Abstract
Background
Systemic immune-inflammation index (SII) has been suggested to be effective to reflect the inflammatory status and thus may be an underlying biomarker for prognosis prediction. This hypothesis has been demonstrated in meta-analyses on several cancer types. However, there was no study to confirm the prognostic roles of SII for gynecological and breast cancers, which was the goal of our study.
Methods
PubMed, EMBASE, and Cochrane Library databases were searched to collect the articles exploring the associations of SII with prognostic outcomes [overall survival (OS), disease-free survival (DFS), progression-free survival (PFS), lymph node metastasis (LNM), and lymphovascular invasion (LVI)] in gynecological and breast cancers. The prognostic value of SII was estimated by hazard ratio (HR) or relative risk (RR) with 95% confidence interval (CI).
Results
Nine articles involving 2724 patients in 11 datasets were included. Meta-analysis showed that a high SII index was significantly associated with poor OS (HR = 2.12, 95% CI, 1.61–2.79, P < 0.001), DFS/PFS (HR = 2.28, 95% CI 1.52–3.41, P < 0.001) and an increased risk for LNM (RR = 1.34, 95% CI 1.20–1.50, P < 0.001) in patients with gynecological and breast cancers. Subgroup analysis confirmed the prognostic role of SII for OS was applicable to all cancer types, but the association with DFS/PFS and LNM was only significant for ovarian cancer and breast cancer, especially triple-negative breast cancer. No significant association was detected between SII and LVI.
Conclusion
High SII may be a promising indicator for the prediction of poor prognosis in patients with gynecological and breast cancers, especially ovarian cancer and triple-negative breast cancer.
Similar content being viewed by others
Background
Gynecological and breast cancers are the two leading causes of death among women [1]. According to the epidemiological investigation in the USA in 2019, breast cancer was responsible for 41,760 deaths, followed by ovarian cancer (13,980), uterine corpus endometrial cancer (12,160), cervical cancer (4250), and vulvar cancer (1280) [2]. Recurrence and metastasis are the main contributors for the treatment failure and poor outcomes of these gynecological and breast cancer patients. Therefore, it may be a pivotal issue to identify the patients at a high risk of unfavorable prognosis in order to early schedule individualized preventive and therapeutic strategies.
In recent years, increasing evidence has shown that activation of inflammation is a crucial mechanism for the recurrence and metastasis of gynecological [3, 4] and breast [5, 6] cancers. Thus, inflammatory-related peripheral cells measured in routine blood test (such as neutrophils, lymphocytes, and platelets) and their derived index [including neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII, platelet count × neutrophil count/lymphocyte count)] may be potential prognostic biomarkers for gynecological and breast cancers. This hypothesis had been demonstrated by previous studies, especially for NLR and PLR [7,8,9,10]. Their prognostic values had been confirmed by an integrated meta-analysis of all updated evidence, that is, elevated NLR or PLR was associated with poor overall survival (OS) and disease-free survival (DFS) of patients with gynecological [7, 8] or breast [9, 10] cancer. For SII, only individual literatures were reported to reveal its prognostic ability for gynecological and breast cancers. For example, a retrospective study in ovarian cancer patients showed that SII was an independent prognostic indicator for OS and progression-free survival (PFS) not only in the training cohort [OS: hazard ratio (HR) = 6.36, 95% confidence interval (CI) = 2.64–15.33; P < 0.001; PFS: HR = 7.61, 95%CI = 3.34–17.35; P < 0.001], but also in the discovery cohort (OS: HR = 1.96, 95%CI = 1.09–3.63; P = 0.024; PFS: HR = 2.71, 95%CI = 1.48–4.93; P = 0.001) [11]. Multivariate analysis proved that increased SII correlated with poor OS (Liu et al.: HR = 2.60, 95% CI = 1.74–3.88; P < 0.001 [12]; Wang et al.: HR = 2.96, 95% CI = 2.18–3.98; P < 0.00 1[13]) and DFS (Liu et al.: HR = 1.46, 95% CI = 1.01–2.12; P = 0.045 [12]; Wang et al.: HR = 2.85, 95% CI = 1.62–3.81; P = 0.005 [13]) in patients with triple-negative breast cancer. Using primary (HR = 2.53, 95% CI = 1.32–4.83; P = 0.005) and validation (HR = 3.99, 95% CI = 1.388–11.47; P = 0.010) cohorts, the study of Huang et al. supported that SII was an independent risk factor for prediction of OS in cervical cancer patients [14]. Furthermore, receiver operating characteristics curve analysis suggested that the prognostic accuracy of SII for 5-year OS in patients with cervical cancer was even higher than NLR [area under the curves (AUC): 0.64 vs 0.59, primary; 0.64 vs 0.59, validation] or PLR (AUC: 0.64 vs 0.60, primary; 0.64 vs 0.60, validation) [14], indicating SII may represent a promising biomarker for predicting survival of gynecological cancer patients clinically. However, the associations between SII and clinical outcomes of gynecological and breast cancer patients were found to be nonsignificant in some other studies [15, 16]. Therefore, it is necessary to re-assess the prognostic value of SII in patients with gynecological and breast cancer patients by performing a meta-analysis like NLR and PLR, which was not reported previously and was the goal of this study.
