Abstract
Exosomes are lipid bilayer vesicles with a diameter of 40–100 nm secreted by almost all cells. They have been found play crucial regulatory roles in various diseases. With the development of exosomes engineering technology, exosome-based drug delivery has also rapidly evolved. Bladder cancer is a worldwide disease with high morbidity and recurrence but lack of funding, so it is also called Cinderella. Some explorations have demonstrated that exosomes are important in the development, prognosis, diagnosis and drug delivery of bladder cancer. With the rapid development of Mass spectrometry and next-generation sequencing, increasing numbers of differentially expressed molecules derived from exosomes have been found in bladder cancer. Exosomes and their contents are largely involved in bladder cancer progression, engineering of these exosomes with the targeted genes improves their potential for drug delivery of bladder cancer. Furthermore, exosomes and their contents are relate to many characteristics of bladder cancer. Herein, we briefly search 59 researches to explore the cargoes encapsuled in exosomes of bladder cancer patients. We also summarize the biogenesis, function, expression profiles, engineering approaches and biological mechanisms of exosomes and their contents for the diagnosis, prognosis and drug delivery for bladder cancer. We aim to make it clear whether exosomes are the glass slippers of Cinderella.
Graphical Abstract

Similar content being viewed by others
Background
Exosomes are spherical lipid bilayer vesicles with a diameter of 40-100 nm [1]. “Exosome” was first put forward by Trams et al. in 1981, referred as vesicles derived from plasma membrane which might play important roles in pathological and physiological function [2]. Though the concept “exosome” was widely used, ISEV 2018 guidelines suggested that it should be replaced by the term “small Extracellular Vesicles (sEVs)” [3]. For better distinction, we still refer it as “exosome” in this review. As a subtype of extracellular vesicles, exosomes distinguish themselves from microvesicles (MVs) and apoptotic bodies based on their biogenesis, size, contents and functions [4].The cargoes of exosomes include nucleic acids, lipids, cytokines and proteins [4]. Exosomes are immunogenic and can protect their contents from lysosomal degradation [5]. Exosomes have been found to play important roles in the occurrence and development of a variety of diseases through the cargoes they wrapped. More and more studies have been conducted to explore the possibility of exosomes as a treatment to cure a variety of diseases [6].
Bladder cancer is the 4th most common male cancer and 9th most common female malignancy, however, the clinical outcomes remained stagnant because of the lack of research funding. So, bladder cancer is also called Cinderella [7]. As a result, there are many unanswered questions associated with bladder cancer and needed to be explored. Recently, increasing studies have shown that exosomes play important roles in the pathological and physiology process of bladder cancer [8]. Exosomes can be used as liquid biopsy markers for diagnosis or prognosis of bladder cancer [9]. Furthermore, exosomes have been proposed as therapies for bladder cancer because they could be used for drug delivery [9]. In this review, we summarize the characteristics of exosomes and applications of engineered exosomes for drugs delivery in diseases, focused on the profiles, functions and clinical applications of exosomes in bladder cancer, we wonder whether exosomes can be the glass slippers of bladder cancer.
Biogenesis of exosomes
The biogenesis of exosomes is intensely regulated by many cell-specific receptors and signaling pathways [10]. The first step of exosome biogenesis is the fusion of endocytic vesicles and then form early endosomes (EE) [11]. There are two pathways for EEs, one way is called “recycling endosomes”, in which EEs can return the cargoes involved in them to the plasma membrane. Or EEs can change into “late endosomes”, which also called multivesicular bodies (MVBs) through Rab5. Multivesicular bodies and late endosomes are a subset of endosomal compartments rich in intraluminal vesicles (ILVs) [12]. ILVs are originated in the inward budding of endosomal membranes, and first discovered by Pan BT et al. in mature reticulocytes [13].
The sorting of cargoes wrapped in ILVs is highly regulated by many specific molecules. Endosomal-sorting complex required for transport (ESCRT) machinery is the main mechanism mediating ubiquitinated proteins sorted into ILVs. ESCRT apparatus are consisted of four complexes, ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III [14]. ESCRT-0 can recognize mono-ubiquitinated proteins via HRS heterodimer which is a cytosolic protein related to Clathrin. Calthrin is responsible for encountering the ubiquitinated proteins [15]. Then, the combination of ESCRT-I, ESCRT-II and ESCRT-0, binding the ubiquitinated substrates more tightly [16]. ESCRT-III finally helps to release the complex into endosome [17]. If the cargoes are not de-ubiquitinated by de-ubiquitinating enzymes (DUBs), the ILVs containing these cargoes will be targeted to fuse with the lysosome for degradation [18].
How are the un-ubiquitinated cargoes sorted into ILVs? As we know, Alix is a marker protein of exosomes and it can bind to ESCRT-III and send un-ubiquitinated molecules [19]. The ESCRT-independent pathway mainly happens in melanosomes with the help of Pmel17 and Tetraspanin CD63 [20].
Carolina Villarroya-Beltri et al. found that the specific motif in non-coding RNA decides whether it will be sorted into ILVs or not. Heterogeneous nuclear ribonucleo protein(hnRNP) is a ubiquitously expressed RNA-binding protein. Sumoylated hnRNP can recognize EXOmotifs of EXOmiRNAs and load them into ILVs. Then hnRNP can interact with cytoskeletal components to help transporting RNA to exosomes [21].
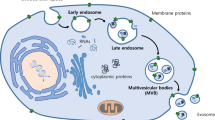
Finally, MVBs undergo two intracellular destination either fusion with lysosomes or they can move toward the plasma membrane and release ILVs to extracellular space as exosomes [22]. MVBs transferred to cell periphery are induced by Rab27A/B [23], then soluble N-ethylmaleimide(NEM)-sensitive factor attachment protein receptor(SNARE) complex drives MVBs to dock and fuse with the plasma membrane, then exosomes are released to the extracellular space [24]. Understanding the biogenesis and release of exosomes is essential for shedding new sights on therapeutic strategies (Fig. 1).
Exosomes biogenesis. In the endosomal system, endocytic vesicles fused with each other to form early endosomes (EE). There were many cargoes sequestered in EE. On one hand, the ubiquitinated proteins in EE could be sorted into intraluminal vesicles (ILVs) via ESCRT machinery. ILVs were formed through inward budding of the membrane with selected cargoes. While RNA-binding proteins heterogeneous nuclear ribonucleoprotein (hnRNP) could recognize the EXOmotifs of miRNAs and help them sorted into ILVs. On the other hand, some cargoes could be returned to the plasma membrane, called recycling endosomes. In addition, cargoes could also originate from trans-Golgi network and cytoplasm. These ILVs constituted the late endosomes /multivesicular body (LE/MVBs). The ubiquitinated targeted ILVs could be degraded within lysosome or rescue by DUBs. MVBs could also be transferred to the cell periphery via Rab27A/B. Finally, SNARE complex could help MVBs dock and fuse with plasma membrane to release ILVs into the extracellular space as exosomes
Function of exosomes
The functions of exosomes are associated with those of mother cells and depend on the cargoes capsuled in them, they can be derived from and transferred to many types of cells mediating the intercellular communication between cells [25]. Several studies have indicated that exosomes might play important roles in immune response and infection, tumor progression, neurodegeneration, metabolic and cardiovascular diseases and inflammatory response [26]. Though no severe immune reaction has been observed elicited by exosomes [27]. Recently, research found that exosomes derived from different sources, including immune cells, epithelial, and mesenchymal cells with cargoes could regulate both the innate and adaptive immune system of recipient cells [28]. C. J. E. Wahlund et al. found that exosomes derived from antigen-presenting cells (APCs) could induce the activation of specific T cells via p-MHC-II (major histocompatibility complex II with antigen peptide(p)) capsuled in exosomes [29]. R. Nandakumar et al. found that the nucleic acid exosomal cargo, namely DNA and miRNA of intercellular bacteria played important roles in regulating immune responses [30]. The studies focused on roles exosomes playing in cancer have increased rapidly. Many studies indicated that exosomes can influence neoplasia, tumor growth and metastasis [31]. K. Stefanius et al. found that exosomes derived from pancreatic cancer can initiate cell transformation by inducing mutations in NIH/3T3 recipient cells [32]. According to M.T.Le et al.,exosomal miR-200 derived from breast cancer cells can enhance the metastasis of breast cancer [33]. What’s more, exosomes are found important in neurodegenerative disorders mainly because of their control of misfolded protein accumulation. It has been found that a-synuclein was rich in cerebrospinal fluid of patients with Parkinson or amyotrophic lateral sclerosis [34]. What’s more, Yingkun Hu et al. demonstrated that exosomes could regulate the inflammatory response mainly through NF-κB signaling pathway [26]. In addition to participating in the pathological and physiological processes of various diseases, exosomes also have numerous applications in clinical settings including designing more effective personalized treatments [35]. Although there have been many studies conducted to explore the function of exosomes, what is the core capsuled in these exosomes that maximally affect the recipient cells remains vague.
