Abstract
Background
Pulsatile GnRH therapy is the gold standard treatment for ovulation induction in women having functional hypothalamic amenorrhea (FHA). The use of pulsatile GnRH therapy in FHA patients with polycystic ovarian morphology (PCOM), called “FHA-PCOM”, has been little studied in the literature and results remain contradictory. The aim of this study was to compare the outcomes of pulsatile GnRH therapy for ovulation induction between FHA and “FHA-PCOM” patients in order to search for an eventual impact of PCOM.
Methods
Retrospective study from August 2002 to June 2015, including 27 patients with FHA and 40 “FHA-PCOM” patients (85 and 104 initiated cycles, respectively) treated by pulsatile GnRH therapy for induction ovulation.
Results
The two groups were similar except for markers of PCOM (follicle number per ovary, serum Anti-Müllerian Hormone level and ovarian area), which were significantly higher in patients with “FHA-PCOM”. There was no significant difference between the groups concerning the ovarian response: with equivalent doses of GnRH, both groups had similar ovulation (80.8 vs 77.7 %, NS) and excessive response rates (12.5 vs 10.6 %, NS). There was no significant difference in on-going pregnancy rates (26.9 vs 20 % per initiated cycle, NS), as well as in miscarriage, multiple pregnancy or biochemical pregnancy rates.
Conclusion
Pulsatile GnRH seems to be a successful and safe method for ovulation induction in “FHA-PCOM” patients. If results were confirmed by prospective studies, GnRH therapy could therefore become a first-line treatment for this specific population, just as it is for women with FHA without PCOM.
Similar content being viewed by others
Background
Functional Hypothalamic Amenorrhea (FHA) is one of the most common causes of secondary amenorrhea [1–3]. FHA is due to a chronic energy deprivation, mostly caused by significant weight loss, severe food restriction and/or excessive exercise. This negative energy balance leads to a reduced frequency of the gonadotropin-releasing hormone (GnRH) pulses, which is responsible for gonadotropin insufficiency and results in anovulation and hypoestrogenism [2]. It has been demonstrated that pulsatile GnRH therapy is the best treatment to induce ovulation and pregnancy in women with FHA [4, 5], but it is available in only few countries worldwide. In women with FHA, by restoring a GnRH secretion and its pulsatility, this therapy results almost in 95 % pregnancy rate in 6 months [6, 7].
Polycystic ovarian morphology (PCOM) is the third criterion of the Rotterdam classification for Polycystic ovarian syndrome (PCOS) [8, 9]. Its definition essentially lies on an excessive number of antral follicles per ovary (FNPO) ≥ 12 and/or ovarian volume ≥ 10 mL [8, 10]. Pulsatile GnRH therapy has been tried in women with PCOS but the results remain mixed [11–13]. Neither the meta-analysis from the Cochrane Database [14], nor the Thessaloniki consensus [15], recommends GnRH pulsatile therapy in PCOS.
Besides PCOS, PCOM may also be observed as an isolated feature in about 30 % of normal women and in patients with FHA [16]. The association between FHA and PCOM has been poorly described in the literature and the studies are heterogeneous (variable diagnostic criteria for FHA and PCOM, confusion between hypogonadotropic hypogonadism and FHA, inconsistent LH level, small series or case reports) and as a result, the conclusions are contradictory [17–25]. More recently Dubourdieu et al. [26] have demonstrated the superiority of pulsatile GnRH therapy over gonadotropin, for ovulation induction in women with ‘FHA-PCOM’ (on-going pregnancy rates: 46.7 % versus 0 %; p = 0.02). However, this study has been restricted to only one treatment cycle, with interestingly similar ovarian responses in both groups.
It is therefore difficult to determine whether the presence of PCOM modifies or not the results of pulsatile GnRH therapy, when used for ovulation induction in women with FHA. Some issues remain unanswered such as: is there a higher risk of excessive ovarian response, of multi follicular responses and finally of multiple pregnancies? Or on the contrary, does the presence of PCOM make the stimulation more difficult, with lower ovulation and on-going pregnancy rates?
The aim of this study was to compare the use of pulsatile GnRH therapy for ovulation induction in patients with FHA and in patients with “FHA-PCOM”, and to obviate any possible impact of the presence of PCOM.
