Abstract
Background
CREB-binding protein (CBP) and p300 represent histone acetyltransferases (HATs) and transcriptional coactivators that play essential roles in tumour initiation and progression. Both proteins are generally thought to function as tumour suppressors, although their distinct roles in colorectal cancer (CRC) remain inconsistent and ambiguous.
Thus, we analysed the expression of these two HATs in human tissue samples from patients with locally advanced rectal cancer via immunohistochemistry and evaluated their potential impacts on future CRC diagnosis and treatment.
Methods
In our analysis, we included ninety-three (n = 93) patients diagnosed with adenocarcinoma in the upper third of the rectum. None of the patients received preoperative chemoradiotherapy, but the patients did undergo primary resection of the tumour within the phase II GAST-05 trial. By using H-scores, the expression of both proteins was visualised via immunohistochemistry in resected specimens from the patients. CBP and p300 expression were correlated with clinical and follow-up data.
Results
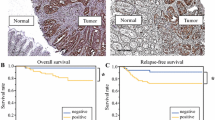
Our analysis showed that high expression of CBP was significantly associated with prolonged cancer-specific survival (CSS; p = 0.002). In univariate analysis, CBP was an independent prognostic parameter for CSS (p = 0.042). High nuclear CBP expression was observed in two-thirds of patients. In contrast, we could not find any significant correlation between the expression of p300 and cancer-specific survival in this cohort of patients (p = 0.09). We did not observe any cooperation between CBP and p300 in our analysis.
Conclusions
High expression of CBP was significantly associated with improved oncological outcomes. This finding could help to stratify patients in the future for CRC treatment. Histone deacetylase (HDAC) inhibitors are increasingly playing a role in oncological treatment and could additionally become therapeutic options in CRC. Our findings need to be further evaluated and verified in future clinical analyses.
Similar content being viewed by others
Background
Colorectal cancer (CRC) represents one of the most common malignancies in the Western world [1]. Although advances in perioperative radiation and chemotherapy have been made, including the implementation of specific monoclonal antibodies, the long-term prognosis of CRC is still limited because patients respond heterogeneously to current standard treatments. Therefore, individualised therapies are desirable. Thus, it is essential to find potential biomarkers and new therapeutic targets to improve patient outcomes.
As epigenetic alterations such as DNA methylation and histone modifications have raised interest regarding the initiation and progression of tumours in recent decades, epigenetic therapies addressing epigenetic modifiers are now being included in clinical trials for cancer treatment [2, 3]. The highly conserved tumour suppressor and transcriptional coactivator CREB binding protein (CBP) as well as its close partner p300 are histone acetyltransferases (HATs) that share approximately 60% homology and play essential roles in gene expression regulation by acetylating chromatin substrates [4, 5].
The acetylation of histones leads to a reduction in the electrostatic interactions between the positive charge of histones and the negative charge of DNA, which reduces the compactness of the chromatin structure and favours transcriptional progression, possibly contributing to carcinogenesis by specific activation of cancer-associated genes [6].
Studies suggest an inverse correlation between the expression of CBP and that of p300 because these molecules correlate positively and negatively with patient survival. Despite the high degree of homology between these proteins, studies have revealed functional differences from other HAT proteins due to differences in substrate specificities [7]. CBP and p300 are involved in several cellular activities such as cell growth, differentiation, DNA repair and apoptosis. They also interact with at least 40 different transcription factors [7,8,9]. Previous studies have shown that the interaction between CBP/p300 and ß-Catenin influences Wnt/ß-Catenin signalling. The influence of CBP and p300 activity on Wnt/ß-Catenin signalling affects cell proliferation and differentiation [10,11,12,13,14]. Mutations in this pathway are responsible for the initiation of many CRC tumours [15].
Somatic alterations in the CBP gene are associated with malignant diseases such as acute myeloid leukaemia and hepatocellular carcinoma, while germline mutations in the CBP gene have been identified in Rubinstein-Taybi disease [16,17,18]. Mutations in p300 have recently been detected in colon cancer and gastric cancer [19].