Approaches for exosomes studies
Exosomes exert their effects depending on the cargo enclosed within them. For a long time, exosomes were considered merely as a mechanism for transporting cellular waste, however, with the development of mass spectrometry and next-generation sequencing, the exploration of exosomal contents has improved a lot [36]. The mostly used methods for verification of exosomes include Western blotting, NTA and TEM. Many approaches, including PCR, Western blotting, Northern blotting and ELISA are widely used to validate the cargoes capsuled in exosomes [37]. Separation of exosomes is the first step to all the exploration and utilization, many methods on exosome isolation and purification poured out in these years, namely ultracentrifugation, ultrafiltration, size-exclusion chromatography, Immunoaffinity, polymer precipitation and many commercial separation kits [38]. The large improvements in methods and experimental approaches help us learn the biogenesis and function of exosomes better (Fig. 2).
Approaches of designing exosomes used for drug delivery
The characteristics of exosomes make them a suitable platform for drug delivery [39]. Although natural exosomes have many advantages, they still have many limitations for clinical application, such as low targeting capability and a low concentration of functional molecules [40]. Engineered exosomes can effectively overcome these limitations. There are two main approaches to designing exosomes: parental cell-based exosome engineering and direct exosome engineering. The engineering procedures of the former occur before the isolation of exosomes, while the latter occurs after the isolation of exosomes. Parental cell-based exosome engineering can be divided into two classes. In the first class, non-specific way, it can be conducted through the transfection of parental cells with plasmids or mimics of interest. These procedures exclude any packaging and sorting. The other class involves specific loading of molecules, which can also be divided into two subgroups: exosomal surface display and loading into the lumen.Exosomal surface display utilizes exosomal signal peptides, including Lamp2b (lysosome-associated membrane protein 2b) fusion proteins [41], tetraspanins (CD63, CD9, CD81) [42], GPI (glycosylphosphatidylinositol) [43], PDGFRs (platelet-derived growth factor receptors) [44], lactadherin (C1C2 domain) [45], and VSVG (vesicular stomatitis virus glycoprotein) [46]. Fuse an interested protein to the signal peptide can present the protein on the exosomes’ surface. Loading of the molecules into the lumen of exosomes is based on molecule sorting modules (MSMs). Various methods with different MSMs exist, including engineered ubiquitin tags [47], WW tags [48], non-functional mutants of the HIV-I Nef protein [49], EXPLORs (exosomes for protein loading via optically reversible protein–protein interaction) for loading proteins, and EXOtic (exosomal transfer into cells) devices, TAT (Trans-activator of transcription)—TAR (Trans-activating response RNA loop) protein-RNA interaction strategies, and RNA binding modules for loading RNA into exosomes [50].Technically, direct exosome engineering is simpler compared to methods based on parental cells. In this approach, electroporation, sonication, incubation, bio-conjugation, freeze–thaw, and extrusion can be applied directly to design exosomes after their isolation from cells [51] (Fig. 3). The emergence and application of increasingly numerous and advanced engineered methods provide better tools and prospects for exosome-based drug delivery.
Engineering approaches of exosomes. Two main approaches of designer exosomes include parental cell-based exosome engineering and direct exosome engineering, in which the engineering procedures of parental cell-based exosomes occur before exosomes isolation from cells while direct exosome engineering occur after exosomes isolation. There are also many different methods in each class. GPI Glycosylphosphatidylinositol, PDGFRs Platelet-derived growth factor receptors, VSVG Vesicular stomatitis virus glycoprotein, MSMs Molecule sorting modules, EXPLORs Exosomes for protein loading via optically reversible PPIs, EXOtic Exosomal Transfer Into cells, TAT-TAR Trans-activator of transcription, Trans-activating response RNA loop, RBP RNA binding proteins;
Bladder cancer(Cinderella)
Bladder cancer is the 4th most common cancer in male and 9th in female. The prevalence and incidence keep increasing worldwide. However, the clinical outcomes have stayed static for 25 years for the small investment in bladder cancer research, therefore, bladder cancer is also known as “Cinderella”, often neglected though important [7]. There are three main pathological types of bladder cancer of which bladder urothelial carcinoma (BUC) accounting for 90% [52]. BUC can be composed of muscle-invasive BCa (MIBC) and non-muscle-invasive BCa (NMIBC) and NMIBC accounts for approximately 75% [53]. The treatments of bladder cancer were often endoscopic resection and adjuvant intravesical therapy and patients with advanced disease were treated with immunotherapy. Though the treatments for BCa have improved a lot through years, postoperative recurrence and distant metastasis are still severe, making it of great importance to explore the potential ways for treatment, early diagnosis, prognosis and prevention [54, 55].
Researches of exosomes in BCa
A full review was conducted with Web of science, PubMed and Embase to search reports with the key words (“exosomes” or “extracellular vesicles”) and (“bladder cancer” or “bladder urothelial carcinoma” or “bladder neoplasm” or “bladder tumor”) for 10 years since January 2013-March 2023 Additional file 1. The studies finally involved in this review are listed in Additional file 2: Table S1. Research associated with exosomes and their contents involved in bladder cancer has increased annually. Collectively, recent studies validate exosomes derived from bladder cancer cells, or biofluid of bladder cancer patients can wrap up mRNAs, miRNAs, lncRNAs, proteins and bacteria which are crucial in the formation and metastasis of bladder cancer [56, 57].
Increasing methods have been conducted to explore the contents of exosomes. RNA sequencing (RNA-seq), microarray, 16S metagenomic sequencing and Mass Spectrometry are widely used for identification and quantification of exosomes. Western Blotting, Reverse transcription polymerase chain reaction (RT-PCR), and Enzyme linked immunosorbent assay (ELISA) are main approaches used to further verify the contents of exosomes.
To explore the function and application of exosomes more comprehensively, conveniently and efficiently, numerous exosomes-associated public databases have been established, including EVmiRNA, ExoRBase, ExoCarta, EV-TRACK, MiRandola and so on. For example, ExoRBase contains the information of exosomal circRNA, lncRNA and mRNA from human serum samples. EV-miRNA provides organ- and disease-associated miRNA annotations [58,59,60,61,62,63,64,65,66]. The characterization of other databases is listed in Table 1.
