Abstract
Background and objectives
One of the possible male sterility risk factors are polymorphisms of Methylenetetrahydrofolate reductase (MTHFR). However, the epidemiologic investigations described inconsistent results regarding MTHFR polymorphism and the risk of male infertility. For that reason, we carried out a meta-analysis of published case-control studies to re-examine the controversy.
Methods
Electronic searches of Cochrane, EMBASE, Google Scholar, and PubMed were conducted to select eligible studies for this meta-analysis (updated to May 2019). According to our exclusion and inclusion criteria, only high-quality studies that remarked the association between MTHFR polymorphisms and male infertility risk were included. The Crude odds ratio (OR) with a confidence interval of 95% (CI) was used to assess the relationship between MTHFR polymorphism and male infertility risk.
Results
Thirty-four case-control studies with 9662 cases and 9154 controls concerning 677C/T polymorphism and 22 case-control studies with 5893 cases and 6303 controls concerning 1298A/C polymorphism were recruited. Both MTHFR polymorphisms had significant associations with male infertility risk (CT + TT vs. CC: OR = 1.37, 95% CI: 1.21–1.55, P = 0.00, I2 = 41.9%); (CC vs. CA + AA: OR = 0.82, 95% CI: 0.52–1.30, P = 0.04, I2 = 50.1%). Further, when stratified by ethnicity, the significant association results were observed in Asians and Caucasians for 677C/T and just Asians for 1298A/C.
Conclusions
Some of MTHFR polymorphisms like MTHFR 677C > T are associated with an elevated male infertility risk. To confirm our conclusion and to provide more accurate and complete gene-environment communication with male infertility risk, more analytical studies are needed.
Similar content being viewed by others
Introduction
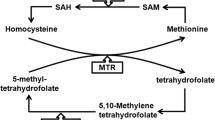
Infertility is a global problem and according to the World Health Organization, almost one in seven couples are affected by fertility complications [1, 2]. Male infertility is a heterozygous disorder caused by numerous genetic and environmental factors that lead to defects in spermatogenesis [3, 4]. This kind of fertility disorder accounts for 20–50% of causes. According to studies, there is a positive correlation between serum folate concentrations, density, and normal morphology of sperm [5]. Therefore, the Folate pathway is likely to be important in male fertility [6]. Methylenetetrahydrofolate reductase (MTHFR) is a key enzyme in folate metabolism and plays a vital role in balancing the storage of methyl groups between DNA synthesis and its methylation [7]. DNA methylation is one of the important epigenetics features that play an essential role in regulating gene expression in spermatogenesis [8]. The mutations of C677T and A1298C in the MTHFR gene reduce the enzyme activity and cause male sterility in some populations [9].
The catalytic enzyme- encoding gene MTHFR is located at the end of the short arm of chromosome 1 (1p36.3) and has 33 exons [10].
Two C677T and A1298C polymorphisms that significantly alter MTHFR enzyme activity are recognized in this gene [11]. The conversion of cytosine into thymine as a result of a point mutation in nucleotide 733 in exon 4 of the MTHFR gene results in the replacement of alanine by valine [12]. This point mutation leads to the formation of an unstable and heat-sensitive MTHFR enzyme with low activity. Due to the decreased enzymatic activity of mutant MTHFR, increased serum homocysteine levels are achieved. The MTHFR enzyme has a 19% function in homozygotes and 71% in heterozygotes compared to normal people [13].
The adenine to cytosine conversion at nucleotide position 3289 in exon 3 of the MTHFR gene (A1298C), also leads to the replacement of glutamine by alanine [14]. There are few studies on A1298C polymorphism; however, it has been shown that CC genotype has an equivalent function to 79% of the AA genotype. Homozygotes do not show high serum homocysteine levels for the A1298C allele. But individuals with combined heterozygote A1298C and C677T polymorphisms have biochemical characteristics similar to C677T homozygotes with elevated levels of homocysteine and decreased levels of folate [15, 16]. Numerous studies have examined the association between MTHFR polymorphism and male infertility, but the conclusions are argumentative [17]. The reason for this can be attributed in part to the ethnicity differences. There are only four meta-analyses that have evaluated the effect of MTHFR C677T polymorphism on male infertility in Asians [18].