Although dysfunction in CBP and/or p300 is considered to be associated with tumourigenesis in several human malignancies, their roles in CRC remain unclear and somewhat controversial. Therefore, we investigated the expression of CBP and p300 in patients with rectal adenocarcinoma via immunohistochemistry, and the findings were compared with clinicopathological parameters, including patient outcome, to investigate the clinical impacts and functions of both the tumour suppressor CBP and the potential oncogene p300. In addition, molecular aspects in the context of potential downstream targets were analysed. Herein, we show for the first time that CBP overexpression in CRC but not p300 overexpression is associated with an improved outcome.
Methods
Patients
Specimens from patients with locally advanced UICC (Union International Contre le Cancer) II/III colorectal adenocarcinoma in the upper third of the rectum included in the phase II GAST-05 trial were assessed using immunohistochemistry. Study details of the GAST-05 trial are described elsewhere [20].
Patients with complete follow-up were further analysed. Approval from the local ethics committee and informed consent from patients were given (study number 9/8/08). Written consent was obtained from all 93 patients.
Patients were treated at the Department of General, Visceral and Paediatric Surgery, University Medical Center Göttingen (UMG), Germany, between March 2007 and September 2012.
Histopathological assessment
Histopathological and clinical staging included TNM staging as well as grading and tumour stage classification [21]. Nodal staging was evaluated histopathologically by examining all detected lymph nodes and determining the lymph node ratio in all cases. Complete lymph node dissection data were included once 12 or more lymph nodes were found in the resected tissue and were taken for further analysis as recommended. Tumour tissue was collected at the time of surgery.
Immunohistochemical determination of CBP/p300 statuses
CBP and p300 expression were assessed using formalin-fixed, paraffin-embedded (FFPE) tissue samples from resection specimens cut into sections with a thickness of 2 μm. Standardised immunohistochemical staining was performed using a polyclonal rabbit anti-CBP antibody (Catalogue No. IHC-00023, Bethyl, Montgomery, TX, USA, 1:50 dilution). Heat-mediated epitope retrieval was performed for 90 min at 100 °C. The anti-CBP antibody was incubated at room temperature for 30 min. Staining was visualised by means of alkaline phosphatase using the ultraView Universal Fast Red Kit (Ventana Medical Systems).
The monoclonal mouse anti-p300 antibody ab3164 (Abcam, Cambridge, Great Britain, 1:500 dilution) was incubated at 37 °C for 40 min. For p300, heat-mediated epitope retrieval was performed for 56 min at 100 °C. Horseradish peroxidase was used for visualisation, and staining was analysed using the optiView Universal DAB Detection Kit (Ventana Medical Systems) (Fig. 1).
Standard immunohistochemical staining was performed on a Ventana Bench-Mark XT immunostainer (Ventana, Tucson, AZ, USA). More than 100 tumour cells were needed in resection specimens to define CBP and p300 positivity. Since both CBP and p300 are located in the nucleus, nuclear staining was exclusively analysed. In order to quantify immunohistochemical staining, H-score was implemented as described before ranging from 0 to 300 (y-axis). For nuclear staining, four different staining intensity grades were defined: 0 (very weak staining), I (weak staining), II (strong staining) and III (very strong staining). For every immunohistochemical slide, we chose three different areas, which were localised (a) at the basis of the tumour on the boundary layer to healthy tissue, (b) at the centre of the tumour and (c) at the apex towards the gut lumen. This area approximately covers 7.500 μm2 (Fig. 2).
Statistical analysis
The expression of CBP and p300 was correlated with clinicopathological parameters as described. The impacts of CBP and p300 on disease-free survival (DFS) and cancer-specific survival (CSS) were determined using Kaplan-Meier analysis and assessed for statistical significance using Kendall’s Tau (Cox proportional hazard model). Statistical analysis was performed using the R package (version 2.14.2), and survival analysis was carried out after grouping patients into three groups (high, medium, and low) as described above [22]. The significance level was set at α = 5%. To quantify immunohistochemical staining, the H-score was implemented as described previously [23].