The profiles of exosomal cargoes in bladder cancer
Many novel dysregulated exosomal cargoes have been found in bladder cancer cell lines and biofluid of bladder cancer patients, demonstrating that exosomes play important roles in bladder cancer development and progression. From Additional file 2: Table S1, we found Joanne L et al. presented the first proteomics analysis of exosomes derived from bladder cancer cell lines in 2010, they reported 353 high quality identifications of which 72 proteins were not found by other human exosome studies before, what’s more, authors found that basigin 5T4 and galectin-3 were confirmed positive in exosomes derived from urine of bladder cancer patients, indicating they might play important roles in bladder cancer formation [67]. Dennis et al. found 58 significantly different exosomal proteins derived from bladder cancer cells with or without the metastatic process, indicating exosomes could affect the metastasis and progression of bladder cancer [68]. Microarray showed urinary exosomal miR-375 and miR-146a could be used as biomarkers for high-grade and low-grade bladder cancer [69]. In another study, next generation sequencing revealed that HOTAIR and four additional lncRNAs, including HYMAI, LINC00477, LOC100506688 and OTX2-AS1 enriched in the exosomes of UBC patients, suggesting that UE-derived lncRNA could be served as biomarkers and therapeutic targets [70]. In addition, secondary bioinformatic analyses based on Gene Expression Omnibus (GEO), the Cancer Genome Atlas (TCGA) and exosome-related databases were used to identify differentially expressed exosomal protein, mRNAs and non-coding RNAs. Nitu Kumari et al. found that exosomal catanin, PAK1, CDC42 and NF2 were overexpressed in bladder cancer patients via Exocarta database and verified them in the urine samples of bladder cancer patients [71]. Bioinformatic analysis of the tissues of bladder cancer patients constructed a panel of five urinary exosomal mRNAs, then exosomes derived from urine samples were used to validate the ROC of the panel, indicating the panel a potential diagnosis of bladder cancer [70]. RNA-seq, or Mass spectrometry data analysis, paired t tests or non-parametric Mann–whitney U tests are conducted to analyse differences between groups in microarray. Fold change ≥ 2.0 is treated as significantly different and the false discovery rate (FDR) is recommended to be < 0.05. For RT-PCR or Western blotting of exosomes, an external reference is usually used instead of internal reference.
Biological functions of exosomes in bladder cancer
Exosomes regulate the hallmarks of bladder cancer
Proliferative signaling, Growth suppressors, Cell death, Replicative immortality, Angiogenesis and Invasion and metastasis are important hallmarks of bladder cancer [72]. Here, we summarize the exosomes involved in the progression of bladder cancer to explore the association between exosomes and the hallmark features of cancer (Fig. 4). There are more and more studies indicated that exosomes could be involved in cell proliferation, apoptosis, invasion, migration, metastasis, angiogenesis and cisplatin chemoresistance of bladder cancer. We summarize the main signaling pathways involved in these processes in Fig. 5.
The signaling pathways involved in exosomes regulating bladder cancer progression. Exosomes and their contents can regulate cell proliferation, cell cycle, invasion and migration, metastasis, angiogenesis and cisplatin chemoresistance in bladder cancer. The main signaling pathways involved in these processes including Wnt/β-catenin pathway, PI3K/AKT pathway, STAT3 pathway and NF-κB signaling pathway
Cell proliferation
Bladder cancer can sustain proliferative states through activating cell proliferation signaling pathways [55]. Normal cells derived exosomes could regulate NF2 to inhibit tumor growth and progression of bladder cancer [71]. The PI3K/AKT/NF-κB/STAT3 signaling pathway is an important regulatory pathway. FAN LIN et al. found that exosomal miR-21 derived from bladder cancer cells could promote M2 phenotypic polarization through inhibiting phosphatase and tensin homolog activation of PI3K/AKT pathway, and finally lead to cancer progression [73]. Phosphatase and tensin homologue (PTEN) is a negative regulator of PI3K/AKT pathway. Rui Zheng et al. found that exosomes derived from normal cells transferred PTENP1 to bladder cancer cells, then exosomal PTENP1 acted as a miR-17 decoy to regulate PTEN, suppressing bladder cancer progression [74]. Consistent with the results, Shu -Cheng Liu et al. revealed BMSC-derived exosomal PTENP1 suppressed the bladder cancer by upregulating the expression of SCARA5, making it a potential target for bladder cancer therapy [75]. Exosomes derived from MB49, a kind of mouse bladder cancer cells, induced macrophage M2 polarization via down-regulation of PTEN and activation of AKT/STAT3/6 signaling [76]. MiR-663b generated from exosomes of bladder cancer cells could act as a tumor promoter via targeting Ets2-repressor factor [77]. Exosomal miR-133b could suppress bladder cancer proliferation by upregulating dual-specificity protein phosphatase1(DUSP1) [78]. Cheng Shuo Huang et al. presumed that exosome-derived LINC00960 and LINC02470 from high-grade bladder cancer cells promote the malignant progression by upregulating β-catenin signaling, Notch signaling, and Smad2/3 signaling [79]. Exosomal miR-375-3p and LINC01133 were also found to be a suppressor of bladder cancer and could inhibit proliferation and metastasis via Wnt/β-catenin pathway [80, 81]. What’s more, exosomal miR-93-5p suppressed BTG2 expression and promoted bladder cancer cells progression, exosomal EphA2 promoted the invasion and migration of bladder cancer cells, exosomal CDC6 effectively repressed the malignant process of bladder cancer cells [82,83,84].
Additionally, dysregulated of cell cycle regulators played important roles in bladder cancer cell growth and progression [85].C-MYC and Cyclin D1 are two key genes regulating the cell growth [56]. As reported by Qi Li, exosomal miR-375-3p could block the expression of Cyclin D1 and c-Myc and then inhibited cell growth [80]. Jian-Hong Wu et al. also provided the first evidence that the exosome-mediated delivery of miR-4792 could down-regulate c-Myc, inhibiting aerobic glycolysis [86].
Apoptosis
Apoptosis is one of the major mechanisms resulting in controlled cell death which can be controlled by cancer cells. Many tumor cells can avoid apoptosis, thus they can multiplicate infinitely [87]. Qi Li et al. found that exosomal miR-375-3p suppressed bladder cancer growth through promoting apoptosis in BC cells [80]. Bladder cancer cell-derived exosomes could inhibit tumor cell apoptosis via activating Akt and ERK pathways [88]. Chia-Hao Wu et al. demonstrated that tumor-derived extracellular vesicles (TEVs) could promote malignant transformation of predisposed cells by inhibiting pro-apoptotic signals [89]. According to Xiaoxiao Cai et al., exosomal miR-133b could induce apoptosis in BC cells [78].