Materials and methods
This meta-analysis was performed based on the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement. Because the present study was a meta-analysis of articles published previously, ethical approval and patient consent were not required.
Search strategy
Two authors independently searched eligible articles in PubMed, EMBASE, and Cochrane Library from the date of establishment to January 1, 2020. The search strategy included (“gynecological” OR “breast” OR “cervical” OR “ovarian” OR “endometrial”) AND (“cancer” or “carcinoma” or “tumor”) AND (“systemic immune-inflammation index” or “SII”). Additionally, references of included publications and reviews were manually reviewed for potential trials.
Inclusion and exclusion criteria
The study selection was completed by two independent investigators. Publications were eligible if they met the following inclusion criteria: (1) the enrolled patients suffered from gynecological and breast cancers which were pathologically diagnosed; (2) patients did not have an active infection, inflammatory, or comorbid diseases or undergo anti-inflammatory medication before blood examination; (3) neutrophil, platelet, and lymphocyte counts were measured prior to any treatments and SII was calculated; (4) the associations between SII and prognostic outcomes of patients were assessed; (5) the HRs with their 95%CIs were reported or could be calculated from raw data; (6) the cut-off value of SII was provided; and (7) articles were published in English. The exclusion criteria were as follows: (1) studies were duplicated or data were overlapped; (2) letters, case reports, editorials, or reviews; (3) non-human studies; and (4) insufficient data for estimating HRs and 95%CIs for prognosis outcomes.
Data extraction and quality assessment
Two authors extracted the following data independently: the name of the first author, year of publication, country, sample size, cancer type, study design, treatment, follow-up period, SII cut-off, source of HRs, outcomes, and HR with 95%CI for each outcome. HR based on multivariate analysis was preferentially extracted if available. The Newcastle-Ottawa Scale (NOS) criteria [17] was used by two independent researchers to evaluate the quality of enrolled studies, with scores ≥ 6 suggested to be of high quality.
Statistical analysis
The HR and 95%CI of each study were calculated using STATA 13.0 (STATA Corporation, College Station, TX, USA) to assess the associations of SII with OS, PFS, and DFS. The relative risk (RR) and 95%CI were calculated for lymph node metastasis (LNM) and lymphovascular invasion (LVI). A pooled HR or RR > 1 indicated a poor prognosis for patients with high SII. Statistical difference was determined by using a z test (P < 0.05) with 95%CI (range not including the value of 1). Cochrane’s Q and I2 statistic tests were used to measure the heterogeneity of included studies. P < 0.10 and I2 > 50% indicated the presence of heterogeneity among studies, so that the pooled HR was calculated by a random-effects model; otherwise, a fixed-effects model was applied. Subgroup analyses were also performed by country, sample size, cut-off, cancer type, follow-up length, and source of HR. The values for dividing the subgroup of sample size, cut-off, and follow-up were selected according to the integer value of the median. Publication bias was estimated by Egger’s linear regression test (P < 0.05 indicated a significant publication bias) [18]. Publication bias was adjusted using the trim-and-fill procedure [19]. The robustness of the results was assessed by sensitivity analysis in which each study was removed in turn.