The N. Gupta and colleagues studied the Indian population [18], Wiener’s worked on the men’s idiopathic infertility in Russian population [19], Z. Ren and colleagues studied the Chinese population [20], and V. Rai and P. Kumar research [21] focused on the relationship between one type of MTHFR A1298C and male infertility.
In this study, with the help of eligible findings, we carried out a meta-analysis to provide a comprehensive assessment of the association between C677T and A1298C MTHFR polymorphisms with male infertility.
Materials and methods
Literature search
A comprehensive literature quest in Cochrane, EMBASE, Web of Science, PubMed, Scopus, as well as Google Scholar databases was conducted for all articles regarding the impact of C677T and A1298C polymorphisms on male infertility published up to May 2019“ Include from when the analysis was done. Articles were obtained with the following keywords: “methylenetetrahydrofolate reductase” or “MTHFR”, ‘polymorphism” or “variant”, “677C > T”, “1298A > C” and “male infertility”. The inclusion criteria were: 1) Well defined case-control study design; 2) sufficient data for examining an odds ratio (OR) with 95% confidence interval (CI). The exclusion criteria were: 1) non-human studies; 2) articles not available in English and Farsi languages; 3) duplicate study by the same group with lower sample number; 4) cases only studies; v) insufficient genotyping data.
Data extraction
According to the inclusion and exclusion criteria, data extraction was achieved by two independent investigators. Any disagreements of the studies were resolved through a comprehensive reassessment by the other author and only high- quality studies can be included in our meta-analysis. Reference lists of all included full-text manuscripts were screened for additional articles. Authors of papers were contacted to ask clarification where inadequate information was provided. The following data were collected from studies: first author, year of publication, ethnicity, sample size, allele distribution in cases and controls and, a genotyping method used. The different ethnic groups were classified as Caucasian and Asian.
Statistical analysis
All analyses were performed using STATA 14.1 software (Stata Corporation, College Station, TX, USA). In this research, P- values were calculated two-sided and P = 0.05 was statistically considered as significant. The Hardy–Weinberg equilibrium (HWE) was calculated by the Chi-square test in control groups, to verify the representation of the study population. The correlation between the polymorphisms and male infertility risk was calculated via assessment of odds ratios (ORS) and 95% confidence interval (CI). Pooled ORs and their 95% CIs for dominant, codominant, and recessive inheritance models were calculated.
The significance of the pooled OR was assessed by Z-test and P < 0.05 (Forest plot). To indicate the presence of heterogeneity, the random effect model was selected; otherwise, the fixed-effects model was chosen.
Of all the models available, a funnel plot was designed to assess the publication bias, and an asymmetrical plot was considered a sign of these impresses. The degree of the asymmetry in these plots was measured with the help of Egger’s test and a p-value less than 0.05 was introduced as a significant publication bias. Sensitivity analysis was conducted to measure the effect by ignoring a single study at a time.
Results
Study characteristics
According to the present investigation, for both polymorphisms, 137 relevant articles were identified.
The process of literature retrieval and selection is shown in Fig. 1. There were 34 case-control studies with 9662 cases and 9154 controls concerning 677C/T polymorphism and 22 case-control studies with 5893 cases and 6303 controls concerning 1298A/C polymorphism. Also, for the 677C/T polymorphism, there were 23 studies of the Asian population and ten studies of the Caucasian population, and for1298A/C polymorphism, fifteen and five, respectively. The specifications and data for the considered studies are summarized in Table 1.
MTHFR 677C > T
Overall, we realized that the 677C > T polymorphism was associated with the risk of male infertility. In this study, the frequency of the TT genotype to CC showed a significant increase (P = 0.00). Also, the frequency of TC genotype to CC, the frequency of TT genotype to the combination of TC + CC genotypes, and the frequency of TT + TC combined genotype to CC showed a significant increase (P = 0.006) (Table 2, Fig. 2).