Invasion and metastasis
The invasion of tumor cells into lymphatic and blood vessels is important for the metastasis of solid tumor to distant organs [90]. While epithelial-mesenchymal transition (EMT) plays an important role in the invasion and metastasis process, for which epithelial cells lose their cell polarity and cell–cell adhesion [91]. Dennis et al. found the exosomal proteins derived from bladder cancer cells with or without metastasis were significantly different, indicating the important roles these proteins might play in the metastasis process [68]. Carla et al. revealed that exosomal EDIL3 derived from bladder cancer could activate epidermal growth factor receptor signaling which induced cell migration [92]. CA Franzen et al. demonstrated that exosomes derived from bladder cancer cell were able to induce the expression of several mesenchymal markers in recipient urothelial cells [93]. Claudia et al. showed that IncRNA HOX transcript antisense RNA(HOTAIR) was increased in exosomes derived from the serum of bladder cancer patients, loss of this lncRNA in UBC cells altered expression of epithelial-to-mesenchyme (EMT) [70]. EV-mediated ELNAT1 was proved to promote lymphangiogenesis and LN metastasis in bladder cancer via UBC9/SOX18 regulatory axis, EV-mediated ELNAT1 was also correlated with a poor prognosis [94]. Consistent with these results, Changhao Chen et al. declared that bladder cancer cell-derived exosome-mediated lymphangiogenesis promoted LN metastasis in bladder cancer through a VEGF-C-independent manner [95]. MicroRNA(miR)-663b was found increased in plasma from patients with bladder cancer (BC), while it could promote epithelial-mesenchymal transition via targeted Ets2-repressor factor [78]. KRT6B, a molecule significantly related to epithelial-mesenchymal transition and immune mechanisms, was detected elevated in bladder cancer-derived exosomes, indicating its crucial role in the invasion and metastasis of bladder cancer process [96].
Angiogenesis
The growth and progression of tumor are highly relied on the nutrients and oxygen supplied by angiogenesis. Without angiogenesis, the size of tumor will only be limited to 200 μm [97]. Vascular endothelial growth factor (VEGF) is one of the most potent inducers of angiogenesis [98]. Exosomal GFAT1 derived from bladder cancer was reported to promote tumor angiogenesis by inducing HBP-related metabolic reprogramming and SerRS O-GlcNAcylation in endothelial cells, this may shed light on novel targets for bladder cancer antiangiogenetic therapy [99]. As illustrated by Carla J et al., exosomes isolated from high grade bladder cancer cells could promote angiogenesis and migration of bladder cancer cells. Exosomal EDIL-3 was one of the proteins that activated epidermal growth factor receptor signaling, inducing bladder cancer cell migration [92]. According to Xinyuan Li, cathepsin B (CTSB) was upregulated in exosomes derived from serum of bladder cancer patients, directly ingesting EV-CTSB prominently activated TPX2-mediated phosphorylation of the AURKA-PI3K-AKT axis, increased VEGFA expression, finally promoted angiogenesis [100].
Cisplatin chemoresistance
Cisplatin resistance is a problem for bladder cancer although bladder cancer is relatively sensitive to chemotherapy. Previous studies have indicated that exosomes can promote chemotherapy resistance [101]. Consistent with these results, Guangyue Luo found that exosomal LINC00355 derived from CAFs promoted the cisplatin chemoresistance of bladder cancer via the miR-34b-5p/ABCB1 axis [102].
Clinical significance of exosome in Bca
Bladder cancer is the second most common urology malignancy worldwide [103]. The high mortality makes it important to promote its early diagnosis and prognosis. Currently, the gold standard of the diagnosis in bladder cancer is cystoscopic examination of bladder and histological evaluation of the bladder tissue [104]. However, it is an invasive examination. Urine cytology is another common method for bladder cancer diagnosis, however its low sensitivity for low-grade tumors prevents it from widely used [105]. Exosomes are membrane-bound vesicles that most cells release into body fluids and they have been treated as mediators of tumor progression over past decades [106]. What’s more, exosomes are stable and they can protect their cargoes from degradation by enzymes [107]. Therefore, many studies have focused on the clinical applications of exosomes (Fig. 6). Exosomes with the potential of diagnostic, prognostic value of bladder cancer are listed in Table 2. As mentioned before, exosomes play key roles in bladder cancer, promoting the release of exosomes or inhibiting the secretion of exosomes might be an effective strategy for inhibiting the progression of bladder Cancer [108]. What’s more, exosomes can be designed to be loaded with exogenous RNAs and proteins for targeted therapy [109]. Engineered exosomes have been widely applied in bladder cancer, Liu et al. found Exo-miR-138-5p engineered from adipose derived mesenchymal stem cells(ADSCs) could penetrate tumor tissues and suppress the growth of xenograft tumors, what’s more, Mesenchymal stem cells-derived exosomal microRNA-139-5p restrained tumorigenesis in bladder cancer [110, 111].
Exosomes are significantly related to characteristics of bladder cancer. Fathia et al. observed that urine and serum exosome level is correlated with the tumor stages, indicating it can be used as biomarker for prognosis and diagnosis [108]. The contents wrapped in exosomes have been found to be involved in the clinical applications of bladder cancer. a2M (alpha-2-macroglobulin) has been reported to be upregulated in the urine exosomes of bladder cancer patients [112]. Moreover, a three exosomal lncRNA panel (RMRP, UCA1 and MALAT1) are elevated in bladder cancer, and is correlated with the tumor stage of bladder cancer [113]. Furthermore, the exosomal proteins derived from bladder cancer urine and healthy controls are significantly different, indicating their potential as a noninvasive biomarker [114]. Similarly, urine exosomal NMP22 is upregulated in bladder cancer than normal samples [115]. Chenchen et al. demonstrated that exosomal TERC is significantly upregulated in the urine of bladder cancer patients, what’s more, it has a tight correlation with tumor grade [116]. Exosomal EDIL-3 has been shown to be overexpressed in urine samples of bladder cancer patients and its levels are associated with pathologic grade [92]. Similarly, exosomal miR-375 is overexpressed in bladder cancer, and its levels are correlated with high-grade tumor. In contrast, miR-146a is downregulated in bladder cancer, and its expression levels are significantly correlated with low-grade tumor [69]. Sophie et al. revealed that exosomal miR-146b-5p and miR-155-5p derived from urine of bladder cancer patients have a positive correlation with muscle invasion of tumor [117]. According to Hao Lin et al., the expression of exosomal miR-93-5p and miR-516a-5p is higher in bladder cancer, and the level of exosomal miR-93-5p is associated with muscle invasion of tumor [82]. The expression levels of exosomal KLHDC7B, CASP14, PRSS1, MIR205HG and GAS5 have been found increased in bladder cancer urine samples, furthermore, the expression of these five molecules are significantly related to tumor stage, grade and hematuria degree [118]. In addition, exosomal TUG-1 is detectable in bladder cancer urine and serum at an early stage [119]. Exosomal BCYRN1 has been reported to be associated with lymph node metastasis of bladder cancer, and, higher expression of BCYRN1 represented poorer prognosis [120]. Alexandru et al. indicated that exosomal miR-4508 and piR-has-5936 have a tight association of risk class and tumor grade, while miR-4508 has a downward trend as the risk class increased, piR-has-5936 has a upward trend as the risk class increased [121]. Dong hyeon Lee and Xunian Zhou both found that the unique somatic variants of exoDNA are positively correlated with bladder cancer [122, 123]. In addition, label-free optic redox ratio of exosomes can also tell bladder cancer patients from normal controls [124].
