Abstract
Background
The aim of this study was to identify predictors of mortality in elderly patients undergoing colorectal cancer surgery and to develop a risk score.
Methods
This was an observational prospective cohort study. Individuals over 80 years diagnosed with colorectal cancer and treated surgically were recruited in 18 hospitals in the Spanish National Health Service, between June 2010 and December 2012, and were followed up 1, 2, 3, and 5 years after surgery. Sociodemographic and clinical data were collected. The primary outcomes were mortality at 2 and between 2 and 5 years after the index admission.
Results
The predictors of mortality 2 years after surgery were haemoglobin ≤ 10 g/dl and colon locations (HR 1.02; CI 0.51–2.02), ASA class of IV (HR 3.55; CI 1.91–6.58), residual tumour classification of R2 (HR 7.82; CI 3.11–19.62), TNM stage of III (HR 2.14; CI 1.23–3.72) or IV (HR 3.21; CI 1.47–7), LODDS of more than − 0.53 (HR 3.08; CI 1.62–5.86)) and complications during admission (HR 1.73; CI 1.07–2.80). Between 2 and 5 years of follow-up, the predictors were no tests performed within the first year of follow-up (HR 2.58; CI 1.21–5.46), any complication due to the treatment within the 2 years of follow-up (HR 2.47; CI 1.27–4.81), being between 85 and 89 and not having radiotherapy within the second year of follow-up (HR 1.60; CI 1.01–2.55), no colostomy closure within the 2 years of follow-up (HR 4.93; CI 1.48–16.41), medical complications (HR 1.61; CI 1.06–2.44), tumour recurrence within the 2 years of follow-up period (HR 3.19; CI 1.96–5.18), and readmissions at 1 or 2 years of follow-up after surgery (HR 1.44; CI 0.86–2.41).
Conclusion
We have identified variables that, in our sample, predict mortality 2 and between 2 and 5 years after surgery for colorectal cancer older patients. We have also created risks scores, which could support the decision-making process.
Trial registration
Similar content being viewed by others
Introduction
Populations are ageing all around the world. In Spain, in 2016, 18% of the population was over 60 years, with octogenarians representing 6% of the total, and these percentages will grow in the coming years [1].
Cancer is one of the main causes of morbidity and mortality worldwide, with 18 million new cases and 9.6 million deaths in 2018 [2]. Colorectal cancer was the most frequent cancer, with 1.8 million new cases and almost 861,000 deaths [2]. As for the incidence of cancer in the elderly, a review stated that it is 11-fold compared with younger patients [3]. There is therefore an increase in the average age at the time of cancer diagnosis.
The relationship between age and mortality due to cancer is complex, as it can be confounded by other factors, such as differences in stage at presentation, tumour site, or type of treatment received [4, 5]. Therefore, it becomes important to have systems of stratification of these patients to see who could have a better long-term prognosis and/or who could benefit from certain treatments.
Several publications have described factors influencing mortality in the older patients due to colorectal cancer [6,7,8], but most of this research has focused on the short term. Studies that have analysed factors in the long term have identified complications, American Society of Anaesthesiologists (ASA) class, tumour stage, or increased age as having a significant influence on mortality in older patients [5, 9,10,11,12,13]. Nevertheless, the majority of these studies have employed multivariable analysis, without any classification or scoring system. The aim of this study was to detect variables that have the most weight in the prediction of mortality in older colorectal cancer patients and to develop a risk score to stratify this population.
Materials & methods
The data presented in this manuscript is a post hoc analysis that comes from a prospective observational cohort study that recruited patients diagnosed with colorectal cancer who were treated surgically. Patients were recruited in 18 hospitals in the Spanish National Health Service, between June 2010 and December 2012, and they were followed up 1, 2, and 5 years after the surgery.
The inclusion criteria were that patients were diagnosed with cancer of the colon or rectum (between the anal margin and 15 cm above it), had curative or palliative surgery performed for first time, and signed the informed consent form to participate in the study. For this manuscript, we added an age criterion, selecting patients 80 years or older. Patients were excluded if they had in situ cancer, inoperable tumours, a severe mental or physical condition that prevented the patient from responding to questionnaires, or terminal illness.
Patients were identified from the surgical waiting lists and were invited to participate during a clinical appointment or by letter. After this selection process, clinical data were collected at baseline, 1 month, and 1, 2, and 5 years after the surgery.