Forest plot of male infertility risk associated with MTHFR 677C > T polymorphism [a for TT vs. CC; b for TC vs. CC; c for TT + TC vs. CC; d for TT vs. TC + CC]. The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI
When the results were stratified by ethnicity, the following positive associations were also observed in the Caucasian population where TT genotype had significantly elevated frequency to the CC genotype (P = 0.002), TC had significant increased frequency to CC (P = 0.038), TT frequency to the TC + CC genotypes (P = 0.007), and TT + TC showed higher frequency than CC genotype (P = 0.005). In addition, we found that 677C/T polymorphism was significantly associated with the male infertility risk in the Asian population where the TT to CC genotype (P = 0.01), TC to CC (P = 0.002), TT to TC + CC (P = 0.084), and combination of TT + TC genotype frequencies to the CC (P = 0.00) showed significant difference.
MTHFR 1298A/C
We attained that the 1298A/C polymorphism was not associated with risk of male infertility where the frequency of CC to the AA genotype (P = 0.09), CA to the AA (P = 0.210), CC to the CA + AA (P = 0.194), and CC + CA to the AA (P = 0.084) genotype was not significantly different, Table 3, Fig. 3. When the analysis was stratified by ethnicity, the subsequent negative associations were also observed in Caucasian population: CC to AA genotype (P = 0.530), CA to AA genotype (P = 0.167), CC to CA + AA genotypes (P = 0.405), and CC + CA genotype to AA (P = 0.237) did not show significant increase in the frequency. Also, in Asian population, there was no significant difference between the examined genotypes as the frequency of CC to AA (P = 0.168); CA to AA (P = 0.071); CC to CA + AA (P = 0.305), and CC + CA to AA (P = 0.073) was observed in the population.
Forest plot of male infertility risk associated with MTHFR 1298A/C polymorphism [a for CC vs. AA; b for CA vs. AA; c for CC + CA vs. AA; d for CC vs. CA + AA]. The squares and horizontal lines correspond to the study-specific OR and 95% CI. The area of the squares reflects the weight (inverse of the variance). The diamond represents the summary OR and 95% CI
Evaluation of heterogeneity
We used Egger’s test and Begg’s funnel plot to assess the publication bias. The shape of funnel plots has shown in Fig. 4, and in most cases funnel plot symmetry was observed. Also, the statistical results confirmed the results of the plots. For the 677C/T polymorphism, the heterogeneity was reckoned between each of the studies using the chi-square test, p-value of 0.024 for TT vs. CC; p-value = 0.281 for TC vs. CC; p-value = 0.196 for the dominant model; and p-value = 0.008 for the recessive model, respectively. For the 1298A/C polymorphism, the heterogeneity was reckoned between each of the studies using the Chi-square test, p-value of 0.403 for CC vs. AA; p-value = 0.235 for CA vs. AA; p-value = 0.200 for dominant model; and p-value =0.235 for recessive model, respectively.
Funnel plot for publication bias test [for 677C > T polymorphism: a for TC vs. CC, p = 0.281; b for TT + TC vs. CC, p = 0.196; for 1298A > C polymorphism: c for CC vs. AA, p = 0.403; d for CC vs. CA + AA, p = 0.235]. Each point represents a separate study for the indicated association. Log [or], natural logarithm of OR. Horizontal line, mean effect size
Sensitivity analysis
In the sensitivity analysis, the effect of each survey on the pooled OR was assayed by repeating the meta-analysis while deleting each study, one at a time. This method verified the stability of our total results.
Publication bias
Begg’s funnel plot and Egger’s test were conducted to assess the publication bias of the literature. The shape of funnel plots reveals a bit of evidence of funnel plot asymmetry (Fig. 4). The statistical results still show publication bias (for 677C > T polymorphism: p-value = 0.02 and p-value =0.00 for 1298A/C).