Results
Patient characteristics and recurrence
All analysed patients were registered in the prospective multi-centre phase II GAST-05 trial. Overall, 93 patients with a confirmed adenocarcinoma in the upper third of the rectum underwent surgery, partially followed by postoperative chemotherapy. In this analysis, 63 patients (67.7%) were male, while 30 patients were female (32.3%). Patient ages ranged from 38 to 88 years (median 70 years). In this analysis, anterior rectal resection (ARR) was performed in 37 patients (39.8%), while 53 patients (57.0%) underwent lower anterior rectal resection (LARR). In three patients (3.2%), Hartmann’s procedure was performed. Regarding the extent of mesorectal excision, total mesorectal excision (TME) was executed in 38 patients (40.9%), while 52 patients (55.9%) experienced a partial mesorectal excision (PME).
Within this cohort of patients, 3 patients (3.2%) presented with early pT1-stage disease, while 18 patients (19.4%) were diagnosed with pT2 tumours. Within the patients with locally advanced rectal tumours, 61 patients (65.6%) had pT3 tumours, while in 11 patients (11.8%), a pT4 tumour was present. Considering the lymph node status of the patients, 53 patients (57%) did not show any lymph node metastasis, while 22 patients (23.7%; pN1) and 18 patients (19.3%; pN2) were diagnosed with positive lymph nodes. Regarding long-term follow-up, 3 patients (3.2%) developed local recurrences, while 15 patients (16.1%) showed distant metastases (for details, see Table 1).
CBP expression in resection specimens evaluated by immunohistochemistry
CBP expression was exclusively nuclear, and no significant correlation was observed between CBP expression and apical, central or basal localisation of CBP (see Fig. 3). High expression of CBP was significantly associated with prolonged CSS (p = 0.002; see Fig. 4). Furthermore, subgroup analysis showed a correlation between high and medium CBP expression (p < 0.05), but not between low expression and CSS (p > 0.05). In this cohort of patients, CBP expression represented an independent prognostic parameter for CSS by univariate analysis (p = 0.042). There were no further significant associations between CBP expression and clinicopathological parameters, e.g. size of the tumour (pT), postoperative nodal status (pN), distant metastasis status, pUICC or grade. Additionally, there was no significant correlation between the expression of CBP and local relapse (p > 0.05).
p300 expression in resection specimens evaluated by immunohistochemistry
The expression of p300 was exclusively nuclear. There was no predominant localization in the apical, central or basal side (see Fig. 5). Low expression of p300 was not significantly associated with poor CSS (p = 0.09; see Fig. 6). In our hands, p300 did not represent a prognostic parameter. We could not find any correlations between p300 expression and clinicopathological parameters such as tumour size (pT), lymph node status (pN), distant metastasis status, pUICC or grade. Furthermore, we could not find any association between p300 expression and local relapse.
Discussion
CBP and p300 function as transcriptional coactivators and HATs, which may favour euchromatin formation and therefore activate transcriptional activity. Studies have shown that both enzymes are involved in numerous cellular activities such as cell growth, differentiation, DNA repair and apoptosis. However, their distinct roles in CRC remain unclear.
Previous studies suggested an inverse correlation between the expression of CBP and p300 in CRC with regard to overall survival (OS) [24]. Correlations have also been observed in other cancers, such as prostate cancer, where genetic deletion of CBP and p300 results in the promotion or perturbation of tumourigenesis induced by phosphatase and tensin homologue (PTEN) deficiency [25, 26].
Although somatic mutations such as the translocation or loss of heterozygosity of CBP and p300 have been observed in leukaemia as well as in solid tumours such as hepatocellular carcinoma, breast cancer and CRC, genetic mutations in these two genes remain rare [27,28,29,30,31,32,33].
In an analysis of 222 cancer samples, truncating mutations in p300 were only observed in six out of 107 (5.6%) cell lines and two out of 115 (1.7%) primary tumours [34,35,36]. CBP mutations are even rarer; only two heterozygous truncations and no further mutations were discovered in 63 cell lines. In 116 primary tumours, truncating mutations could not be detected [36]. These findings support our hypothesis regarding the roles of CBP and p300 as central chromatin modifiers and suggest that epigenetic therapies specifically targeting CBP or p300 may serve as a potential option for the treatment of a subset of colorectal tumours.