Methods
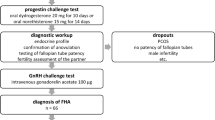
This is a retrospective study in which data were collected from August 2002 to June 2015 in the Department of Endocrine Gynaecology and Reproductive Medicine, University Hospital of Lille, France. All women included were adults, wishing a pregnancy and presenting FHA only or “FHA-PCOM”. The patients were stimulated for ovulation induction with pulsatile GnRH therapy. This study was approved by the International Review Board of the University of Lille and all patients had given consent for the use of their clinical record.
Population
Patients’ data, including their medical background, history of body weight, eating habits and the type and frequency of sports activity, were collected. Age, size, weight (with recent evolution) and body mass index were reported, as well as signs of hypometabolism (fatigue, chilliness, coldness of the body’s extremities, bradycardia, lanugo). All patients benefited from a full psycho-nutritional support, in order to identify any potential eating disorder. Patients with important eating disorders or metabolic complications were excluded from the study as their condition contraindicated pregnancy.
FHA was defined as secondary amenorrhea over 6 months, failure to bleed after progesterone withdrawal test, in a context of low body weight (BMI < 18) and/or history of important weight loss and/or intense physical activity, leading to a negative energy balance.. As FHA is a diagnosis of exclusion, every patient had a normal pituitary magnetic resonance imaging, normal prolactin and TSH levels and a non-elevated basal FSH level.
PCOM was defined by antral follicle excess on ultrasound and/or high serum AMH level. The threshold was ≥ 12 FNPO until 2008, then ≥ 19 FNPO with new ultrasound equipment (see below) [27]. Serum AMH level ≥ 35 pmol/L was considered as a surrogate to the excessive FNPO [27].
Ovulation induction was then carried out provided that the patient had a strictly normal hysterosalpingogram and the partner, a normal semen analysis.
Exclusion criteria were tubal obstruction, endometriosis, any other aetiology of central hypogonadism or sperm abnormalities.
Assay and ultrasound procedures
For each patient, ultrasound and serum assays were performed a week after a progesterone challenge test (dydrogesterone 10 mg/day for 10 days). Patients remained in amenorrhea. Biological assessment included serums of FSH, E2, LH, prolactin, TSH, FT4, FT3, testosterone, dehydroepiandrosterone sulfate (DHEAS), Delta-4-androstenedione and 17-hydroxyprogesterone, as described previously [27–29]. Serum AMH levels were measured by ELISA technique with a second generation immunoassay kit, using monoclonal antibodies directed against the human AMH, called AMH-MIS (Beckmann Coulter, Immunotech Marseille, France), as previously described [27].
The ultrasound device used between 2002 and 2008 was a Logic 400 General Electric Milwaukee, replaced in 2008 by a General Electric Voluson E8 (probe frequencies were 5–7 MHz and 5–13 MHz, respectively). All follicles between 2 to 9 mm were included in the assessment of AFC.
Treatment protocols
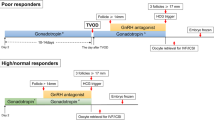
The GnRH pump (Lutrepulse®, Ferring, SAS, Gentilly, France) was placed intravenously (IV) or subcutaneously (SC) by the nurse of the department and was programmed to deliver 1 pulse of GnRH every 90 min (gonadoréline, Lutrelef® 3.2 mg, Ferring, SAS, Gentilly, France). The starting dose was the standard 15 μg SC and 5 μg IV, then adapted to the ovarian response.
The ovarian response was systematically controlled by serum E2 and LH assays and pelvic ultrasound. The first monitoring was made on the 8th day of the stimulation, looking for multifollicular response and, if needed, treatment was adapted: decreased in case of multifollicular response or increased if no response. Patients were monitored under the same conditions until the emergence of at least one dominant follicle. The response was considered as excessive if there were more than 2 dominant follicles on ultrasound and/or E2 serum level ≥1835 pmol/L [30], leading to the cancellation of the cycle. Monitoring was ended once 1 or 2 dominant follicle(s) exceeded 13 mm in diameter. The pump remained in place until a spontaneous ovulation, as indicated by a serum progesterone assay (≥5 ng/mL) seven days after the presumed date of ovulation.