There have been many studies focusing on exosomes treated as diagnostic biomarker for bladder cancer. The area under the receiver operating characteristic (ROC) curve (AUC) of exosomal CEACAM1 is 0.907 [125]. The AUC for exosomal miR-96-5p is 0.87, with a sensitivity of 82.4% and a specificity of 91.8% [126]. The AUC of combined RMRP, UCA1 and MALAT1 is 0.875, with the sensitivity of 80% and specificity of 81.4%, respectively [113]. The AUC of combined exosomal UCAI-201, UCAI-203, MALAT1 and LINC00355 is 0.96, with a sensitivity of 92% and a specificity of 91.7%, respectively [127]. The AUC for exosomal CA9 is 0.837, with a sensitivity of 85.18% and a specificity of 83.15%, respectively [128]. The AUC of exosomal TERC is 0.836, with the sensitivity of 78.65% and specificity of 77.78%, respectively The AUC of combined exosomal KLHDC7B, CASP14, PRSS1, MIR205HG and GAS5 is 0.924 [118]. The AUC of exosomal ANRIL is 0.7229, with a sensitivity of 46.67% and specificity of 87.5%, respectively [129].
Exosomes can also be used to predict the prognostic of bladder cancer. We found that upregulated exosomal H19, BCYRN1, periostin and miR-10b-5p were reported to predict poor overall survival (OS) [120, 121, 130, 131], while downregulated of exosomal TALDO1, miR-185-5p and miR-106a-5p were reported to predict poor OS [119, 121]. Cheng-shuo huang et al. revealed that the expression of exosomal LINC00960 and LINC02470 can be used as prognostic surveillance [79]. In addition, patients with high exosomal PCAT-1, UBC1, SNHG16 were reported to have a lower recurrence-free survival [132]. Similarly, two studies revealed that higher expression of exosomal miR-451a with miR-486-5p and MALAT1, PCAT1 are associated with poorer recurrence-free survival [133, 134]. In addition to these published studies, we searched the registered clinical trials website and found that SunYat-Sen Memorial Hospital has been conducting a prospective, multicenter cohort study in blaader cancer to explore the predictive value of exosomal ELNAT1 for lymphatic metastasis of bladder cancer (Additional file 1: Fig S1).
Discussion
Bladder cancer is a worldwide disease with high morbidity and recurrence, however, there are not many studies explored on bladder cancer for the lack of funding, so it is also called “Cinderella” [7]. The mechanism and progression of bladder cancer still remain vague. What’s more, a noninvasive and accurate diagnosis or prognosis biomarker and engineered exosomes for drug delivery of bladder cancer is urgently needed. As we outlined above, exosomes and their contents are deeply involved in the formation and metastasis of bladder cancer, they can also be used as the liquid biomarker for bladder cancer. Does that mean exosome is the glass slipper of Cinderella? This question still needs further explorations.
Exosomes are spherical lipid bilayer vesicles with a diameter of 40-100 nm, they can be secreted from most cells through a period of processes [135], the contents wrapped into exosomes are sorted through ESCRT-dependent pathway or ESCRT-independent pathway [136]. The exosomes can protect their contents from degradation by RNase. The isolation and purification methods for exosomes have improved a lot over the past decades, In addition to the methods described above, combined application of those methods, such as combined ultracentrifugation and ultrafiltration can lead to the higher purity and quality of exosomes [137]. Furthermore, more and more Isolation Kits have been invented.
The cargoes wrapped in exosomes include almost all kinds of RNA, proteins, lipids and so on, they play crucial roles in the progression and metastasis of bladder cancer, they can also be used for diagnosis or prognosis in bladder cancer. The studies over the past 10 years share some common exosomal contents including MALAT1, PCAT-1 and PTENP1. Many studies have demonstrated that these three molecules play key roles in bladder cancer and can be used as accurate biomarker for bladder cancer [113, 127, 129, 132, 133]. The phosphatase and tensin homologue (PTEN) is an essential tumor suppressor [138]. It is reported to be pivotal to regulate the receptor tyrosine kinase (RTK) PI-3 kinase (PI3K)/Akt pathway [139]. PTENP1, the pseudogene of PTEN, is a novel modulator of PTEN expression [140]. The relative expression of PTEN and PTENP1 change according to the variable stages and histological grades of different tumors [141,142,143]. Prostate cancer associated transcript-1(PCAT-1) is an oncogenic lncRNA, high expression of PCAT-1 is associated with poor overall survival of cancer. It is also involved in Wnt/β-catenin-signaling pathway and participates in the cancer cell proliferation, apoptosis, invasion and metastasis [144].
Metastasis associated lung adenocarcinoma transcript 1(MALAT1) is a ubiquitous lncRNA in mammals, it is widely explored in cancer and crucial for the regulation of cancer-related pathways. MALAT1 can modulate many chief tumourigenesis pathways including MAPK/ERK, PI3K/AKT, β-catenin/Wnt, Hippo, VEGF, YAP signaling pathways, etc. [145]. What’s more, MALAT1 is reported to correlate with poor OS, RFS, DFS in various cancers [146]. However, MALAT1 also plays a key role in many other diseases like diabetes and neurologic disorders, which make MALAT1 not an ideal tumor biomarker [147, 148]. Combined some other molecules might make the detection more accurate.
In addition to the contents wrapped in exosomes, the properties of the exosomes themselves are also worth exploring. The exosomes level derived from urine samples are significantly correlated with the tumor grade and stage [108]. Jaena Park et al. found the label-free optical redox ratio of exosomes can be used for diagnosis for bladder cancer [124]. What’s more, engineered exosomes have been widely used for targeted delivery of drugs in bladder cancer, the approaches of engineered exosomes include parental cell-based exosome engineering and direct exosome engineering, Exo-miR-138-5p engineered from adipose derived mesenchymal stem cells(ADSCs) and Mesenchymal stem cells-derived exosomal microRNA-139-5p have been found restrain the growth of bladder cancer.
Conclusion
Exosomes are spherical lipid bilayer vesicles with a diameter of 40–100 nm, the contents wrapped into exosomes are sorted through ESCRT-dependent pathway or ESCRT-independent pathway. Engineered exosomes have been used for targeted delivery of drugs in many diseases. They have been found to play crucial roles in bladder cancer progression and immigration, they can also be noninvasive biomarkers for prognosis or diagnosis of bladder cancer. Exosomal MALAT1, PCAT-1 and PTENP1 have been found in many studies focused on the link between exosomes and bladder cancer, indicating these three molecules participate in the progression of bladder cancer in depth. What’s more, engineered exosomes have been widely found to play important roles in bladder cancer. Exosomes seem to be the glass slippers of Cinderella, although it still needs further exploration whether the shoes fit well.
Availability of data and materials
Not applicable.
References
Farooqi AA, Desai NN, Qureshi MZ, Librelotto DRN, Gasparri ML, Bishayee A, Nabavi SM, Curti V, Daglia M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol Adv. 2018;36(1):328–34. https://doi.org/10.1016/j.biotechadv.2017.12.010.
Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645(1):63–70. https://doi.org/10.1016/0005-2736(81)90512-5.
Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. https://doi.org/10.1080/20013078.2018.1535750.PMID:30637094;PMCID:PMC6322352.
Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8(7):727. https://doi.org/10.3390/cells8070727.PMID:31311206;PMCID:PMC6678302.
Li B, Cao Y, Sun M, Feng H. Expression, regulation, and function of exosome-derived miRNAs in cancer progression and therapy. FASEB J. 2021;35(10): e21916. https://doi.org/10.1096/fj.202100294RR.
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;22(15):6917–34. https://doi.org/10.2147/IJN.S264498.PMID:33061359;PMCID:PMC7519827.
Van Hemelrijck M, Patel P, Mouw KW. Editorial: bladder cancer—a cinderella cancer: advances and remaining research questions. Front Oncol. 2020;4(10):1749. https://doi.org/10.3389/fonc.2020.01749.PMID:33014863;PMCID:PMC7499472.