Patients were informed of the objectives of the study, and they were asked to provide written informed consent before inclusion. The Institutional Review Boards of the participating hospitals approved this project. More details of this study can be found in an earlier publication [14].
Data collection
Data were collected from the medical records by trained reviewers, employing data collection forms and an instruction manual to ensure consistency.
Patients who did not survive within the 30-day period after the index surgery were excluded in this study.
Baseline data included sociodemographic characteristics; clinical history; preoperative findings, in particular, laboratory test results, diagnostic test results, and tumour site; and data from the outpatient preoperative anaesthesia appointment, including ASA class [15].
Data related to hospital admission included data on the surgical intervention; histopathological data, including TNM stage; residual tumour classification after surgery; number of organ invasion; lymph node involvement, expressed as the log odds of positive lymph nodes (LODDS) [16]; length of stay; presence and degree of complications, grouped in surgical, medical, infectious, and haematological (haemorrhage/thrombosis/embolism); types of treatment given, including need for reintervention; and death.
Finally, data were collected on relevant variables up to 30 days after surgery (laboratory and diagnostic test results, presence of complications, readmissions, reintervention, or death) and through the first, second, and fifth postoperative years (radiation therapy, chemotherapy, laboratory and diagnostic test results, colostomy closure within the 2 years follow-up, presence of complications, tumour recurrence, readmission or reoperation, and death).
Outcome measures
The primary outcomes were mortality at 2 and between 2 and 5 years after the patient was first admitted to the hospital for surgery for colorectal cancer (index admission). Vital status was established by reviewing medical records and examining the hospital database and public registers of deaths. Deaths were considered confirmed if the name, sex, date of birth, and identity card number on the record matched those of the participant.
Statistical analysis
Descriptive statistics of the retrieved variables were calculated using means and standard deviations (SD) and median and interquartile ranges for quantitative data and frequencies and percentages for categorical variables. Chi-square and Fisher’s exact tests were used for comparing categorical variables and Student’s t test or the nonparametric Wilcoxon test for assessing the relationship of mortality up to 2 and between 2 and 5 years with potentially relevant continuous variables.
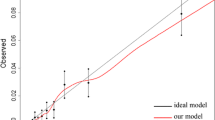
In the multivariable analysis, a Cox regression model was developed that used mortality up to 2 years and between 2 and 5 years (excluding those patients who did not survive at 2 years) as the dependent variable. In addition, the hospital effect was added to the model, in order to analyse any variation caused by differences between centres. When that effect showed no statistical significant differences, it was removed from the model. The goodness of fit of the models was assessed with the Greenwood-Nam-D'Agostino (GND) test [17] and the C-index.
Two mortality risk scores were developed, one for each of the studied outcomes. To develop these predictive risk scores and therefore, to determine the scoring weights related to the variable categories of each predictor involved in the punctuation, we first assigned a weight to each risk factor in relation to each β parameter based on the multivariate Cox regression model; as the first step, regression coefficients that turned out to be statistically significant were selected. The smallest beta coefficient was identified to divide each of the significant betas by this value. The resulting value led to the corresponding weight of each of the predictor categories. Then, weights of each of the risk factors presented by a patient were summed, with a higher score corresponding to a higher likelihood of death. Considering the optimal cut-points [18], three severity categories were created for each score, and Kaplan–Meier curves were plotted for each risk group. As for internal validations of our models, a total of 500 bootstrap samples were generated to calculate the C-index and their corresponding confidence intervals of the scores. The GND test was also used to assess the goodness of fit of the models.
All effects were deemed statistically significant at p < 0.05. All statistical analyses were performed using SAS Software, version 9.4 (SAS Institute, Inc., Carey, NC, USA), and figures were depicted using R statistical software, version 3.5.
Results
The sample of patients aged 80 years or older was comprised of 426 individuals, of whom, 21 (4.92%) died within the first 30 days after the index surgery. Out of those who fulfilled the criteria (n = 405), 92 (22.71%) died during the first 2 years and a further 99 (31.63%) between the second and the fifth year of follow-up. These 405 patients had a mean age of 83.24 years (SD 2.93) and the 92% had an ASA classification lower than class IV. Descriptive data and univariate analyses are shown in Table 1.