Discussion
Male infertility is a disorder that is affected by environmental and genetic factors. MTHFR gene plays a crucial role in folate metabolism as a result of spermatogenesis. Studies in different populations have shown contradictory results for the association between MTHFR gene polymorphisms and male infertility [52, 53]. The present meta-analysis from 34 published studies, including 9662 cases and 9154 controls for 677C/T and 22 published case-control studies with 5893 cases and 6303 controls for1298A/C, explored the association between two potentially functional polymorphisms in the MTHFR gene and male infertility risk. The numbers of included studies in this meta-analysis are much higher than the prior meta-analyses which dramatically increased the statistical power of the analysis due to the low number of pieces of evidence. Overall, we found that the variant genotypes of the MTHFR 677C/T were significantly associated with male infertility risk. In stratified analysis by ethnicity, we found that the variant genotype of the MTHFR 677C/T polymorphism was significantly associated with the risk of male infertility in the Caucasian and Asian populations.
On the other hand, there was no significant association between the variant genotype of the MTHFR 1298A/C polymorphism and the risk of male infertility in Caucasian and Asian populations.
In a meta-analysis study by H. Nikzad, et al. 2015, an MTHFR 677C/T polymorphism showed a significant association between allelic, dominant and codominant models and the risk of male infertility (P < 0.001) [54]. Concerning 677C/T variation, our results were consistent with other meta-analysis results (M. Gong, et al. [55], F. Tüttelmann, et al. [56], N. Gupta, et al. [18], and W. Wu, et al. [57], but in contradiction with the results of B. Wei, et al. [58] and A.S.Weiner, et al. [19].
In another study, B. Wei, et al. [58] showed that both 677C/T and 1298A/C polymorphisms were not significantly associated with male infertility risk. However, in the classified analysis by ethnicity, it was reported that 677C / T polymorphism had a significant association with the risk of male infertility in the Asian population. However, the results of our studies were in contrast to the results of B. Wei, et al. meta-analysis and showed a clear relationship between both polymorphisms and male infertility. The reasons for these contradictions between the mentioned studies could be the presence of other unknown causal genes and/or their variations in combination to some environmental factors which may strongly influence male infertility, ethnic differences, selection bias, and different matching criteria.
In recent years, new genetics techniques such as GWAS have been used to evaluate the genetic variation of many diseases, including infertility, especially in idiopathic cases [55]. Concerning MTHFR common polymorphism and its association with male infertility, two studies have been conducted by Aston et al. and the results are different from our study [59, 60]. The reasons for this difference are:(1) The small sample size in the GWAS studies makes it difficult to interpret; (2) Our study includes the totality of studies conducted in various populations, while in Aston studies, only European population have been investigated.
Some of the limitations in this article were: lack of sufficient studies for the African and Latin American populations, unadjusted estimates, bias publication, and heterogeneities for MTHFR polymorphisms among all the studies. Although some modest bias could not be eliminated, this meta-analysis suggests that the MTHFR 677 T and 1298C alleles might be effective risk factors for male infertility, especially in the Asian population. We suggest screening genetic test for the MTHFR SNP’s as new male infertility biomarkers for Asian people.
Also, nutritional management and folate administration under proper medical care could lessen the risk of infertility in such populations.
In summary, this meta-analysis supports the hypothesis that both MTHFR polymorphisms, MTHFR 677C > T and MTHFR 1298A/C, might be markers of male infertility susceptibility, especially in the Asian population. However, more comprehensive studies are warranted to validate our findings.
Conclusion
Our meta-analysis results showed that the MTHFR 677C > T polymorphism was associated with an enhanced risk of male infertility, and supporting the hypothesis that the most common MTHFR polymorphisms may be a potential cause of male infertility.
However, there was no relation between MTHFR 1298A/C and male sterility. Subsequent investigations should use standardized unbiased genotyping methods, homogeneous infertility patients, well-matched controls, and subgroup analysis according to the sperm concentration to confirm our findings in the future.
Availability of data and materials
The original data from the survey is available.
