghai). 2018;50(8):817–25.
Cao Y, Ye Q, Zhuang M, Xie S, Zhong R, Cui J, et al. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS ONE. 2017;12(11):e0186520.
Murillo MM, Zelenay S, Nye E, Castellano E, Lassailly F, Stamp G, et al. RAS interaction with PI3K p110alpha is required for tumor-induced angiogenesis. J Clin Invest. 2014;124(8):3601–11.
Gong G, Yang XX, Li YY, Geng HY, Wang H, Wang LS, et al. Protective effects of PI3KCG gene on acute myocardial infarction. J Thorac Dis. 2018;10(2):941–53.
Zhu Y, Song D, Song Y, Wang X. Interferon gamma induces inflammatory responses through the interaction of CEACAM1 and PI3K in airway epithelial cells. J Transl Med. 2019;17(1):147.
Zhou Y, Li S, Li J, Wang D, Li Q. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol Biochem. 2017;42(4):1431–46.
Schauerte C, Hubner A, Rong S, Wang S, Shushakova N, Mengel M, et al. Antagonism of profibrotic microRNA-21 improves outcome of murine chronic renal allograft dysfunction. Kidney Int. 2017;92(3):646–56.
Zununi Vahed S, Omidi Y, Ardalan M, Samadi N. Dysregulation of urinary miR-21 and miR-200b associated with interstitial fibrosis and tubular atrophy (IFTA) in renal transplant recipients. Clin Biochem. 2017;50(1–2):32–9.
Zununi Vahed S, Poursadegh Zonouzi A, Ghanbarian H, Ghojazadeh M, Samadi N, Omidi Y, et al. Differential expression of circulating miR-21, miR-142-3p and miR-155 in renal transplant recipients with impaired graft function. Int Urol Nephrol. 2017;49(9):1681–9.
Song D, Xu M, Qi R, Ma R, Zhou Y, Wu D, et al. Influence of gene modification in biological behaviors and responses of mouse lung telocytes to inflammation. J Transl Med. 2019;17(1):158.
Song D, Tang L, Wang L, Huang J, Zeng T, Fang H, et al. Roles of TGFbeta1 in the expression of phosphoinositide 3-kinase isoform genes and sensitivity and response of lung telocytes to PI3K inhibitors. Cell Biol Toxicol. 2019. https://doi.org/10.1007/s10565-019-09487-3.
Lopez-Romero P. Pre-processing and differential expression analysis of Agilent microRNA arrays using the AgiMicroRna Bioconductor library. BMC Genomics. 2011;12:64.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47.
Valero-Mora PM. ggplot2: elegant graphics for data analysis. J Stat Softw. 2010. https://doi.org/10.18637/jss.v035.b01.
Sticht C, De La Torre C, Parveen A, Gretz N. miRWalk: an online resource for prediction of microRNA binding sites. PLoS ONE. 2018;13(10):e0206239.
Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015. https://doi.org/10.7554/eLife.05005.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–13.
Dennis G Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, et al. DAVID: database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504.
Zhang Y, Chen Z, Feng L, Jiang P, Li X, Wang X. Ionizing radiation-inducible microRNA-21 induces angiogenesis by directly targeting PTEN. Asian Pac J Cancer Prev. 2019;20(5):1587–93.
Zhang Y, Yuan F, Liu L, Chen Z, Ma X, Lin Z, et al. The Role of the miR-21/SPRY2 axis in modulating proangiogenic factors, epithelial phenotypes, and wound healing in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2019;60(12):3854–62.
Weijts B, Westendorp B, Hien BT, Martinez-Lopez LM, Zijp M, Thurlings I, et al. Atypical E2Fs inhibit tumor angiogenesis. Oncogene. 2018;37(2):271–6.
Weijts BG, Bakker WJ, Cornelissen PW, Liang KH, Schaftenaar FH, Westendorp B, et al. E2F7 and E2F8 promote angiogenesis through transcriptional activation of VEGFA in cooperation with HIF1. EMBO J. 2012;31(19):3871–84.
Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L379–99.
Zheng Y, Zhang M, Qian M, Wang L, Cismasiu VB, Bai C, et al. Genetic comparison of mouse lung telocytes with mesenchymal stem cells and fibroblasts. J Cell Mol Med. 2013;17(4):567–77.
Wang S, Olson EN. AngiomiRs—key regulators of angiogenesis. Curr Opin Genet Dev. 2009;19(3):205–11.
Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108(9):3068–71.