The independent predictors of mortality in patients 80 years or older 2 years after surgery were having haemoglobin ≤ 10 g/dl and colon cancer (vs haemoglobin > 10 and colon cancer), an ASA class of IV (vs I, II, III), a residual tumour classification of R2 (vs R0), a TNM stage of III or IV (vs 0, I, II), LODDS of more than – 0.53 (vs less than − 1.36), and complications during admission (Table 2). The model showed good discrimination, with a C-index of 0.80 (95% CI: 0.78–0.87). Likewise, the GND test was < 0.001.
Between the second and the fifth year of follow-up, the predictors of mortality were no tests performed within the first year of follow-up (vs CAT, colonoscopy and CAT + colonoscopy), any complication due to the treatment within the 2 years of follow-up, being between 85 and 89 and not having radiotherapy within the second year of follow-up, not having the colostomy closed within the 2 years of follow-up, having medical complications, tumour recurrence within the 2 years of follow-up period, and having readmissions at 1 or 2 years of follow-up (Table 3). In this model, the C-index was 0.73 (95% CI 0.68–0.78), which demonstrates good discrimination of the model. The GND test was < 0.001.
Table 4 shows that the risk of 2 years mortality was significantly higher in those patients whose score was ≥ 8 (hazard ratio, 10.50; 95% CI, 6.02–18.29; p < 0.001) and for patients with a score of 4–7 (hazard ratio, 3.08; 95% CI, 1.71–5.55; p < 0.001), compared to those in the lowest risk group. With regards to the 2- to 5-year mortality risk, it was higher in the patients with a score ≥ 17 (hazard ratio, 6.10; 95% CI, 3.19–11.66; p < 0.001) and in the moderate risk group (hazard ratio, 1.99; 95% CI, 1.15–3.45; p = 0.015), compared with the patients with the lowest risk.
This same pattern can be seen in the Kaplan–Meier curves, shown in the Online Resource. The probability of surviving 2 years after the intervention was below 0.4 for patients classified as having a high risk of death, rising to near 0.8 for those with moderate risk and higher for patients with a low risk of mortality. The probabilities of surviving between the second and the fifth year of follow-up years after the surgery were 0.4, 0.8, and 0.9 for each risk group, respectively (Figs. 1 and 2).
Internal validation of all the models were performed where it can be seen that those variables selected for our two models are the ones which were selected more frequently by bootstrap (Table 5).
Discussion
This study has identified variables that, in our sample, predict mortality at 2 and between 2 and 5 years after surgery for colorectal cancer, in patients 80 years or older. In addition, we have created risk scores for each follow-up time point, which can help classify patients by mortality risk.
Some of these variables have also been found to be mortality predictors in other studies, namely, cancer stage [9,10,11, 13], ASA class [7, 9,10,11], LODDS [19], tumour recurrence [20], and complications [9, 10, 21], with similar results to those found in our study.
Regarding haemoglobin, Kim and Kim [11] did not find it to be predictive of mortality in their sample, and in other studies, it was evaluated as predictor of mortality and/or complications on the short term [22, 23].
We have not found studies analysing two other variables identified in our models, i.e., residual tumour classification and colostomy closure. The residual tumour classification is usually employed to assess the presence of tumour tissue remaining after surgery [21]. As has been found in our study, patients with a poorer response to treatment, reflected in a higher score in residual tumour classification, generally have a poorer prognosis [21]. As far as colostomy closure is concerned, the results seem to indicate that the evolution is worse in those where the closure has not been performed.
Some variables have been identified as predictors of mortality in other studies with older patients, but not in ours: increased age [5, 7, 9, 12, 13], operative urgency [7], no cancer excision vs resection [7], living in an institution [10], and male sex [9]. Regarding age, although some authors have reported poorer outcomes in older patients [9, 24], others have not found differences in prognosis as a function of age [4, 25], so further studies are needed to provide more evidence on the evolution of cancer in this patient profile [26, 27].
Studies on colorectal cancer prognosis regardless of age limit have not found very different prognostic variables to those already mentioned for older patients [28,29,30,31]. Specifically, the predictors found in these studies were the following: tumour site (mixed results), advanced tumour stage, blood transfusion, older age, high grade, male sex, Chinese ethnicity, high carcinoembryonic antigen levels, emergency surgery, bowel obstruction, blood or lymphatic vessel invasion, and positive radial margins.