This potential targeting is further supported by our finding that approximately two-thirds of tumour cells from our patients highly expressed CBP and p300, stressing their importance in the development and progression of cancer as transcriptional coactivators and HATs.
At least in our hands, stronger expression of CBP, but not p300, seems to increase CSS. Furthermore, our results revealed CBP as an independent prognostic factor regardless of tumour stage or localization, which could not be shown for p300. Cooperation between CBP and p300 was not verifiable in this cohort of patients, supporting current evidence that the two HATs play different roles in tumourigenesis. This hypothesis is strengthened by in vitro and in vivo analyses that showed different specificities and selectivities for CBP and p300 in the acetylation of histones, the inability of CBP to rescue the growth of p300-deficient carcinoma cell lines and an inverse prognostic correlation in CRC [24, 37, 38].
Our results may introduce CBP as a potential target in a subset of colorectal cancer patients. Our findings are further supported by an analysis by Du et al. [39]. Their results demonstrated global histone deacetylation in CRC cell lines caused by 5-fluorouracil (5-FU), which is the standard chemotherapeutic agent in colorectal cancer. Additionally, they showed that 5-FU was capable of reducing the ability of CBP and p300 to bind to chromatin and thereby inducing their degradation. Interestingly, blocking CBP and p300 degradation resulted in an enhancement in 5-FU’s cytotoxicity to CRC cells, indicating that the degradation of CBP and p300 is relevant to cellular resistance to 5-FU. By analysing 262 samples from colorectal cancer patients receiving 5-FU treatment via immunohistochemistry, Du et al. showed that high expression of CBP and p300 significantly correlated with prolonged disease-free survival (DFS) and decreased early progression. Taken together, CBP and p300 might represent not only prognostic biomarkers but also predictive biomarkers of chemo-sensitivity to 5-FU treatment, thereby distinguishing responders from non-responders to stratify patients for CRC therapy.
Taken together, the prognostic capacities of CBP and p300 have been investigated in previous studies with partially controversial results [24, 39,40,41]. Both of these highly homologous transcriptional coactivators are essential in apoptosis, cell transformation, differentiation and growth, as well as in CRC [42]. CBP and p300 both acetylate a variety of transcription-regulating proteins, including oncogenes and tumour suppressors such as p53 [43,44,45]. In recent years, efforts have been made to target CBP and p300, including designing small-molecule inhibitors with heterogeneous efficacy [46,47,48]. However, our findings may provide better insight into the clinical significance of CBP and p300 in patients suffering from CRC, although the distinct roles of these two HATs in CRC remain incompletely understood.
Conclusions
In this patient cohort, high expression of CBP was correlated with improved long-term outcomes. This histone acetyltransferase could therefore represent a potential biomarker for stratifying therapeutic regimens for patients suffering from colorectal cancer. Inhibitors of CBP have already been implemented in preclinical trials. It is desirable to find not only prognostic biomarkers but also particularly predictive biomarkers to predict the success of therapies and to prevent severe side effects of therapies from which not every patient benefits. At least in our hands, CBP may represent both.
As genetic mutations in these two genes are known to be rare, we and others postulate central epigenetic functions for these two proteins in tumour initiation and progression, and both enzymes may therefore be feasible targets for anticancer treatment.
The small number of patients and the fact that immunohistochemistry detects only expression, not activity, represent the limitations of our study.
Further studies on CBP and p300 are desirable to evaluate the future potential of these two proteins in cancer therapy. Our findings should be further evaluated and verified in upcoming clinical trials.