Oliveira MC, Caires HR, Oliveira MJ, Fraga A, Vasconcelos MH, Ribeiro R. Urinary biomarkers in bladder cancer: where do we stand and potential role of extracellular vesicles. Cancers. 2020;12(6):1400. https://doi.org/10.3390/cancers12061400.PMID:32485907;PMCID:PMC7352974.
Georgantzoglou N, Pergaris A, Masaoutis C, Theocharis S. Extracellular vesicles as biomarkers carriers in bladder cancer: diagnosis, surveillance, and treatment. Int J Mol Sci. 2021;22(5):2744. https://doi.org/10.3390/ijms22052744.PMID:33803085;PMCID:PMC7963171.
Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. https://doi.org/10.1186/s12943-019-0991-5.PMID:30940145;PMCID:PMC6444571.
Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–500. https://doi.org/10.1038/emboj.2011.286.PMID:21878991;PMCID:PMC3181477.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47. https://doi.org/10.1186/s12964-021-00730-1.PMID:33892745;PMCID:PMC8063428.
Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78. https://doi.org/10.1016/0092-8674(83)90040-5.
Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012;22(4):R116–20. https://doi.org/10.1016/j.cub.2012.01.028.PMID:22361144;PMCID:PMC3314914.
van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21. https://doi.org/10.1093/jb/mvj128.
Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458(7235):172–7. https://doi.org/10.1038/nature07836.
McCullough J, Frost A, Sundquist WI. Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes. Annu Rev Cell Dev Biol. 2018;6(34):85–109. https://doi.org/10.1146/annurev-cellbio-100616-060600.
Mathieu J, Michel-Hissier P, Boucherit V, Huynh JR. The deubiquitinase USP8 targets ESCRT-III to promote incomplete cell division. Science. 2022;376(6595):818–23. https://doi.org/10.1126/science.Abg2653.
Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26(19):4215–27. https://doi.org/10.1038/sj.emboj.7601850.
van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–21. https://doi.org/10.1016/j.devcel.2011.08.019.
Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, Pérez-Hernández D, Vázquez J, Martin-Cofreces N, Martinez-Herrera DJ, Pascual-Montano A, Mittelbrunn M, Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. https://doi.org/10.1038/ncomms3980.PMID:24356509;PMCID:PMC3905700.
Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: a hypothesis. Biochem Soc Trans. 2011;39(2):559–62. https://doi.org/10.1042/BST0390559.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):6977. https://doi.org/10.1126/science.aau6977.
Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. https://doi.org/10.1038/s41556-018-0250-9.
Yue B, Yang H, Wang J, Ru W, Wu J, Huang Y, Lan X, Lei C, Chen H. Exosome biogenesis, secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020;53(7):e12857. https://doi.org/10.1111/cpr.12857.
Hu Y, Wang Y, Chen T, Hao Z, Cai L, Li J. Exosome: function and application in inflammatory bone diseases. Oxid Med Cell Longev. 2021;31(2021):6324912. https://doi.org/10.1155/2021/6324912.PMID:34504641;PMCID:PMC8423581.
Mendt M, Kamerkar S, Sugimoto H, McAndrews KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, Marszalek JR, Maitra A, Yee C, Rezvani K, Shpall E, LeBleu VS, Kalluri R. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8): e99263. https://doi.org/10.1172/jci.insight.99263.PMID:29669940;PMCID:PMC5931131.
Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, Chalmers J, Schmittgen TD, Phelps MA. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 2017;6(1):1324730. https://doi.org/10.1080/20013078.2017.1324730.PMID:28717420;PMCID:PMC5505007.
Wahlund CJE, Güclüler G, Hiltbrunner S, Veerman RE, Näslund TI, Gabrielsson S. Exosomes from antigen-pulsed dendritic cells induce stronger antigen-specific immune responses than microvesicles in vivo. Sci Rep. 2017;7(1):17095. https://doi.org/10.1038/s41598-017-16609-6.PMID:29213052;PMCID:PMC5719080.
Nandakumar R, Tschismarov R, Meissner F, Prabakaran T, Krissanaprasit A, Farahani E, Zhang BC, Assil S, Martin A, Bertrams W, Holm CK, Ablasser A, Klause T, Thomsen MK, Schmeck B, Howard KA, Henry T, Gothelf KV, Decker T, Paludan SR. Intracellular bacteria engage a STING-TBK1-MVB12b pathway to enable paracrine cGAS-STING signalling. Nat Microbiol. 2019;4(4):701–13. https://doi.org/10.1038/s41564-019-0367-z.
Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, Breakefield XO, Skog JK. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466–77. https://doi.org/10.1016/j.annonc.2021.01.074.
Stefanius K, Servage K, de Souza SM, Gray HF, Toombs JE, Chimalapati S, Kim MS, Malladi VS, Brekken R, Orth K. Human pancreatic cancer cell exosomes, but not human normal cell exosomes, act as an initiator in cell transformation. Elife. 2019;28(8): e40226. https://doi.org/10.7554/eLife.40226.PMID:31134894;PMCID:PMC6538373.
Le MT, Hamar P, Guo C, Basar E, Perdigão-Henriques R, Balaj L, Lieberman J. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124(12):5109–28. https://doi.org/10.1172/JCI75695.
Spencer B, Kim C, Gonzalez T, Bisquertt A, Patrick C, Rockenstein E, Adame A, Lee SJ, Desplats P, Masliah E. α-Synuclein interferes with the ESCRT-III complex contributing to the pathogenesis of Lewy body disease. Hum Mol Genet. 2016;25(6):1100–15. https://doi.org/10.1093/hmg/ddv633.
Gao Y, Qin Y, Wan C, Sun Y, Meng J, Huang J, Hu Y, Jin H, Yang K. Small extracellular vesicles: a novel avenue for cancer management. Front Oncol. 2021;15(11): 638357. https://doi.org/10.3389/fonc.2021.638357.PMID:33791224;PMCID:PMC8005721.
Zhao S, Wu M, Yang S, Wu Y, Gu Y, Chen C, Ye J, Xie Z, Tian Z, Bachman H, Huang PH, Xia J, Zhang P, Zhang H, Huang TJ. A disposable acoustofluidic chip for nano/microparticle separation using unidirectional acoustic transducers. Lab Chip. 2020;20(7):1298–308. https://doi.org/10.1039/d0lc00106f.
Dong Q, Han Z, Tian L. Identification of serum exosome-derived circRNA-miRNA-TF-mRNA regulatory network in postmenopausal osteoporosis using bioinformatics analysis and validation in peripheral blood-derived mononuclear cells. Front Endocrinol. 2022;9(13): 899503. https://doi.org/10.3389/fendo.2022.899503.PMID:35757392;PMCID:PMC9218277.
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, Du L. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2022;5(9): 811971. https://doi.org/10.3389/fbioe.2021.811971.PMID:35071216;PMCID:PMC8766409.
Zhu F, Chong Lee Shin OLS, Pei G, Hu Z, Yang J, Zhu H, Wang M, Mou J, Sun J, Wang Y, Yang Q, Zhao Z, Xu H, Gao H, Yao W, Luo X, Liao W, Xu G, Zeng R, Yao Y. Adipose-derived mesenchymal stem cells employed exosomes to attenuate AKI-CKD transition through tubular epithelial cell dependent Sox9 activation. Oncotarget. 2017;8(41):70707–26. https://doi.org/10.18632/oncotarget.19979.
Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–96. https://doi.org/10.1016/j.apsb.2016.02.001.
Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. https://doi.org/10.1016/j.pharmthera.2017.02.020.