The variables mentioned so far are mainly clinical and related to the evolution of patients after the intervention. However, in our mortality prediction model at 5 years, other variables have been identified that evaluate the follow-up of these patients, in terms of the use of health resources such as specialist consultations, tests, treatments, emergency visits, or readmissions. In addition to the variables mentioned above, our study has identified others that predict mortality between 2 and 5 years from diagnosis, such as colostomy closure and readmissions during the years following surgery. There are already guidelines and recommendations on medium–long-term follow-up of these patients [32, 33], some of which indicate that survival increases when more intensive follow-ups are performed [33]. Therefore, it is important that future studies consider this type of variable so that health services can make decisions about the surveillance to be carried out on these patients.
Another difference between our study and others is that we have found identifying predictors of mortality in colorectal cancer is the follow-up period. Most other studies have chosen shorter follow-up periods, and only Hessman et al. [10] presented data for 5 years after the surgery. That is, our study provides mortality prediction information on a relatively long follow-up period.
This study presents some strengths that should be highlighted. First of all, it is a large prospective cohort study, with 18 participating hospitals, which ensures more variability. Notably, the main studies we have mentioned in comparisons with our results were all retrospective. Second, this cohort has been followed for 5 years, which is a considerably long period that allows observing the evolution of these patients and detecting other factors that influence mortality, in addition to those more directly related to the surgery. Third, we have identified common variables on which data can be obtained easily as predictors of mortality, and these have been combined into single scores, providing information on patient prognosis, which could help in the decision-making process regarding treatment and/or follow-up. Finally, most previous studies focused on the prediction of mortality from colorectal cancer in older patients have used multivariable models, but have not explored other ways of analysing, interpreting, and presenting the data. In our study, we have developed a risk score, which could help clinicians interpret the data more easily. Only Heriot et al. [7] conducted this kind of analysis, and they followed patients until just 30 days after surgery, not considering longer-term factors.
On the other hand, we should also recognise some limitations of our research. First, as in any prospective study, missing data is a source of bias. We attempted to reduce this bias by training the reviewers in each centre, to strengthen consistency in the collection of the information. Secondly, we have presented the results together for colon and rectal cancer, despite some authors having pointed out special features of these types of cancer that make them suitable for separate analysis [34, 35]. Most of the studies we have found in the literature present results for colorectal cancer, and as do some of the reviews and reports that study these types of cancer in older patients [4]. Besides, we have not found significant differences in our results when considering cancer localisation. Future studies should investigate this issue, with larger sample sizes and multicentre approaches, seeking to obtain more generalizable data. Finally, an external validation would help confirming the robustness of the risk models that have been developed.
Conclusions
The risk scores developed could be easily employed by clinicians as prediction rules helping the decision-making process following at long term older colorectal cancer patients as they contain important information that could condition decisions that need to be made at that point regarding, for example, which treatments to administer and when to follow these patients in order to reduce mortality.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Abellán García A, Ayala García A, Pujol RR. Un perfil de las personas mayores en España, 2017. Indicadores estadísticos básicos, vol. 15. Madrid: Informes Envejecimiento en red; 2017. p. 48.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Pallis AG, Fortpied C, Wedding U, Van Nes MC, Penninckx B, Ring A, et al. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer. 2010;46(9):1502–13. https://doi.org/10.1016/j.ejca.2010.02.022.
Colorectal Cancer Collaborative Group. Surgery for colorectal cancer in elderly patients: a systematic review. Lancet. 2000;356:968–74. https://doi.org/10.1016/S0140-6736(00)02713-6.
van den Berg I, van den Braak RRJC, van Vugt JLA, Ijzermans JNM, Buettner S. Actual survival after resection of primary colorectal cancer: results from a prospective multicenter study. World J Surg Oncol. 2021;19. https://doi.org/10.1186/s12957-021-02207-4.
Arnaud JP, Schloegel M, Ollier JC, Adloff M. Colorectal cancer in patients over 80 years of age. Dis Colon Rectum. 1991;34(10):896–8. https://doi.org/10.1007/BF02049704.
Heriot A, Tekkis P, Smith J, Cohen CR, Montgomery A, Audisio R, et al. Prediction of postoperative mortality in elderly patients with colorectal cancer. Dis Colon Rectum. 2006;49(6):816–24. https://doi.org/10.1007/s10350-006-0523-4.