Abbreviations
- MTHFR :
-
Methylenetetrahydrofolate reductase
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- HWE:
-
Hardy–Weinberg equilibrium
References
Anawalt, B.D., S.T. Page, and A.M. Matsumoto. Approach to the male with infertility. UpToDate. Snyder PJ, Matsumoto AM (Eds), UpTodate, Waltham, MA. Accessed 7 Feb 2019.
Azizi F, et al. Gene polymormisms and prostate cancer: a systematic review. Mens Health J. 2018;2(1):6.
Skakkebaek NE, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2015;96(1):55–97.
Azizi F, Ghafouri-Fard S. Outer dense fiber proteins: bridging between male infertility and cancer. Arch Iran Med. 2017;20(5):320.
Aarabi M, et al. High-dose folic acid supplementation alters the human sperm methylome and is influenced by the MTHFR C677T polymorphism. Hum Mol Genet. 2015;24(22):6301–13.
Kurzawski M, et al. Association study of folate-related enzymes (MTHFR, MTR, MTRR) genetic variants with non-obstructive male infertility in a polish population. Genet Mol Biol. 2015;38(1):42–7.
Shi T-L, et al. The relevance of MTHFR C677T, A1298C, and MTRR A66G polymorphisms with response to male infertility in Asians: a meta-analysis. Medicine. 2019;98(8):e14283.
Rahiminia T, et al. Relation between sperm protamine transcripts with global sperm DNA methylation and sperm DNA methyltransferases mRNA in men with severe sperm abnormalities. Hum Fertil. 2019:1–7.
Momenzadeh M. Analysis of methylenetetrahydrofolate reductase (mthfr) c677t and a1298c polymorphisms on male infertility. Indian J Fundam Appl Life Sci. 2016.
Karimian M, Colagar AH. Association of C677T transition of the human methylenetetrahydrofolate reductase (MTHFR) gene with male infertility. Reprod Fertil Dev. 2016;28(6):785–94.
Liew S-C, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet. 2015;58(1):1–10.
McEwen BJ. Methylenetetrahydrofolate reductase (MTHFR): mythology or polymorphism (ology)? Adv Integr Med. 2016;3(3):79–81.
Long S, Goldblatt J. MTHFR genetic testing: controversy and clinical implications. Aust Fam Physician. 2016;45(4):237.
Nefic H, Mackic-Djurovic M, Eminovic I. The frequency of the 677C> T and 1298A> C polymorphisms in the methylenetetrahydrofolate Reductase (MTHFR) gene in the population. Med Arch. 2018;72(3):164.
Saad MN, et al. Genetic case-control study for eight polymorphisms associated with rheumatoid arthritis. PLoS One. 2015;10(7):e0131960.
Yang Y, et al. Association between C677T and A1298C polymorphisms of the MTHFR gene and risk of male infertility: a meta-analysis. Genet Mol Res. 2016;15(2):10-4238.
Hong HH, et al. Associations of C677T polymorphism in methylenetetrahydrofolate reductase (MTHFR) gene with male infertility risk: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017;212:101–9.
Gupta N, et al. Strong association of 677 C> T substitution in the MTHFR gene with male infertility-a study on an Indian population and a meta-analysis. PLoS One. 2011;6(7):e22277.
Weiner AS, et al. Polymorphisms in folate-metabolizing genes and risk of idiopathic male infertility: a study on a Russian population and a meta-analysis. Fertil Steril. 2014;101(1):87–94. e3.
Ren Z, et al. MTHFR C677T, A1298C and MS A2756G gene polymorphisms and male infertility risk in a Chinese population: a meta-analysis. PLoS One. 2017;12(1):e0169789.
Rai V, Kumar P. Methylenetetrahydrofolate Reductase C677T polymorphism and risk for male infertility in Asian population. Indian J Clin Biochem. 2017;32(3):253–60.
Irfan M, et al. Association of the MTHFR C677T (rs1801133) polymorphism with idiopathic male infertility in a local Pakistani population. Balkan J Med Genet. 2016;19(1):51–62.
Stuppia L, et al. The methylenetethrahydrofolate reductase (MTHFR) C677T polymorphism and male infertility in Italy. J Endocrinol Investig. 2003;26(7):620–2.