The luteal phase support was provided by one injection of hCG 1500 IU every 3 days, starting on the day of the withdrawal of the pump. A blood hCG pregnancy test was carried out systematically 14 days after ovulation. An ultrasound was performed around 6 or 7 weeks of amenorrhea in order to control the localisation of the pregnancy and its evolution, and to identify multiple pregnancies.
Statistics
Quantitative variables were expressed as mean with standard deviation, or as median with 5th and 95th percentiles, according to the Gaussian or non-Gaussian distribution of the variables, respectively. To compare the two groups, the student T-test or the non-parametric Mann Whitney test was used, respectively. Qualitative variables were expressed in percentages and compared by a Chi2 or a Fisher test, according to the size of the population. Results were considered significant when p was < 0.05.
Results
Sixty-seven patients were included in the study: 27 in the group “FHA” and 40 in the group “FHA-PCOM” (85 and 104 initiated cycles, respectively).
Both groups were similar except for the ultrasound aspect of PCOM and for serum AMH levels, which were significantly higher in “FHA-PCOM” patients (Table 1).
There was no significant difference concerning the ovulation rate (80.8 vs 77.7 %, NS), the cancelled cycle an excessive ovarian response rates (12.5 vs 10.6 %, NS) (Table 2). The dose of GnRH was not different between the groups, either for the first cycle or the mean of all cycles (Tables 2 and 4).
There was no significant difference concerning pregnancy rates, whether it was per initiated cycle (26.9 vs 20.0 %, NS; Table 2), or per ovulatory cycles (33.3 vs 28.5 %, NS; Table 3) or per patient (70.0 vs 63.0 %, NS; Fig. 1).
There was no significant difference between FHA and “FHA-PCOM” for biochemical pregnancy, miscarriage and multiple pregnancy rates.
There was no difference between the 2 groups when ovulatory cycles were compared (Table 4): same doses of GnRH, same stimulation duration, equivalent monofollicular response (78.6 vs 71.2 %, NS), and no more bifollicular reponse (20.2 vs 24.2 %, NS).
Discussion
This study shows that the groups were not different from each other, except for PCOM features and serum AMH. The gonadotropin insufficiency was therefore similar in our patients with FHA only and with “FHA-PCOM”. Ovulation induction with pulsatile GnRH therapy seemed to take place exactly the same way in both groups. Indeed, the doses of GnRH and the stimulation duration were equivalent. Ovarian response also appeared to be identical in both groups as there were the same monofollicular and bifollicular response rates. There was no more excessive ovarian response in patients with “FHA-PCOM”. And there was no difference between the two groups concerning on-going pregnancy rates, as well as miscarriage, multiple pregnancy and biochemical pregnancy rates.
The study observed about 20 % of bi-follicular response with pulsatile GnRH therapy in both groups. However and in agreement with the literature [4], the multiple pregnancy rate reported was low. The risk of multiple pregnancy is therefore not null, which imposes a fair information to couples, and a careful monitoring based on ultrasound and biology, rather than a simple clinical monitoring, as recommended in some previous studies [31].
This study demonstrates that the presence of PCOM in patients with FHA does not influence the management of the pulsatile GnRH therapy, nor the ovarian response to the treatment and the pregnancy rates. The pump appears therefore to be as efficient in “FHA-PCOM’” patient as it is in FHA patients [4]. This disagrees with previous studies [20, 22, 23] or case reports [17, 18], which showed the revelation of true PCOS in patients with “FHA-PCOM”, when treated with pulsatile GnRH therapy.
This study raises the question whether PCOM is actually indicative of a pre-existing but latent PCOS, “switched off” because of the gonadotropin insufficiency, or simply an ultrasound manifestation. The existence of PCOM is quite frequent in general population, including normo-ovulatory women with no hyperandrogenism [32–35]. In FHA, Robin et al. [16] identified (by cluster analysis) 3 groups of patients, depending on serum AMH level (as a surrogate for PCOM). The first two groups (normal serum AMH level (52 %) and moderate increase (about two-fold) of serum AMH level (38 %)) were in line with findings in female controls. The third group (10 %) with significantly higher serum AMH levels (about four-fold) was not found in controls. This suggests that this third group could match pre-existing PCOS “masked” by the gonadotropin deficiency of the FHA and would correspond to the cases reported by others [17, 20, 22, 23]. Our study is retrospective and cannot attest the revelation of true PCOS in some of our “FHA-PCOM” patients. To do so, it would be interesting to run a prospective trial and to systematically look for revelation of PCOS during the stimulation (elevation of serum androgens levels, increased number of follicles on ultrasound and/or serum AMH level…) [36].