Vather R, Zargar-Shoddhtari K, Agegbola S, Hill AG. Comparison of the POSSUM, P-POSSUM and CR-POSSUM scoring systems as predictors of postoperative mortality in patients undergoing major colorectal surgery. ANZ J Surg. 2006;76(9):812–6. https://doi.org/10.1111/j.1445-2197.2006.03875.x.
Duraes LC, Stocchi L, Dietz DW, Kalady MF, Kessler HP, Remzi FH. The disproportionate effect of perioperative complications on mortality with 1 year after colorectal cancer resection in octogenarians. Ann Surg Oncol. 2016;23(13):4293–301. https://doi.org/10.1245/s10434-016-5445-3.
Hessman O, Bergkvist L, Ström S. Colorectal cancer in patients over 75 years of age--determinants of outcome. Eur J Surg Oncol. 1997;23(1):13–9. https://doi.org/10.1016/s0748-7983(97)80136-9.
Kim YW, Kim IY. Factors associated with postoperative outcomes and 1-year mortality after surgery for colorectal cancer in octogenarians and nonagenarians. Clin Interv Aging. 2016;11:689–97. https://doi.org/10.2147/CIA.S104783.
Kunitake H, Zingmond DS, Ryoo J, Ko CY. Caring for octogenarian and nonagenarian patients with colorectal cancer: what should our standards and expectations be? Dis Colon Rectum. 2010;53(5):735–43. https://doi.org/10.1007/DCR.0b013e3181cdd658.
Wang Z, Wang Y, Yang Y, Luo Y, Liu J, Xu Y, et al. A competing-risk nomogram to predict cause-specific death in elderly patients with colorectal cancer after surgery (especially for colon cancer). World J Surg Oncol. 2020;18(1):30. https://doi.org/10.1186/s12957-020-1805-3.
Quintana JM, Gonzalez N, Anton-Ladislao A, Redondo M, Bare M, de Larrea NF, et al. Colorectal cancer health services research study protocol: the CCR-CARESS observational prospective cohort project. BMC Cancer. 2016;16(1):435. https://doi.org/10.1186/s12885-016-2475-y.
Hightower CE, Riedel BJ, Feig BW, Morris GS, Ensor JE, Woodruff VD, et al. A pilot study evaluating predictors of postoperative outcomes after major abdominal surgery: physiological capacity compared with the ASA physical status classification system. Brit J Anaesth. 2010;104(4):465–71. https://doi.org/10.1093/bja/aeq034.
Sun Z, Xu Y, Li DM, Wang ZN, Zhu GL, Huang BJ, et al. Log odds of positive lymph nodes: a novel prognostic indicator superior to the number-based and the ratio-based N category for gastric cancer patients with R0 resection. Cancer. 2010;116(11):2571–80. https://doi.org/10.1002/cncr.24989.
Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness of fit in the survival setting. Stat Med. 2015;34(10):1659–80. https://doi.org/10.1002/sim.6428.
Barrio I, Arostegui I, Rodríguez-Álvarez MX, Quintana JM. A new approach to categorising continuous variables in prediction models: proposal and validation. Stat Methods Med Res. 2017;26(6):2586–602. https://doi.org/10.1177/0962280215601873.
Fielding LP, Phillips RK, Fry JS, Hittinger R. Prediction of outcome after curative resection for large bowel cancer. Lancet. 1986;2(8512):904–7. https://doi.org/10.1016/s0140-6736(86)90422-8.
Mäkelä JT, Klintrup KH, Rautio TT. Mortality and survival after surgical treatment of colorectal cancer in patients aged over 80 years. Gastrointest Tumors. 2017;4:36–44. https://doi.org/10.1159/000477721.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4. https://doi.org/10.1245/s10434-010-0985-4.
Yang J, Zhang T, Feng D, Dai X, Lv T, Wang X, et al. A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Colorectal Dis. 2019;21(5):538–47. https://doi.org/10.1111/codi.14558.
Leung E, McArdle K, Wong LS. Risk-adjusted scoring systems in colorectal surgery. Int J Surg. 2011;9(2):130–5. https://doi.org/10.1016/j.ijsu.2010.10.016.
Jensen H, Nielsen J, Baslev I. Carcinoma of the colon in old age. Ann Surg. 1970;171(1):107–15. https://doi.org/10.1097/00000658-197001000-00016.