Availability of data and materials
All data generated or analysed during this study are included in the published article.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- ARR:
-
Anterior rectal resection
- CBP:
-
CREB-binding protein
- CRC:
-
Colorectal cancer
- CSS:
-
Cancer-specific survival
- DNA:
-
Deoxyribonucleic acid
- FFPE:
-
Formalin-fixed, paraffin-embedded
- HAT:
-
Histone acetyltransferase
- HDAC:
-
Histone deacetylase
- LARR:
-
Lower anterior rectal resection
- OS:
-
Overall survival
- PME:
-
Partial mesorectal excision
- PTEN:
-
Phosphatase and tensin homologue
- UICC:
-
Union International Contre le Cancer
- UMG:
-
University Medical Center Göttingen
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66:7–30.
Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis; epigenetic joins genetics. Trends Genet. 2000;16:168–74.
van Engeland M, Derks S, Smits KM, Meijer GA, Herman JG. Colorectal cancer epigenetics: complex simplicity. J Clin Oncol. 2011;29:1382–91.
Arany Z, Sellers WR, Livingston DM, Eckner R. E1A-associated p300 and CREB-associated CBP belong to a conserved family of coactivators. Cell. 1994;77:799–800.
Shikama N, Lee CW, France S, Delavaine L, Lyon J, Krstic-Demonacos M, La Thangue NB. A novel cofactor for p300 that regulates the p53 response. Mol Cell. 1999;4:365–76.
Sun WJ, Zhou X, Zheng JH, Lu MD, Nie JY, Yang XJ, Zheng ZQ. Histone acetyltransferases and deacetylases: molecular and clinical implications to gastrointestinal carcinogenesis. Acta Biochim Biophys Sin. 2012;44:80–91.
Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol. 2004;68:1145–55.
Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276:13505–8.
Goodman RH, Smolik S. CBP/p300 in cell growth, transformation and development. Genes Dev. 2000;14:1553–77.
Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of ß-catenin/CREB-binding protein transcription. Proc Natl Acad Sci U S A. 2004;101:12682–7.
Lenz HJ, Kahn M. Safely targeting cancer stem cells via selective catenin coactivator antagonism. Cancer Sci. 2014;105:1087–92.
Teo JL, Ma H, Nguyen C, Lam C, Kahn M. Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci U S A. 2005;102:12171–6.
Ma H, Nguyen C, Lee KS, Kahn M. Differential roles for the coactivators CBP and p300 on TCF/ß-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–31.
Bordonaro M, Lazarova DL. CREB-binding protein, p300, butyrate, and Wnt signaling in colorectal cancer. World J Gastroenterol. 2015;21:8238–48.
Lazarova DL, Chiaro C, Wong T, Drago E, Rainey A, O’Malley S, Bordonaro M. CBP activity mediates effects of the histone deacetylase inhibitor butyrat on WNT activity and apoptosis in colon cancer cells. J Cancer. 2013;4:481–90.
Sakai K, Nagahara H, Abe K, Obata H. Loss of heterozygosity on chromosome 16 in hepatocellular carcinoma. J Gastroenterol Hepatol. 1992;7:288–92.
Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, Tommerup N, van Ommen GJ, Goodman RH, Peters DJ. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–51.
Murata T, Kurokawa R, Krones A, Tatsumi K, Ishii M, Taki T, Masuno M, Ohashi H, Yanagisawa M, Rosenfeld MG, Glass CK, Hayashi Y. Defect of histone acetyltransferase activity of the nuclear transcriptional coactivator CBP in Rubinstein-Taybi disease. Hum Mol Genet. 2001;91:1154–60.
Muraoka M, Konishi M, Kikuchi-Yanoshita R, Tanaka K, Shitara N, Chong JM, Iwama T, Miyaki M. p300 gene alterations in colorectal and gastric carcinomas. Oncogene. 1996;12:1565–9.
Liersch T, Rothe H, Ghadimi BM, Becker H. Individualizing treatment for locally advanced rectal cancer. Chirurg. 2009;80:281–93.
Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International union against cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336–9.
Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;3:299–314.
Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, Takizawa T, Sunamori M, Handa M, Kondo T, Sasano H. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88:2309–17.