Xu CH, Liu Y, Xiao LM, Chen LK, Zheng SY, Zeng EM, et al. Silencing microRNA-221/222 cluster suppresses glioblastoma angiogenesis by suppressor of cytokine signaling-3-dependent JAK/STAT pathway. J Cell Physiol. 2019. https://doi.org/10.1002/jcp.28794.
Seo HH, Lee SY, Lee CY, Kim R, Kim P, Oh S, et al. Exogenous miRNA-146a enhances the therapeutic efficacy of human mesenchymal stem cells by increasing vascular endothelial growth factor secretion in the ischemia/reperfusion-injured heart. J Vasc Res. 2017;54(2):100–8.
Hu Y, Rao SS, Wang ZX, Cao J, Tan YJ, Luo J, et al. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics. 2018;8(1):169–84.
Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82(1):21–9.
Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, et al. miR-21 improves the neurological outcome after traumatic brain injury in rats. Sci Rep. 2014;4:6718.
Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65(6):1053–61.
Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19(6):326–38.
Chen HZ, Tsai SY, Leone G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat Rev Cancer. 2009;9(11):785–97.
Morgunova E, Yin Y, Jolma A, Dave K, Schmierer B, Popov A, et al. Structural insights into the DNA-binding specificity of E2F family transcription factors. Nat Commun. 2015;6:10050.
Li J, Ran C, Li E, Gordon F, Comstock G, Siddiqui H, et al. Synergistic function of E2F7 and E2F8 is essential for cell survival and embryonic development. Dev Cell. 2008;14(1):62–75.
Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–7.
Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8(6):464–78.
Luo Z, Shang X, Zhang H, Wang G, Massey PA, Barton SR, et al. Notch signaling in osteogenesis, osteoclastogenesis, and angiogenesis. Am J Pathol. 2019;189(8):1495–500.
Zhao Q, Huang J, Wang D, Chen L, Sun D, Zhao C. Endothelium-specific CYP2J2 overexpression improves cardiac dysfunction by promoting angiogenesis via Jagged1/Notch1 signaling. J Mol Cell Cardiol. 2018;123:118–27.
Boucher JM, Harrington A, Rostama B, Lindner V, Liaw L. A receptor-specific function for Notch2 in mediating vascular smooth muscle cell growth arrest through cyclin-dependent kinase inhibitor 1B. Circ Res. 2013;113(8):975–85.
Ho RX, Meyer RD, Chandler KB, Ersoy E, Park M, Bondzie PA, et al. MINAR1 is a Notch2-binding protein that inhibits angiogenesis and breast cancer growth. J Mol Cell Biol. 2018;10(3):195–204.
Wang Y, Yu T, Jin H, Zhao C, Wang Y. Knockdown MiR-302b alleviates LPS-induced injury by targeting Smad3 in C28/I2 chondrocytic cells. Cell Physiol Biochem. 2018;45(2):733–43.
Taylor KL, Henderson AM, Hughes CC. Notch activation during endothelial cell network formation in vitro targets the basic HLH transcription factor HESR-1 and downregulates VEGFR-2/KDR expression. Microvasc Res. 2002;64(3):372–83.
Acknowledgements
The authors would like to thank Dr. Dongli Song (Zhongshan Hospital Institute for Clinical Science, Shanghai Institute of Clinical Bioinformatics, Shanghai Engineering Research for AI Technology for Cardiopulmonary Diseases, Shanghai Medical College, Fudan University, Shanghai, China the Central Laboratory, Zhongshan Hospital Affiliated to Fudan University, Shanghai, China) for kindly providing mouse TCs.
Funding
This work was supported by the National Natural Science Foundation of China (nos. 81971863, 81700008, 81873409, 2017YFSF090207), and Shanghai Science Foundation (17ZR1404300).
Author information
Authors and Affiliations
Contributions
YZ, DS and HF designed the study. YY, YH and TL completed the experimental process, literature search. DS and YZ wrote and edited the manuscript. YY and HT completed generation of figures. All authors reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Zhongshan Hospital Biomedical Research Department.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
The morphology of TCs. The pictures were gathered by Cell-IQ every 8 h. The white arrow showed the typical telopode.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, Y., Yang, Y., Liang, T. et al. The regulatory effect of microRNA-21a-3p on the promotion of telocyte angiogenesis mediated by PI3K (p110α)/AKT/mTOR in LPS induced mice ARDS. J Transl Med 17, 427 (2019). https://doi.org/10.1186/s12967-019-02168-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-019-02168-z