Jin KM, Wang K, Bao Q, Wang HW, Xing BC. Liver resection for colorectal liver-limited metastases in elderly patients: a propensity score matching analysis. World J Surg Oncol. 2020;18(1):275. https://doi.org/10.1186/s12957-020-02055-8.
Ramesh HSJ, Pope D, Gennari R, Audisio RA. Optimising surgical management of elderly cancer patients. World J Surg Oncol. 2005;3:17. https://doi.org/10.1186/1477-7819-3-17.
Roque-Castellano C, Fariña-Castro R, Nogués-Ramia EM, Artiles-Armas M, Marchena-Gómez J. Colorectal cancer surgery in selected nonagenarians is relatively safe and it is associated with a good long-term survival: an observational study. World J Surg Oncol. 2020;18(1):120. https://doi.org/10.1186/s12957-020-01895-8.
Magaji BA, Moy FM, Roslani AC, Law CW. Survival rates and predictors of survival among colorectal cancer patients in a Malaysian tertiary hospital. BMC Cancer. 2017;17(1):339. https://doi.org/10.1186/s12885-017-3336-z.
Mehrkhani F, Nasiri S, Donboli K, Meysamie A, Hedayat A. Prognostic factors in survival of colorectal cancer patients after surgery. Colorectal Dis. 2009;11(2):157–61. https://doi.org/10.1111/j.1463-1318.2008.01556.x.
Ratto C, Sofo L, Ippoliti M, Merico M, Doglietto GB, Crucitti F. Prognostic factors in colorectal cancer. Literature review for clinical application. Dis Colon Rectum. 1998;41(8):1033–49. https://doi.org/10.1007/BF02237397.
Wolters U, Stützert H, Keller HW, Schröder U, Pichlmaier H. Colorectal cancer a multivariate analysis of prognostic factors. Eur J Surg Oncol. 1996;22(6):592–7. https://doi.org/10.1016/S0748-7983(96)92320-3.
Vera R, Aparicio J, Carballo F, Esteva M, González-Flores E, Santianes J, et al. Recommendations for follow-up of colorectal cancer survivors. Clin Transl Oncol. 2019;21(6):1302–11. https://doi.org/10.1016/s0748-7983(96)92320-3.
Pita-Fernández S, Alhayek-Aí M, González-Martín C, López-Calviño B, Seoane-Pillado T, Pértega-Díaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26(4):644–56. https://doi.org/10.1093/annonc/mdu543.
Chiappa A, Zbar AP, Bertani E, Biella F, Audisio RA, Staudacher C. Surgical outcomes for colorectal cancer patients including the elderly. Hepatogastroenterology. 2001;48(38):440–4.
Vironen JH, Sainio P, Husa AI, Kellokumpu IH. Complications and survival after surgery for rectal cancer in patients younger than and aged 75 years or older. Dis Colon Rectum. 2004;47(7):1225–31. https://doi.org/10.1007/s10350-004-0557-4.
Acknowledgments
We thank the participating patients who voluntarily took part in this study. We also thank the doctors and all the interviewers from the participating hospitals (Hospital de Antequera, Hospital Costa del Sol, Hospital Universitario de Valme, Hospital Universitario Virgen del Rocío, Hospital Universitario Virgen de las Nieves, Hospital Universitario de Canarias, Corporació Sanitaria Parc Taulí, Althaia, Hospital del Mar, Hospital Clínico San Carlos, Hospital Universitario La Paz, Hospital Infanta Sofía, Hospital Universitario Fundación Alcorcón, Hospital Galdakao-Usansolo, Hospital Universitario Araba, Hospital Universitario Basurto, Hospital Universitario Cruces, Hospital Hospitalario Donostia, Hospital Bidasoa, Hospital de Mendaro, Hospital de Zumarraga and Hospital Universitario Doctor Peset) for their invaluable collaboration in patient recruitment, and to the Research Committee of the participating hospitals.