Ishihama K, Yamakawa M, Semba S, Takeda H, Kawata S, Kimura S, Kimura W. Expression of HDAC1 and CBP/p300 in human colorectal carcinomas. J Clin Pathol. 2007;60:1205–10.
Ding L, Chen S, Liu P, Pan Y, Zhong J, Regan KM, Wang L, Yu C, Rizzardi A, Cheng L, Zhang J, Schmechel SC, Cheville JC, Van Deursen J, Tindall DJ, Huang H. CBP loss cooperates with PTEN haploinsufficiency to drive prostate cancer: implications for epigenetic therapy. Cancer Res. 2014;74:2050–61.
Zhong J, Ding L, Bohrer LR, Pan Y, Liu P, Zhang J, Sebo TJ, Karnes RJ, Tindall DJ, van Deursen J, Huang H. p300 acetyltransferase regulates androgen receptor degradation and PTEN-deficient prostate tumorigenesis. Cancer Res. 2014;74:1870–80.
Borrow J, Stanton VP Jr, Andresen JM, Becher R, Behm FG, Chaganti RS, Civin CI, Disteche C, Dubé I, Frischauf AM, Horsman D, Mitelman F, Volinia S, Watmore AE, Housman DE. The translocation t(8;16) (p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41.
Giles RH, Dauwerse JG, Higgins C, Petrij F, Wessels JW, Beverstock GC, Döhner H, Jotterand-Bellomo M, Falkenburg JH, Slater RM, van Ommen GJ, Hagemeijer A, van der Reijden BA, Breuning MH. Detection of CBP rearrangements in acute myelogenous leukemia with t(8;16). Leukemia. 1997;11:2087–96.
Panagopoulos I, Fioretos T, Isaksson M, Samuelsson U, Billström R, Strömbeck B, Mitelman F, Johansson B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13). Hum Mol Genet. 2001;10:395–404.
Takahashi K, Kudo J, Ishibashi H, Hirata Y, Niho Y. Frequent loss of heterozygosity on chromosome 22 in hepatocellular carcinoma. Hepatology. 1993;17:794–9.
Allione F, Eisinger F, Parc P, Noguchi T, Sobol H, Birnbaum D. Loss of hetrozygosity at loci from chromosome arm 22Q in human sporadic breast carcinomas. Int J Cancer. 1998;75:181–6.
Chen LC, Kurisu W, Ljung BM, Goldman ES, Moore D 2nd, Smith HS. Heterogeneity for allelic loss in human breast cancer. J Natl Cancer Inst. 1992;84:506–10.
Duriez C, Schmitz A, Fouchet P, Buecher B, Thuille B, Lerebours F, Léger R, Boman F, Fléjou JF, Monges G, Paraf F, Bedossa P, Sabourin JC, Salmon RJ, Laurent-Puig P, Thomas G, Olschwang S. Localization of a tumor suppressor gene distal to D22S270 in colorectal cancer. Gastroenterol Clin Biol. 1997;21:358–64.
Gayther SA, Batley SJ, Linger L, Bannister A, Thorpe K, Chin SF, Daigo Y, Russell P, Wilson A, Sowter HM, Delhanty JD, Ponder BA, Kouzarides T, Caldas C. Mutations truncating the EP300 acetylase in human cancers. Nat Genet. 2000;24:300–3.
Iyer NG, Ozdag H, Caldas C. p300/CBP and cancer. Oncogene. 2004;23:4225–31.
Özdağ H, Batley SJ, Försti A, Iyer NG, Daigo Y, Boutell J, Arends MJ, Ponder BA, Kouzarides T, Caldas C. Mutation analysis of CBP and PCAF reveals rare inactivating in cancer cell lines but not in primary tumours. Br J Cancer. 2002;87:1162–5.
Henry RA, Kuo YM, Andrews AJ. Differences in specificity and selectivity between CBP and p300 acetylation of histone H3 and H3/H4. Biochemistry. 2013;52:5746–59.
Suganuma T, Kawabata M, Ohshima T, Ikeda MA. Growth suppression of human carcinoma cells by reintroduction of the p300 coactivator. Proc Natl Acad Sci U S A. 2002;99:13073–8.