Results
Study characteristics
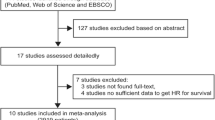
As shown in Fig. 1, a total of 126 records were initially yielded through an electronic search on online databases. After removing duplicates (n = 84) and screening titles and abstracts (n = 32), 10 studies were assessed by full text for eligibility. One study was excluded due to lack of relevant data. No additional records were identified through manual searching. Finally, 9 published articles involving 2724 patients were included in this meta-analysis [11,12,13,14,15,16, 20,21,22]. Among these 9 studies, two [11, 14] contained the training and validation cohorts from different hospitals, and thus, 11 datasets were totally used for statistical analysis (Table 1). Two studies [14, 20] evaluated the relationship between SII index and prognosis in cervical cancer patients, five [12, 13, 16, 21, 22] focused on breast cancer, and two [11, 15] investigated ovarian cancer. In the study of De Giorgi et al., triple-negative, HER2+, and HER2–ER+ subtypes of breast cancer were also independently analyzed, in addition to the overall results [16]; while all the other studies [12, 13, 21, 22] on breast cancer only focused on specific breast cancer subtypes. The endpoint was OS in eight studies and PFS/DFS in six studies. Furthermore, the association of the SII index with LNM and LVI was also reported in five [11, 13, 14, 21, 22] and two [14, 22] studies, respectively. All these studies were retrospectively performed in China (n = 6), Italy (n = 1), USA (n = 1), or Spain (n = 1). Most of the studies (7/9, 77.8%) extracted the HR and 95%CI from the multivariate analysis and only 2 from the univariate analysis [16, 20]. The SII cut-off values ranged from 475 to 1000. The other characteristics of all cohort studies could be seen in Table 1. The NOS was 9 for six articles, 8 for two studies, and 7 for one study, indicating the included literature was overall of high-quality (Table 1).
Meta-analysis for OS
As there was obvious heterogeneity among the eight studies with ten datasets, the random-effects model was used (I2 = 72.0%, P < 0.001). The pooled results indicated that a high SII index was significantly associated with shorter OS in patients with gynecological and breast cancers (HR = 2.12, 95% CI = 1.61–2.79; P < 0.001) (Fig. 2). In order to explore the potential source of heterogeneity, subgroup analysis was conducted by country, sample size, cut-off, cancer type, follow-up length, and source of HR. The results demonstrated that these subgroup factors did not change the prognostic roles of SII index for OS (Table 4), with HR > 1 and P < 0.05 for all subgroups (Table 2).
Meta-analysis for DFS/PFS
The PFS was integrated with DFS for the meta-analysis as these outcomes are similar. The random-effects model was used to analyze the prognostic value of SII index for DFS/PFS because significant heterogeneity was present (I2 = 81.3%, P < 0.001) (Table 3). The meta-analysis revealed that a high SII index was a negative predictor of DFS/PFS for patients with gynecological and breast cancers (HR = 2.28, 95% CI = 1.52–3.41; P < 0.001) (Fig. 3). This prognostic significance of SII index was also confirmed in subgroup analyses according to country (Asian, P < 0.001), sample size (< 200, P = 0.022; > 200, P = 0.004), cut-off (< 600, P = 0.022; > 600, P = 0.004), cancer type (ovarian cancer, P = 0.042; overall breast cancer, P = 0.002; triple-negative breast cancer, P = 0.035), and follow-up length (< 48 months, P = 0.010; > 48 months, P = 0.005) (Table 3).
Meta-analysis for LNM
There was no heterogeneity observed among studies (I2 = 0%, P = 0.544); therefore, a fixed-effects model was used (Table 4). As shown in Fig. 4, the patients with a high SII index were at a significantly increased risk of LNM compared with those with a low SII index (RR = 1.34, 95% CI = 1.20–1.50; P < 0.001) (Fig. 4). Subgroup meta-analysis showed that the prognostic role of SII was only significant for patients with ovarian cancer and breast cancer (regardless of subtype), but not for cervical cancer (P = 0.807). Furthermore, in the subgroup with cut-off less than 600, the risk differences of LNM were not statistically significant between high and low SII index (P = 0.094) (Table 4).