The REDISSEC CARESS-CCR (Results and Health Services Research in Colorectal Cancer) group
Jose María Quintana1,2, Marisa Baré2,3, Maximino Redondo2,4, Eduardo Briones5, Nerea Fernández de Larrea6,7, Cristina Sarasqueta2,8, Antonio Escobar2,9, Francisco Rivas2,10, Maria M. Morales-Suárez-Varela7,11,12, Juan Antonio Blasco13, Isabel del Cura14, Inmaculada Arostegui2,15, Irantzu Barrio2,15, Amaia Bilbao2,9, Nerea González1, Susana García-Gutiérrez1, Iratxe Lafuente1, Urko Aguirre1, Miren Orive1, Josune Martin1, Ane Antón-Ladislao1, Núria Torà2,16, Marina Pont2,16, María Purificación Martínez del Prado17, Alberto Loizate18, Ignacio Zabalza19, José Errasti20, Antonio Z Gimeno21, Santiago Lázaro22, Mercè Comas23, Jose María Enríquez24, Carlos Placer24, Amaia Perales25, Iñaki Urkidi26, Jose María Erro27, Enrique Cormenzana28, Adelaida Lacasta29, Pep Piera29, Elena Campano30, Ana Isabel Sotelo31, Segundo Gómez-Abril32, F. Medina-Cano33, Julia Alcaide34, Arturo Del Rey-Moreno35, Manuel Jesús Alcántara36, Rafael Campo37, Alex Casalots38, Carles Pericay2,39, Maria José Gil40, Miquel Pera40, Pablo Collera41, Josep Alfons Espinàs42, Mercedes Martínez43, Mireia Espallargues2,44, Caridad Almazán7,44, Paula Dujovne Lindenbaum45, José María Fernández-Cebrián45, Rocío Anula46,47, Julio Mayol46,47, Ramón Cantero48, Héctor Guadalajara49, María Alexandra Garceau49, Damián García49, Mariel Morey2,50, Alberto Colina51
1Unidad de InvestigaciónHospital Galdakao-UsansoloGaldakao, Bizkaia, Spain
2Red de Investigación en Servicios de Salud en Enfermedades Crónicas (REDISSEC)Galdakao, Bizkaia, Spain
3Epidemiologia Clínica y Cribado de CancerCorporació Sanitaria Parc Taulí, SabadellBarcelona, Spain
4Servicio de LaboratorioHospital Costa del SolMarbella, Málaga, Spain
5Unidad de EpidemiologíaServicio Andaluz de SaludDistrito Sevilla, Andalusia, Spain
6Centro Nacional de EpidemiologíaInstituto de Salud Carlos IIIMadrid, Spain
7CIBER de Epidemiología y Salud Pública (CIBERESP)Instituto de Salud Carlos IIIMadrid, Spain
8Unidad de Investigación, Hospital Universitario DonostiaInstituto de Investigación Sanitaria BiodonostiaDonostia, Spain
9Unidad de InvestigaciónHospital Universitario BasurtoBilbao, Spain
10Servicio de EpidemiologíaHospital Costa del SolMarbella, Málaga, Spain
11Department of Preventive Medicine and Public HealthUniversity of ValenciaValencia, Spain
12CSISP-FISABIOValencia, Spain
13Unidad de Evaluación de Tecnologías SanitariasAgencia Laín EntralgoMadrid, Spain
14Unidad Apoyo a Docencia-Investigación, Dirección Técnica Docencia e Investigación, Gerencia Adjunta PlanificaciónGerencia Asistencial de Atención Primaria de la Comunidad de MadridMadrid, Spain
15Departamento de Matemática Aplicada, Estadística e Investigación OperativaUPV/EHUBilbao, Spain
16Epidemiologia Clínica y Cribado de CancerCorporació Sanitaria Parc TaulíSabadell, Spain
17Servicio de OncologíaHospital Universitario BasurtoBilbao, Spain
18Servicio de Cirugía GeneralHospital Universitario BasurtoBilbao, Spain
19Servicio de Anatomía PatológicaHospital Galdakao-UsansoloGaldakao, Spain
20Servicio de Cirugía GeneralHospital Universitario ArabaVitoria-Gasteiz, Spain
21Servicio de GastroenterologíaHospital Universitario de CanariasSan Cristóbal de La Laguna, Spain
22Servicio de Cirugía GeneralHospital Galdakao-UsansoloGaldakao, Spain
23IMAS-Hospital del MarBarcelona, Spain
24Servicio de Cirugía General y DigestivaHospital Universitario DonostiaDonostia, Spain
25Instituto de Investigación Sanitaria BiodonostiaDonostia, Spain
26Servicio de Cirugía General y DigestivaHospital de MendaroMendaro, Spain
27Servicio de Cirugía General y DigestivaHospital de ZumárragaZumarraga, Spain
28Servicio de Cirugía General y DigestivaHospital del BidasoaHondarribia, Gipuzkoa, Spain
29Servicio de Oncología MédicaHospital Universitario DonostiaDonostia, Spain
30Instituto de Biomedicina de SevillaHospital Universitario Virgen del RocíoSeville, Spain
31Servicio de CirugíaHospital Universitario Virgen de ValmeSevilla, Spain