The limitations of our study are mainly linked to its retrospective design. Although it might be the largest sample of patients with “FHA-PCOM” (40 patients, 84 initiated cycles), it remains a retrospective study with insufficient methodology to recommend pulsatile GnRH therapy as first-line treatment for ovulation induction. Also, the high prevalence of “FHA-PCOM” patients in this series was presumably due to a referral bias. Also, there are some controversies about the definition of PCOM, which is commonly defined by an excessive FNPO ≥ 12. However, this cut-off is highly dependent on ultrasound equipment and operator skill. Therefore, with the latest ultrasound generation, Dewailly et al. [27] have proposed a new threshold of 19 FNPO. Similarly, a panel of international experts has recently suggested a threshold of 25 follicles, when the ultrasound probe provides a maximum frequency greater that 8 MHz [37]. Serum AMH concentration is strongly correlated with the FNPO since it is mostly secreted from the small antral follicles from 2 to 9 mm, counted on ultrasound [37–40]. Dewailly et al. [27] have described a correlation between FNPO and serum AMH and defined serum AMH level ≥ 35 pmol/L as a surrogate for PCOM. However, this threshold was established with a specific centre and whether it can be extrapolated to other centres (using different control populations and AMH assays) still has to be verified. Last, the cost of one ovulatory cycle remains more expensive with the pump than with gonadotropins. However, in another study (under submission), we have compared the use of these two treatments for ovulation induction in “FHA-PCOM” patients and we showed that it was faster to induce a pregnancy with pulsatile GnRH therapy. Indeed, the on-going pregnancy rate after one initiated cycle of GnRH therapy was 28.9 %, while 3 subsequent cycles of gonadotropins had to be initiated to reach a close rate of 23.6 %. So, the total final cost for a pregnancy was relatively close between pulsatile GnRH therapy (1643€) and hMG (1421€), but much more expensive with recombinant gonadotropins (4334€). We did not take into account the costs of monitoring and blood samples (heavier with gonadotropins) and of eventual hospitalization for OHSS (more frequent with gonadotropins). As to the eventual discomfort of the pump, in our experience, most of the patients did not consider that carrying the device was worse than the daily injections of gonadotropins, where the stimulation duration was significantly longer.
Conclusion
In conclusion, this study demonstrates that for ovulation induction, pulsatile GnRH therapy yields as good results in women having both FHA and PCOM as in women with FHA only. Therefore the presence of PCOM should not alter the management of FHA patients wishing a pregnancy. However, these findings need to be confirmed by prospective studies in order to make pulsatile GnRH therapy the first-line treatment for “FHA-PCOM” patients, just as it is for patients with FHA only.
Ethics, consent and permissions
This study was approved by the International Review Board of the University of Lille and all patients had given consent for the use of their clinical record.
References
Santoro N. Update in hyper- and hypogonadotropic amenorrhea. J Clin Endocrinol Metab. 2011;96(11):3281–8. doi:10.1210/jc.2011-1419.
Couzinet B, Young J, Brailly S, Le Bouc Y, Chanson P, Schaison G. Functional hypothalamic amenorrhoea: a partial and reversible gonadotrophin deficiency of nutritional origin. Clin Endocrinol (Oxf). 1999;50(2):229–35.
Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea-an update. J Clin Endocrinol Metab. 2015;100(3):812–24. doi:10.1210/jc.2014-3344.
Christin-Maitre S, de Crecy M. Groupe Français des pompes à GnRH. Pregnancy outcomes following pulsatile GnRH treatment: results of a large multicenter retrospective study. J Gynecol Obstet Biol Reprod. 2007;36(1):8–12. doi:10.1016/j.jgyn.2006.12.001.
Martin KA, Hall JE, Adams JM, Crowley Jr WF. Comparison of exogenous gonadotropins and pulsatile gonadotropin-releasing hormone for induction of ovulation in hypogonadotropic amenorrhea. J Clin Endocrinol Metab. 1993;77(1):125–9. doi:10.1210/jcem.77.1.8325934.
Mais V, Melis GB, Strigini F, Antinori D, de Ruggiero A, Fioretti P. Adjusting the dose to the individual response of the patient during the induction of ovulation with pulsatile gonadotropin-releasing hormone. Fertil Steril. 1991;55(1):80–5.