Du C, Huang D, Peng Y, Yao Y, Zhao Y, Yang Y, Wang H, Cao L, Zhu WG, Gu J. 5-Fluorouracil targets histone acetyltransferases p300/CBP in the treatment of colorectal cancer. Cancer Lett. 2017;400:183–93.
Huh JW, Kim HC, Kim SH, Park YA, Cho YB, Yun SH, Lee WY, Chun HK. Prognostic impact of p300 expression in patients with colorectal cancer. J Surg Oncol. 2013;108:374–7.
Krubasik D, Iyer NG, English WR, Ahmed AA, Vias M, Roskelley C, Brenton JD, Caldas C, Murphy G. Absence of p300 induces cellular phenotypic changes characteristic of epithelial to mesenchyme transition. Br J Cancer. 2006;94:1326–32.
Giordano A, Avantaggiati ML. p300 and CBP: partners for life and death. J Cell Physiol. 1999;181:218–30.
Soutoglou E, Katrakili N, Talianidis I. Acetylation regulates transcription factor activity at multiple levels. Mol Cell. 2000;5:745–51.
Soutoglou E, Papafotiou G, Katrakili N, Talianidis I. Transcriptional activation by hepatocyte nuclear factor-1 requires synergism between multiple coactivator proteins. J Biol Chem. 2000;275:12515–20.
Sterner DE, Berger SL. Acetylation of histones and transcription-related factors. Microbiol Mol Biol Rev. 2000;64:435–59.
Lee LW, Mapp AK. Transcriptional switches: chemical approaches to gene regulation. J Biol Chem. 2010;285:11033–8.
Michaelides MR, Kluge A, Patane M, Van Drie JH, Wang C, Hansen TM, Risi RM, Mantei R, Hertel C, Karukurichi H, Nesterov A, McElligott D, de Vries P, Langston JW, Cole PA, Marmorstein R, Liu H, Lasko L, Bromberg KD, Lai A, Kesicki EA. Discovery of spiro oxazolidinediones as selective, orally bioavailable inhibitors of p300/CBP histone acetyltransferases. ACS Med Chem Lett. 2017;9:28–33.
Lasko LM, Jakob CG, Edalji RP, Qiu W, Montgomery D, Digiammarino EL, Hansen TM, Risi RM, Frey R, Manaves V, Shaw B, Algire M, Hessler P, Lam LT, Uziel T, Faivre E, Ferguson D, Buchanan FG, Martin RL, Torrent M, Chiang GG, Karukurichi K, Langston JW, Weinert BT, Choudhary C, de Vries P, Van Drie JH, McElligott D, Kesicki E, Marmorstein R, Sun C, Cole PA, Rosenberg SH, Michaelides MR, Lai A, Bromberg KD. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature. 2017;550:128–32.
Acknowledgements
We want to thank Birgit Jünemann for providing outstanding slides for immunohistochemical staining.
Funding
Funding was provided by the Department of General, Visceral and Paediatric Surgery at University Medical Center Göttingen, Germany. This funding body did not participate in the study design, data analysis or manuscript preparation.
Author information
Authors and Affiliations
Contributions
FR contributed to the design and conception of the study, the analysis, the interpretation of data and the writing of the manuscript. IMWJ contributed to the design of the study, the selection of antibodies and the revision of the manuscript. CM contributed to the immunohistochemical analysis of protein expression. TB contributed to the statistical analysis of the study. HB contributed to the immunohistochemical analysis of protein expression and the revision of the manuscript. BMG contributed to the design and conception of the study. SD contributed to the design and conception of the study, the analysis, the interpretation of data and the revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Approval from the local ethics committee and informed consent from patients was given (study number 9/8/08). Written consent was obtained from all 93 patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Rühlmann, F., Windhof-Jaidhauser, I.M., Menze, C. et al. The prognostic capacities of CBP and p300 in locally advanced rectal cancer. World J Surg Onc 17, 224 (2019). https://doi.org/10.1186/s12957-019-1764-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-019-1764-8