Meta-analysis for LVI
Two studies with three datasets investigated the prognostic impact of SII on LVI. Meta-analysis using a fixed-effects model (I2 = 43.6%, P = 0.170) showed that SII index could not predict the LVI for patients with gynecological and breast cancers (RR = 0.99, 95% CI = 0.64–1.54; P = 0.972) (Fig. 5).
Publication bias and sensitivity analyses
Egger tests were carried out to assess the potential publication bias for studies with OS and DFS/PFS because obvious heterogeneities were seen among them as above described. The results showed that there was no evidence of publication bias for OS (P = 0.154). For DFS/PFS, publication bias seemed to be present (P = 0.007); however, the prognostic significance of SII remained unchanged (HR = 1.72, 95% CI = 1.112.67; P < 0.001) after trim-and-fill adjustment. The sensitivity analyses also demonstrated that the pooled results could not be affected after the removal of any one study (Fig. 6).
Discussion
SII is a recently proposed new inflammatory index, which is calculated based on the count of neutrophils, platelets, and lymphocytes in the peripheral blood. Thus, SII may be effective to reflect the inflammatory status which is an important mechanism for the development of cancers and may be an underlying biomarker for prognosis prediction. This hypothesis had been demonstrated in meta-analyses on lung cancer [23, 24], esophageal cancer [25], gastrointestinal cancers [26], hepatocellular carcinoma [27], and several other cancer types [28, 29]. All these meta-analyses showed that increased SII predicted poor prognostic outcomes for patients with cancers. However, there was no study to confirm the prognostic roles of SII for gynecological and breast cancers that are two leading causes of death among women, which was the goal of our study. In line with the studies on other cancers [28, 29], we also found that elevated SII was associated with worse OS, DFS/PFS, and LNM of patients with gynecological and breast cancers compared with the low SII group. The conclusion on OS was applicable to all cancer types (cervical cancer, ovarian cancer, breast cancer), but the association with DFS/PFS and LNM was only significant for ovarian cancer and breast cancer, especially triple-negative breast cancer. These findings suggest that high SII may be a promising predictor for OS in patients with gynecological and breast cancers. For ovarian cancer and triple-negative breast cancer, SII may also serve as a useful prognostic indicator for their progression and survival.
Although the exact mechanisms remain poorly understood, the tumor-promoting functions of neutrophils and platelets, and the tumor-suppressing roles of lymphocytes may explain the prognostic values of high SII in cancers. For example, Coffelt et al. reported that tumor-induced neutrophils suppressed the activation of cytotoxic CD8+ T lymphocytes and then facilitated the establishment of metastases; while the absence of neutrophils profoundly reduced pulmonary and lymph node metastases of breast cancer cells [30]. Lee et al. demonstrated that ovarian tumor-derived neutrophils via forming neutrophil extracellular traps (NET) stimulated ovarian cancer cells colonized in the omentum to realize omental metastasis. Omental colonization and metastasis were found to be significantly decreased in mice with neutrophil-specific deficiency of peptidylarginine deiminase 4 (PAD4, an enzyme that is essential for NET formation) or undergoing the PAD4 inhibitor treatment (CI-amidine or GSK484) [31]. Yao et al. detected that the expression of tropomyosin 3 was significantly increased in platelets of patients with breast cancer compared with age-matched healthy controls. Overexpression of platelet tropomyosin 3 enhanced the migratory ability of breast cancer cells [32]. Hu et al. supported that platelet increased the growth of ovarian cancer in murine models due to high expression of transforming growth factor β1 (Tgfβ1); lack of platelet-specific Tgfβ1 in mice reduced tumor growth, neoangiogenesis, and platelet extravasation [33]. By co-incubation of platelets with breast or ovarian cancer cell lines, the study of Zuo et al. [34] and Guo et al. [35] directly proved that platelets exerted pro-metastatic functions via activation of epithelial-mesenchymal transition transformation. Thus, high levels of neutrophils/platelets and low levels of lymphocytes that led to an increased SII may ultimately contribute to the development and progression of gynecological (especially ovarian cancer) and breast cancers and related with poor prognosis of patients. Although several clinical trials [36] verified the prognostic roles of neutrophils, platelets, and lymphocytes for cervical cancer, rare in vitro and in vivo studies were performed to explore their functions in cervical cancer and further experiments are required.