Ebisch IM, et al. C677T methylenetetrahydrofolate reductase polymorphism interferes with the effects of folic acid and zinc sulfate on sperm concentration. Fertil Steril. 2003;80(5):1190–4.
Park JH, et al. MTHFR C677T polymorphism associates with unexplained infertile male factors. J Assist Reprod Genet. 2005;22(9–10):361–8.
Lee H-C, et al. Association study of four polymorphisms in three folate-related enzyme genes with non-obstructive male infertility. Hum Reprod. 2006;21(12):3162–70.
Singh K, et al. Mutation C677T in the methylenetetrahydrofolate reductase gene is associated with male infertility in an Indian population 1. Int J Androl. 2005;28(2):115–9.
Paracchini V, Garte S, Taioli E. MTHFR C677T polymorphism, GSTM1 deletion and male infertility: a possible suggestion of a gene–gene interaction? Biomarkers. 2006;11(1):53–60.
A, Z.C, et al. Single nucleotide polymorphism C677T in the methylenetetrahydrofolate reductase gene might be a genetic risk factor for infertility for Chinese men with azoospermia or severe oligozoospermia. Asian J Androl. 2007;9(1):57–62.
Dhillon VS, Shahid M, Husain SA. Associations of MTHFR DNMT3b 4977 bp deletion in mtDNA and GSTM1 deletion, and aberrant CpG island hypermethylation of GSTM1 in non-obstructive infertility in Indian men. Mol Hum Reprod. 2007;13(4):213–22.
Tetik A, et al. Influence of methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C gene polymorphisms on male infertility in Turkish infertile men with azoospermia and oligozoospermia. Eur Urol Suppl. 2008;7(3):92.
Ravel C, et al. Lack of association between genetic polymorphisms in enzymes associated with folate metabolism and unexplained reduced sperm counts. PLoS One. 2009;4(8):e6540.
Farcas M-F, et al. Methylenetetrahydrofolate reductase A1298C polymorphism and male infertility in a Romanian population group. Maedica-a J Clin Med. 2009;4(4).
Yang Z, et al. MTHFR C677T polymorphism and colorectal cancer risk in Asians, a meta-analysis of 21 studies. Asian Pac J Cancer Prev. 2012;13(4):1203–8.
Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res/Rev Mutat Res. 2011;727(3):62–71.
Safarinejad MR, Shafiei N, Safarinejad S. Genetic susceptibility of methylenetetrahydrofolate reductase (MTHFR) gene C677T, A1298C, and G1793A polymorphisms with risk for bladder transitional cell carcinoma in men. Med Oncol. 2011;28(1):398–412.
Gava MM, et al. Polymorphisms in folate-related enzyme genes in idiopathic infertile Brazilian men. Reprod Sci. 2011;18(12):1267–72.
Vani G, et al. Methylenetetrahydrofolate reductase C677T polymorphism is not associated with male infertility in a south Indian population. Andrologia. 2012;44:252–9.
Eloualid A, et al. Association of the MTHFR A1298C variant with unexplained severe male infertility. PLoS One. 2012;7(3):e34111.
Balkan M, et al. The possible association of polymorphisms in MTHFR, MTRR, and MTHFD1 genes with male infertility. Intern Med J. 2013;20(4):404–8.
Herodež ŠS, et al. MTHFR C677T and A1298C genotypes and haplotypes in Slovenian couples with unexplained infertility problems and in embryonic tissues from spontaneous abortions. Balkan J Med Genet. 2013;16(1):31–9.
Mfady DS, et al. Associations of variants in MTHFR and MTRR genes with male infertility in the Jordanian population. Gene. 2014;536(1):40–4.
Naqvi H, et al. Role of 677C→ T polymorphism a single substitution in methylenetetrahydrofolate reductase (MTHFR) gene in north Indian infertile men. Mol Biol Rep. 2014;41(2):573–9.