32Servicio de Cirugía General y Aparato DigestivoHospital Dr. PessetValencia, Spain
33Servicio de Cirugía General y Aparato DigestivoAgencia Sanitaria Costa del SolMarbella, Spain
34Servicio de Oncología MédicaAgencia Sanitaria Costa del SolMarbella, Spain
35Servicio de CirugíaHospital de AntequeraAntequera, Spain
36Coloproctology Unit, General and Digestive Surgery ServiceCorporació Sanitaria Parc TaulíSabadell, Spain
37Digestive Diseases DepartmentCorporació Sanitaria Parc TaulíSabadell, Spain
38Pathology ServiceCorporació Sanitaria Parc TaulíSabadell, Spain
39Medical Oncology DepartmentCorporació Sanitaria Parc TaulíSabadell, Spain
40General and Digestive Surgery ServiceParc de Salut MarBarcelona, Spain
41General and Digestive Surgery ServiceAlthaia-Xarxa Assistencial UniversitariaManresa, Spain
42Catalonian Cancer Strategy Unit, Department of HealthInstitut Català d’OncologíaBarcelona, Spain
43Medical Oncology DepartmentInstitut Català d’OncologíaBarcelona, Spain
44Agency for Health Quality and Assessment of Catalonia (AquAS)Barcelona, Spain
45Servicio de Cirugía General y del Aparato DigestivoHospital Universitario Fundación AlcorcónMadrid, Spain
46Servicio de Cirugía General y DigestivaHospital Universitario Clínico San CarlosMadrid, Spain
47Universidad Complutense de MadridMadrid, Spain
48Servicio Cirugía General y del Aparato DigestivoHospital Universitario Infanta SofíaSan Sebastián de los Reyes, Madrid, Spain
49Servicio de Cirugía General y del Aparato DigestivoHospital Universitario La PazMadrid, Spain
50Unidad de Apoyo a la Investigación, Gerencia Asistencial de Atención Primaria de la Comunidad de MadridMadrid, Spain
51Servicio de Cirugía General y del Aparato DigestivoHospital Universitario de CrucesBarakaldo, Spain
Funding
This work was supported in part by grants from the Spanish Health Research Fund (PS09/00314, PS09/00910, PS09/00746, PS09/00805, PI09/90460, PI09/90490, PI09/90397, PI09/90453, PI09/90441; PI13/01692; PI13/00013); Department of Health of the Basque Country (2010111098); KRONIKGUNE, Institute for Health Services Research (KRONIK 11/006); and the European Regional Development Fund. These institutions had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
NG participated in the study concepts, study design, data acquisition, quality control of data and algorithms, data analysis and interpretation, manuscript preparation, editing, and review. AL took part in the quality control of data and algorithms, data analysis and interpretation, statistical analysis, manuscript preparation, editing, and review. UA participated in the quality control of data and algorithms, data analysis and interpretation, manuscript editing, and review. SL took part in the study concepts, quality control of data and algorithms, data analysis and interpretation, and manuscript review. MB, MR, EB, CS, AB, and NFdL participated in the study concepts, study design, data acquisition, quality control of data and algorithms, data analysis and interpretation, and manuscript review. JMQ participated in the study concepts, study design, data acquisition, quality control of data and algorithms, data analysis and interpretation, statistical analysis, manuscript preparation, editing, and review. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Patients were informed of the objectives of the study, and they were asked to provide written informed consent before inclusion. The Institutional Review Boards of the participating hospitals approved this project: the Ethics Committees of Txagorritxu (2009–20), Galdakao, Donostia (5/09), Basurto, La Paz, Clínico San Carlos, Fundación Alcorcón, and Marbella (10/09) hospitals and the Ethics Committee of the Basque Country (PI2014084).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
González, N., Loroño, A., Aguirre, U. et al. Risk scores to predict mortality 2 and 5 years after surgery for colorectal cancer in elderly patients. World J Surg Onc 19, 252 (2021). https://doi.org/10.1186/s12957-021-02356-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-021-02356-6