There were some limitations that should be acknowledged. First, only 9 retrospective articles were included and the sample size for each cancer type was small. Also, there were none on endometrial cancer. Second, most of the studies were performed in China. There were studies to show that body mass index (BMI) varied by ethnicity and BMI was positively correlated with SII [37]. Hereby, ethnicity may affect SII and outcomes in patients with cancers. Third, the cut-off value varied in different articles. Fourth, most of the HRs and 95%CIs extracted from published articles were not adjusted by clinical related covariates. Therefore, the prognostic values of the SII in gynecological and breast cancers needed to be validated by using more trials with a prospective design, larger sample sizes, and patients from other countries.
Conclusion
Our findings provide evidence that high SII may be a promising indicator for prediction of poor prognosis (OS, DFS/PFS, and LNM) in patients with gynecological and breast cancers, especially ovarian cancer and triple-negative breast cancer.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- NLR:
-
Neutrophil-lymphocyte ratio
- PLR:
-
Platelet-lymphocyte ratio
- SII:
-
Systemic immune-inflammation index
- OS:
-
Overall survival
- DFS:
-
Disease-free survival
- PFS:
-
Progression-free survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- AUC:
-
Area under the curves
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- NOS:
-
Newcastle-Ottawa Scale
- RR:
-
Relative risk
- LNM:
-
Lymph node metastasis
- LVI:
-
Lymphovascular invasion
References
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, Sarker M, Huong TT, Allemani C, Dvaladze A, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–60.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis. Cancer Manag Res. 2018;10:6685–93.
Szewczyk G, Maciejewski TM, Szukiewicz D. Current progress in the inflammatory background of angiogenesis in gynecological cancers. Inflamm Res. 2019;68:247–60.
An G, Wu F, Huang S, Feng L, Bai J, Gu S, Zhao X. Effects of CCL5 on the biological behavior of breast cancer and the mechanisms of its interaction with tumor-associated macrophages. Oncol Rep. 2019;42(6):2499–511.
Castaño Z, San Juan BP, Spiegel A, Pant A, DeCristo MJ, Laszewski T, Ubellacker JM, Janssen SR, Dongre A, Reinhardt F, Henderson A, Del Rio AG, Gifford AM, Herbert ZT, Hutchinson JN, Weinberg RA, Chaffer CL, McAllister SS. IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nat Cell Biol. 2018;20(9):1084–97.
Ni L, Tao J, Xu J, Yuan X, Long Y, Yu N, Wu R, Zhang Y. Prognostic values of pretreatment neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2020;301(1):251–61.
Yin X, Wu L, Yang H, Yang H. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: a systematic review and meta-analysis. Medicine. 2019;98(45):e17475.
Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: a meta-analysis. Medicine. 2018;97(49):e13340.
Guo W, Lu X, Liu Q, Zhang T, Li P, Qiao W, Deng M. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for breast cancer patients: an updated meta-analysis of 17079 individuals. Cancer Med. 2019;8(9):4135–48.
Nie D, Gong K, Mao X, Li Z. Systemic immune-inflammation index predicts prognosis in patients with epithelial ovarian cancer: a retrospective study. Gynecol Oncol. 2019;152(2):259–64.
Liu J, Shi Z, Bai Y, Liu L, Cheng K. Prognostic significance of systemic immune-inflammation index in triple-negative breast cancer. Cancer Manag Res. 2019;11:4471–80.
Wang P, Yue W, Li W, Luo Y, Li Z, Shao Y, He Z. Systemic immune-inflammation index and ultrasonographic classification of breast imaging-reporting and data system predict outcomes of triple-negative breast cancer. Cancer Manag Res. 2019;11:813–9.
Huang H, Liu Q, Zhu L, Zhang Y, Lu X, Wu Y, Liu L. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284.
Farolfi A, Petrone M, Scarpi E, Gallà V, Greco F, Casanova C, Longo L, Cormio G, Orditura M, Bologna A, Zavallone L, Ventriglia J, Franzese E, Loizzi V, Giardina D, Pigozzi E, Cioffi R, Pignata S, Giorda G, De Giorgi U. Inflammatory indexes as prognostic and predictive factors in ovarian cancer treated with chemotherapy alone or together with bevacizumab. a multicenter, retrospective analysis by the MITO group (MITO 24). Target Oncol. 2018;13(4):469–79.