Jiang S, et al. Associations of MTHFR and MTRR polymorphisms with serum lipid levels in Chinese hypertensive patients. Clin Appl Thromb Hemost. 2014;20(4):400–10.
Zarebska A, et al. Association of the MTHFR 1298A> C (rs1801131) polymorphism with speed and strength sports in Russian and polish athletes. J Sports Sci. 2014;32(4):375–82.
Ni W, et al. Lack of association between genetic polymorphisms in three folate-related enzyme genes and male infertility in the Chinese population. J Assist Reprod Genet. 2015;32(3):369–74.
Wang T, et al. Association of MTHFR, NFKB1, NFKBIA, DAZL and CYP1A1 gene polymorphisms with risk of idiopathic male infertility in a Han Chinese population. Int J Clin Exp Pathol. 2017;10(7):7640.
Najafipour R, et al. Effect of B9 and B12 vitamin intake on semen parameters and fertility of men with MTHFR polymorphisms. Andrology. 2017;5(4):704–10.
Huang WJ, et al. Effects of folic acid on oligozoospermia with MTHFR polymorphisms in term of seminal parameters, DNA fragmentation, and live birth rate: a double-blind, randomized, placebo-controlled trial. Andrology. 2020;8(1):110–6.
Ullah N, et al. MTHFR polymorphisms as risk for male infertility in Pakistan and its comparison with socioeconomic status in the world. Pers Med. 2019;16(1):35–49.
Murphy LE, et al. Folate and vitamin B12 in idiopathic male infertility. Asian J Androl. 2011;13(6):856.
Azizi F, et al. The genetic causes of male infertility in Iranian population; a systematic review. Mens Health J. 2018;2(1):1.
Karaca M, et al. Association between methylenetetrahydrofolate reductase (MTHFR) gene promoter hypermethylation and the risk of idiopathic male infertility. Andrologia. 2017;49(7):e12698.
Nikzad H, et al. MTHFR-Ala222Val and male infertility: a study in Iranian men, an updated meta-analysis and an in silico-analysis. Reprod BioMed Online. 2015;31(5):668–80.
Gong M, et al. MTHFR 677C> T polymorphism increases the male infertility risk: a meta-analysis involving 26 studies. PLoS One. 2015;10(3):e0121147.
Tüttelmann F, et al. Gene polymorphisms and male infertility–a meta-analysis and literature review. Reprod BioMed Online. 2007;15(6):643–58.
Wu W, et al. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of male infertility: a meta-analysis. Int J Androl. 2012;35(1):18–24.
Wei B, et al. MTHFR 677C> T and 1298A> C polymorphisms and male infertility risk: a meta-analysis. Mol Biol Rep. 2012;39(2):1997–2002.
Aston KI, Carrell DT. Genome-wide study of single-nucleotide polymorphisms associated with azoospermia and severe oligozoospermia. J Androl. 2009;30(6):711–25.
Aston KI, et al. Evaluation of 172 candidate polymorphisms for association with oligozoospermia or azoospermia in a large cohort of men of European descent. Hum Reprod. 2010;25(6):1383–97.
Acknowledgments
We thank our colleagues from Men’s Health & Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences who provided insight and expertise that greatly assisted the research.
Funding
The authors declare that there are no sources of funding.
Author information
Authors and Affiliations
Contributions
F.A.: Designed analyzed data, co-wrote the paper and Critical revision of the manuscript for important intellectual content; F.AA: Study concept and design, performed the searches, Acquisition of data; N.E.: Acquisition of data, co-wrote the paper, Performed proof editing, F.P.: Analysis and interpretation of data, Critical revision of the manuscript for important intellectual content and answered the reviewer’s comments in parallel to the manuscript revision, Z.Z.: statistical Analysis and interpretation of data. All the authors provided their final approval for the completed manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Aliakbari, F., Pouresmaeili, F., Eshghifar, N. et al. Association of the MTHFR 677C>T and 1298A>C polymorphisms and male infertility risk: a meta-analysis. Reprod Biol Endocrinol 18, 93 (2020). https://doi.org/10.1186/s12958-020-00649-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-020-00649-1