Stickney Z, Losacco J, McDevitt S, Zhang Z, Lu B. Development of exosome surface display technology in living human cells. Biochem Biophys Res Commun. 2016;472(1):53–9. https://doi.org/10.1016/j.bbrc.2016.02.058.
Kooijmans SA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. 2016;14(5):31053. https://doi.org/10.3402/jev.v5.31053.PMID:26979463;PMCID:PMC4793259.
Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91. https://doi.org/10.1038/mt.2012.180.
Rountree RB, Mandl SJ, Nachtwey JM, Dalpozzo K, Do L, Lombardo JR, Schoonmaker PL, Brinkmann K, Dirmeier U, Laus R, Delcayre A. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011;71(15):5235–44. https://doi.org/10.1158/0008-5472.CAN-10-4076.
van Dongen HM, Masoumi N, Witwer KW, Pegtel DM. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol Mol Biol Rev. 2016;80(2):369–86. https://doi.org/10.1128/MMBR.00063-15.PMID:26935137;PMCID:PMC4867369.
Cheng Y, Schorey JS. Targeting soluble proteins to exosomes using a ubiquitin tag. Biotechnol Bioeng. 2016;113(6):1315–24. https://doi.org/10.1002/bit.25884.
Sterzenbach U, Putz U, Low LH, Silke J, Tan SS, Howitt J. Engineered exosomes as vehicles for biologically active proteins. Mol Ther. 2017;25(6):1269–78. https://doi.org/10.1016/j.ymthe.2017.03.030.
de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft-associated protein sorting in exosomes. Blood. 2003;102(13):4336–44. https://doi.org/10.1182/blood-2003-03-0871.
Jafari D, Shajari S, Jafari R, Mardi N, Gomari H, Ganji F, Forouzandeh Moghadam M, Samadikuchaksaraei A. Designer exosomes: a new platform for biotechnology therapeutics. BioDrugs. 2020;34(5):567–86. https://doi.org/10.1007/s40259-020-00434-x.PMID:32754790;PMCID:PMC7402079.
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin. 2017;38(6):754–63. https://doi.org/10.1038/aps.2017.12.
Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The national cancer data base report on bladder carcinoma. The american college of surgeons commission on cancer and the american cancer society. Cancer. 1996;78(7):1505–13.
Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, Kiemeney L, Kriegmair M, Montironi R, Murphy WM, Sesterhenn IA, Tachibana M, Weider J. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 Suppl 1):4–34. https://doi.org/10.1016/j.urology.2005.07.062.
Nadal R, Bellmunt J. Management of metastatic bladder cancer. Cancer Treat Rev. 2019;76:10–21. https://doi.org/10.1016/j.ctrv.2019.04.002.
Alifrangis C, McGovern U, Freeman A, Powles T, Linch M. Molecular and histopathology directed therapy for advanced bladder cancer. Nat Rev Urol. 2019;16(8):465–83. https://doi.org/10.1038/s41585-019-0208-0.
Lin F, Yin HB, Li XY, Zhu GM, He WY, Gou X. Bladder cancer cell-secreted exosomal miR-21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int J Oncol. 2020;56(1):151–64. https://doi.org/10.3892/ijo.2019.4933.
Wu JH, Sun KN, Chen ZH, He YJ, Sheng L. Exosome-mediated miR-4792 transfer promotes bladder cancer cell proliferation via enhanced FOXC1/c-Myc signaling and warburg effect. J Oncol. 2022;19(2022):5680353. https://doi.org/10.1155/2022/5680353.PMID:35096062;PMCID:PMC8791735.
Liu T, Zhang Q, Zhang J, Li C, Miao YR, Lei Q, Li Q, Guo AY. EVmiRNA: a database of miRNA profiling in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D89–93. https://doi.org/10.1093/nar/gky985.PMID:30335161;PMCID:PMC6323938.
Lai H, Li Y, Zhang H, Hu J, Liao J, Su Y, Li Q, Chen B, Li C, Wang Z, Li Y, Wang J, Meng Z, Huang Z, Huang S. exoRBase 2.0: an atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022;50(1):118–28. https://doi.org/10.1093/nar/gkab1085.
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, Gangoda L, Mathivanan S. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol. 2016;428(4):688–92. https://doi.org/10.1016/j.jmb.2015.09.019.
EV-TRACK Consortium; Van Deun J, Mestdagh P, Agostinis P, Akay Ö, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, Berx G, Boere J, Boukouris S, Bremer M, Buschmann D, Byrd JB, Casert C, Cheng L, Cmoch A, Daveloose D, De Smedt E, Demirsoy S, Depoorter V, Dhondt B, Driedonks TA, Dudek A, Elsharawy A, Floris I, Foers AD, Gärtner K, Garg AD, Geeurickx E, Gettemans J, Ghazavi F, Giebel B, Kormelink TG, Hancock G, Helsmoortel H, Hill AF, Hyenne V, Kalra H, Kim D, Kowal J, Kraemer S, Leidinger P, Leonelli C, Liang Y, Lippens L, Liu S, Lo Cicero A, Martin S, Mathivanan S, Mathiyalagan P, Matusek T, Milani G, Monguió-Tortajada M, Mus LM, Muth DC, Németh A, Nolte-’t Hoen EN, O’Driscoll L, Palmulli R, Pfaffl MW, Primdal-Bengtson B, Romano E, Rousseau Q, Sahoo S, Sampaio N, Samuel M, Scicluna B, Soen B, Steels A, Swinnen JV, Takatalo M, Thaminy S, Théry C, Tulkens J, Van Audenhove I, van der Grein S, Van Goethem A, van Herwijnen MJ, Van Niel G, Van Roy N, Van Vliet AR, Vandamme N, Vanhauwaert S, Vergauwen G, Verweij F, Wallaert A, Wauben M, Witwer KW, Zonneveld MI, De Wever O, Vandesompele J, Hendrix A. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228–32. https://doi.org/10.1038/nmeth.4185.
Saverimuttu SCC, Kramarz B, Rodríguez-López M, Garmiri P, Attrill H, Thurlow KE, Makris M, de Miranda PS, Orchard S, Lovering RC. Gene ontology curation of the blood-brain barrier to improve the analysis of Alzheimer’s and other neurological diseases. Database. 2021. https://doi.org/10.1093/database/baab067.
Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17(2):170. https://doi.org/10.3390/ijms17020170.PMID:26861301;PMCID:PMC4783904.
Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101(36):13368–73. https://doi.org/10.1073/pnas.0403453101.
Li JR, Tong CY, Sung TJ, Kang TY, Zhou XJ, Liu CC. CMEP: a database for circulating microRNA expression profiling. Bioinformatics. 2019;35(17):3127–32. https://doi.org/10.1093/bioinformatics/btz042.PMID:30668638;PMCID:PMC7963074.
Russo F, Di Bella S, Vannini F, Berti G, Scoyni F, Cook HV, Santos A, Nigita G, Bonnici V, Laganà A, Geraci F, Pulvirenti A, Giugno R, De Masi F, Belling K, Jensen LJ, Brunak S, Pellegrini M, Ferro A. miRandola 2017: a curated knowledge base of non-invasive biomarkers. Nucleic Acids Res. 2018;46(D1):D354–9. https://doi.org/10.1093/nar/gkx854.PMID:29036351;PMCID:PMC5753291.
Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD, Clayton A. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010;9(6):1324–38. https://doi.org/10.1074/mcp.M000063-MCP201.