Homburg R, Eshel A, Armar NA, Tucker M, Mason PW, Adams J, et al. One hundred pregnancies after treatment with pulsatile luteinising hormone releasing hormone to induce ovulation. BMJ. 1989;298(6676):809–12.
Rotterdam ESHRE/ASRM sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod.19(1):41–7
Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685–97. doi:10.1016/S0140-6736(07)61345-2.
Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9(6):505–14.
Burger CW, Korsen TJ, Hompes PG, van Kessel H, Schoemaker J. Ovulation induction with pulsatile luteinizing releasing hormone in women with clomiphene citrate-resistant polycystic ovary-like disease: clinical results. Fertil Steril. 1986;46(6):1045–54.
Eshel A, Abdulwahid NA, Armar NA, Adams JM, Jacobs HS. Pulsatile luteinizing hormone-releasing hormone therapy in women with polycystic ovary syndrome. Fertil Steril. 1988;49(6):956–60.
Corenthal L, Von Hagen S, Larkins D, Ibrahim J, Santoro N. Benefits of continuous physiological pulsatile gonadotropin-releasing hormone therapy in women with polycystic ovarian syndrome. Fertil Steril. 1994;61(6):1027–33.
Bayram N, van Wely M, van der Veen F. Pulsatile gonadotrophin releasing hormone for ovulation induction in subfertility associated with polycystic ovary syndrome. Cochrane Database Syst Rev. 2004;1:CD000412. doi:10.1002/14651858.CD000412.pub2.
Thessaloniki, ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod. 2008;23(3):462–77. doi:10.1093/humrep/dem426.
Robin G, Gallo C, Catteau-Jonard S, Lefebvre-Maunoury C, Pigny P, Duhamel A, et al. Polycystic Ovary-Like Abnormalities (PCO-L) in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2012;97(11):4236–43. doi:10.1210/jc.2012-1836.
Reyss AC, Merlen E, Demerle C, Dewailly D. Revelation of a polymicrocystic ovary syndrome after one month’s treatment by pulsatile GnRH in a patient presenting with functional hypothalamic amenorrhea. Gynecol Obstet Fertil. 2003;31(12):1039–42.
Mattle V, Bilgyicildirim A, Hadziomerovic D, Ott HW, Zervomanolakis I, Leyendecker G, et al. Polycystic ovarian disease unmasked by pulsatile GnRH therapy in a subgroup of women with hypothalamic amenorrhea. Fertil Steril. 2008;89(2):404–9. doi:10.1016/j.fertnstert.2007.02.063.
Rossmanith WG, Wirth U, Benz R, Wolf AS. Endocrine dynamics during pulsatile GnRH administration in patients with hypothalamic amenorrhea and polycystic ovarian disease. Gynecol Endocrinol. 1989;3(1):21–34.
Schachter M, Balen AH, Patel A, Jacobs HS. Hypogonadotropic patients with ultrasonographically detected polycystic ovaries: endocrine response to pulsatile gonadotropin-releasing hormone. Gynecol Endocrinol. 1996;10(5):327–35.
Filicori M, Campaniello E, Michelacci L, Pareschi A, Ferrari P, Bolelli G, et al. Gonadotropin-releasing hormone (GnRH) analog suppression renders polycystic ovarian disease patients more susceptible to ovulation induction with pulsatile GnRH. J Clin Endocrinol Metab. 1988;66(2):327–33. doi:10.1210/jcem-66-2-327.
Adams J, Franks S, Polson DW, Mason HD, Abdulwahid N, Tucker M, et al. Multifollicular ovaries: clinical and endocrine features and response to pulsatile gonadotropin releasing hormone. Lancet. 1985;2(8469–70):1375–9.
Wang JG, Lobo RA. The complex relationship between hypothalamic amenorrhea and polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(4):1394–7. doi:10.1210/jc.2007-1716.
Shoham Z, Conway GS, Patel A, Jacobs HS. Polycystic ovaries in patients with hypogonadotropic hypogonadism: similarity of ovarian response to gonadotropin stimulation in patients with polycystic ovarian syndrome. Fertil Steril. 1992;58(1):37–45.