De Giorgi U, Mego M, Scarpi E, Giordano A, Giuliano M, Valero V, Alvarez RH, Ueno NT, Cristofanilli M, Reuben JM. Association between circulating tumor cells and peripheral blood monocytes in metastatic breast cancer. Ther Adv Med Oncol. 2019;11:1758835919866065.
Andreas S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63.
Holub K, Biete A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clin Transl Oncol. 2019;21(7):836–44.
Sun Y, Li WQ, Li AJ, Su HC, Yue JB, Yu JM. Increased systemic immune-inflammation index independently predicts poor survival for hormone receptor-negative, HER2-positive breast cancer patients. Cancer Manag Res. 2019;11:3153–62.
Li QX, Shi DJ, Zhang LX, Wang DM, Zhao J, Wang T, Deng XN, Fan XY. Association of body mass and systemic immune-inflammation indices with endocrine therapy resistance in luminal breast cancers. J Int Med Res. 2019;47(5):1936–47.
Wang Y, Li Y, Chen P, Xu W, Wu Y, Che G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: a meta-analysis. Ann Transl Med. 2019;7(18):433.
Zhang Y, Chen B, Wang L, Wang R, Yang X. Systemic immune-inflammation index is a promising noninvasive marker to predict survival of lung cancer: a meta-analysis. Medicine. 2019;98(3):e13788.
Zhang Y, Xiao G, Wang R. Clinical significance of systemic immune-inflammation index (SII) and C-reactive protein-to-albumin ratio (CAR) in patients with esophageal cancer: a meta-analysis. Cancer Manag Res. 2019;11:4185–200.
Zhang Y, Lin S, Yang X, Wang R, Luo L. Prognostic value of pretreatment systemic immune-inflammation index in patients with gastrointestinal cancers. J Cell Physiol. 2019;234(5):5555–63.
Wang B, Huang Y, Lin T. Prognostic impact of elevated pre-treatment systemic immune-inflammation index (SII) in hepatocellular carcinoma: a meta-analysis. Medicine. 2020;99(1):e18571.
Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. 2017;8(43):75381–8.
Yang R, Chang Q, Meng X, Gao N, Wang W. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer. 2018;9(18):3295–302.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–8.
Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019;216(1):176–94.
Yao B, Qu S, Hu R, Gao W, Jin S, Ju J, Zhao Q. Delivery of platelet TPM3 mRNA into breast cancer cells via microvesicles enhances metastasis. FEBS Open Bio. 2019;9(12):2159–69.
Hu Q, Hisamatsu T, Haemmerle M, Cho MS, Pradeep S, Rupaimoole R, Rodriguez-Aguayo C, Lopez-Berestein G, Wong STC, Sood AK, Afshar-Kharghan V. Role of platelet-derived Tgfβ1 in the progression of ovarian cancer. Clin Cancer Res. 2017;23(18):5611–21.
Zuo XX, Yang Y, Zhang Y, Zhang ZG, Wang XF, Shi YG. Platelets promote breast cancer cell MCF-7 metastasis by direct interaction: surface integrin α2β1-contacting-mediated activation of Wnt-β-catenin pathway. Cell Commun Signal. 2019;17(1):142.
Guo Y, Cui W, Pei Y, Xu D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β signaling pathway. Gynecol Oncol. 2019;153(3):639–50.
Prabawa IPY, Bhargah A, Liwang F, Tandio DA, Tandio AL, Lestari AAW, Budiana ING, Manuaba IBAP. Pretreatment neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as a predictive value of hematological markers in cervical cancer. Asian Pac J Cancer Prev. 2019;20(3):863–8.
Furuncuoğlu Y, Tulgar S, Dogan AN, Cakar S, Tulgar YK, Cakiroglu B. How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study. Eur Rev Med Pharmacol Sci. 2016;20(7):1300–6.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
YFJ and HYW designed the study, collected the data, and performed the statistical analysis. YFJ drafted the manuscript. HYW contributed to the interpretation of the results and critically reviewed the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ji, Y., Wang, H. Prognostic prediction of systemic immune-inflammation index for patients with gynecological and breast cancers: a meta-analysis. World J Surg Onc 18, 197 (2020). https://doi.org/10.1186/s12957-020-01974-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-020-01974-w