Sum M, Warren MP. Hypothalamic amenorrhea in young women with underlying polycystic ovary syndrome. Fertil Steril. 2009;92(6):2106–8. doi:10.1016/j.fertnstert.2009.05.063.
Dubourdieu S, Freour T, Dessolle L, Barriere P. Prospective, randomized comparison between pulsatile GnRH therapy and combined gonadotropin (FSH + LH) treatment for ovulation induction in women with hypothalamic amenorrhea and underlying polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;168(1):45–8. doi:10.1016/j.ejogrb.2012.12.016.
Dewailly D, Gronier H, Poncelet E, Robin G, Leroy M, Pigny P, et al. Diagnosis of polycystic ovary syndrome (PCOS): revisiting the threshold values of follicle count on ultrasound and of the serum AMH level for the definition of polycystic ovaries. Hum Reprod. 2011;26(11):3123–9. doi:10.1093/humrep/der297.
Dewailly D, Catteau-Jonard S, Reyss AC, Leroy M, Pigny P. Oligoanovulation with polycystic ovaries but not overt hyperandrogenism. J Clin Endocrinol Metab. 2006;91(10):3922–7. doi:10.1210/jc.2006-1054.
Dewailly D, Pigny P, Soudan B, Catteau-Jonard S, Decanter C, Poncelet E, et al. Reconciling the definitions of polycystic ovary syndrome: the ovarian follicle number and serum anti-Mullerian hormone concentrations aggregate with the markers of hyperandrogenism. J Clin Endocrinol Metab. 2010;95(9):4399–405. doi:10.1210/jc.2010-0334.
Hull ME, Moghissi KS, Magyar DM, Hayes MF, Zador I, Olson JM. Correlation of serum estradiol levels and ultrasound monitoring to assess follicular maturation. Fertil Steril. 1986;46(1):42–5.
Gompel A, Mauvais-Jarvis P. Induction of ovulation with pulsatile GnRH in hypothalamic amenorrhoea. Hum Reprod. 1988;3(4):473–7.
Catteau-Jonard S, Bancquart J, Poncelet E, Lefebvre-Maunoury C, Robin G, Dewailly D. Polycystic ovaries at ultrasound: normal variant or silent polycystic ovary syndrome? Ultrasound Obstet Gynecol. 2012;40(2):223–9. doi:10.1002/uog.11202.
Duijkers IJ, Klipping C. Polycystic ovaries, as defined by the 2003 Rotterdam consensus criteria, are found to be very common in young healthy women. Gynecol Endocrinol. 2010;26(3):152–60. doi:10.1080/09513590903247824.
Johnstone EB, Rosen MP, Neril R, Trevithick D, Sternfeld B, Murphy R, et al. The polycystic ovary post-rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95(11):4965–72. doi:10.1210/jc.2010-0202.
Kristensen SL, Ramlau-Hansen CH, Ernst E, Olsen SF, Bonde JP, Vested A, et al. A very large proportion of young Danish women have polycystic ovaries: is a revision of the Rotterdam criteria needed? Hum Reprod. 2010;25(12):3117–22. doi:10.1093/humrep/deq273.
Catteau-Jonard S, Dewailly D. Pathophysiology of polycystic ovary syndrome: the role of hyperandrogenism. Front Horm Res. 2013;40:22–7. doi:10.1159/000341679.
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–85. doi:10.1093/humupd/dmt062.
Fanchin R, Schonauer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-Mullerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18(2):323–7.
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19(8):519–27. doi:10.1093/molehr/gat024.
van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Mullerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–71.
Acknowledgments
The authors thank Sharon Tetlow for assistance in language corrections.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AD: made substantial contributions to conception and design, and acquisition of data, and analysis and interpretation of data, and drafting the article, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. DD: revised the article critically for important intellectual content, and had given final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. PP: revised the article critically for important intellectual content, and had given final approval of the version to be published. SCJ: revised the article critically for important intellectual content, and had given final approval of the version to be published. GR: made substantial contributions to conception and design and interpretation of data, and revising the article critically for important intellectual content and had given final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Dumont, A., Dewailly, D., Plouvier, P. et al. Does polycystic ovarian morphology influence the response to treatment with pulsatile GnRH in functional hypothalamic amenorrhea?. Reprod Biol Endocrinol 14, 24 (2016). https://doi.org/10.1186/s12958-016-0159-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-016-0159-8