Jeppesen DK, Nawrocki A, Jensen SG, Thorsen K, Whitehead B, Howard KA, Dyrskjøt L, Ørntoft TF, Larsen MR, Ostenfeld MS. Quantitative proteomics of fractionated membrane and lumen exosome proteins from isogenic metastatic and nonmetastatic bladder cancer cells reveal differential expression of EMT factors. Proteomics. 2014;14(6):699–712. https://doi.org/10.1002/pmic.201300452.
Andreu Z, Otta Oshiro R, Redruello A, López-Martín S, Gutiérrez-Vázquez C, Morato E, Marina AI, Olivier Gómez C, Yáñez-Mó M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci. 2017;15(98):70–9. https://doi.org/10.1016/j.ejps.2016.10.008.
Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, Fucile C, Richheimer S, Beckham CJ. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS ONE. 2016;11(1): e0147236. https://doi.org/10.1371/journal.pone.0147236.PMID:26800519;PMCID:PMC4723257.
Kumari N, Saxena S, Agrawal U. Exosomal protein interactors as emerging therapeutic targets in urothelial bladder cancer. J Egypt Natl Canc Inst. 2015;27(2):51–8. https://doi.org/10.1016/j.jnci.2015.02.002.
Xu Y, Zhang P, Tan Y, Jia Z, Chen G, Niu Y, Xiao J, Sun S, Zhang X. A potential panel of five mRNAs in urinary extracellular vesicles for the detection of bladder cancer. Transl Androl Urol. 2021;10(2):809–20. https://doi.org/10.21037/tau-20-1057.PMID:33718082;PMCID:PMC7947455.
Yang X, Ye T, Liu H, Lv P, Duan C, Wu X, Jiang K, Lu H, Xia D, Peng E, Chen Z, Tang K, Ye Z. Expression profiles, biological functions and clinical significance of circRNAs in bladder cancer. Mol Cancer. 2021;20(1):4. https://doi.org/10.1186/s12943-020-01300-8.PMID:33397425;PMCID:PMC7780637.
Zheng R, Du M, Wang X, Xu W, Liang J, Wang W, Lv Q, Qin C, Chu H, Wang M, Yuan L, Qian J, Zhang Z. Exosome-transmitted long non-coding RNA PTENP1 suppresses bladder cancer progression. Mol Cancer. 2018;17(1):143. https://doi.org/10.1186/s12943-018-0880-3.PMID:30285771;PMCID:PMC6169076.
Liu SC, Cao YH, Chen LB, Kang R, Huang ZX, Lu XS. BMSC-derived exosomal lncRNA PTENP1 suppresses the malignant phenotypes of bladder cancer by upregulating SCARA5 expression. Cancer Biol Ther. 2022;23(1):1–13. https://doi.org/10.1080/15384047.2022.2102360.PMID:35998226;PMCID:PMC9415615.
Jiang Z, Zhang Y, Zhang Y, Jia Z, Zhang Z, Yang J. Cancer derived exosomes induce macrophages immunosuppressive polarization to promote bladder cancer progression. Cell Commun Signal. 2021;19(1):93. https://doi.org/10.1186/s12964-021-00768-1.PMID:34521440;PMCID:PMC8439012.
Yin X, Zheng X, Liu M, Wang D, Sun H, Qiu Y, Chen J, Shi B. Exosomal miR-663b targets Ets2-repressor factor to promote proliferation and the epithelial-mesenchymal transition of bladder cancer cells. Cell Biol Int. 2020;44(4):958–65. https://doi.org/10.1002/cbin.11292.
Cai X, Qu L, Yang J, Xu J, Sun L, Wei X, Qu X, Bai T, Guo Z, Zhu Y. Exosome-transmitted microRNA-133b inhibited bladder cancer proliferation by upregulating dual-specificity protein phosphatase 1. Cancer Med. 2020;9(16):6009–19. https://doi.org/10.1002/cam4.3263.
Huang CS, Ho JY, Chiang JH, Yu CP, Yu DS. Exosome-Derived LINC00960 and LINC02470 promote the epithelial-mesenchymal transition and aggressiveness of bladder cancer cells. Cells. 2020;9(6):1419. https://doi.org/10.3390/cells9061419.PMID:32517366;PMCID:PMC7349410.
Li Q, Huyan T, Cai S, Huang Q, Zhang M, Peng H, Zhang Y, Liu N, Zhang W. The role of exosomal miR-375-3p: a potential suppressor in bladder cancer via the Wnt/β-catenin pathway. FASEB J. 2020;34(9):12177–96. https://doi.org/10.1096/fj.202000347R.
Yang H, Qu H, Huang H, Mu Z, Mao M, Xie Q, Wang K, Hu B. Exosomes-mediated transfer of long noncoding RNA LINC01133 represses bladder cancer progression via regulating the Wnt signaling pathway. Cell Biol Int. 2021;45(7):1510–22. https://doi.org/10.1002/cbin.11590.
Lin H, Shi X, Li H, Hui J, Liu R, Chen Z, Lu Y, Tan W. Urinary Exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93-5p in bladder Cancer. BMC Cancer. 2021;21(1):1293. https://doi.org/10.1186/s12885-021-08926-x.PMID:34861847;PMCID:PMC8641206.
Tomiyama E, Fujita K, Matsuzaki K, Narumi R, Yamamoto A, Uemura T, Yamamichi G, Koh Y, Matsushita M, Hayashi Y, Hashimoto M, Banno E, Kato T, Hatano K, Kawashima A, Uemura M, Ukekawa R, Takao T, Takada S, Uemura H, Adachi J, Tomonaga T, Nonomura N. EphA2 on urinary extracellular vesicles as a novel biomarker for bladder cancer diagnosis and its effect on the invasiveness of bladder cancer. Br J Cancer. 2022;127(7):1312–23. https://doi.org/10.1038/s41416-022-01860-0.
Shen Y, Ye H, Zhang D, Yang M, Ji Y, Tang L, Zhu X, Yuan L. The role of exosomal CDC6 in the hirudin-mediated suppression of the malignant phenotype of bladder cancer cells. Gene. 2022;821:146269. https://doi.org/10.1016/j.gene.2022.146269.
Rabbani F, Cordon-Cardo C. Mutation of cell cycle regulators and their impact on superficial bladder cancer. Urol Clin North Am. 2000;27(1):83–102. https://doi.org/10.1016/s0094-0143(05)70237-8.
Li X, Tian Z, Jin H, Xu J, Hua X, Yan H, Liufu H, Wang J, Li J, Zhu J, Huang H, Huang C. Decreased c-Myc mRNA stability via the MicroRNA 141–3p/AUF1 Axis Is Crucial for p63α inhibition of Cyclin D1 Gene transcription and bladder cancer cell tumorigenicity. Mol Cell Biol. 2018;38(21):e00273-e318. https://doi.org/10.1128/MCB.00273-18.PMID:30104251;PMCID:PMC6189456.
Choi NR, Choi WG, Kwon MJ, Woo JH, Kim BJ. [6]-Gingerol induces caspase-dependent apoptosis in bladder cancer cells via MAPK and ROS Signaling. Int J Med Sci. 2022;19(7):1093–102. https://doi.org/10.7150/ijms.73077.PMID:35919815;PMCID:PMC9339411.
Yang L, Wu XH, Wang D, Luo CL, Chen LX. Bladder cancer cell-derived exosomes inhibit tumor cell apoptosis and induce cell proliferation in vitro. Mol Med Rep. 2013;8(4):1272–8. https://doi.org/10.3892/mmr.2013.1634.
Wu CH, Silvers CR, Messing EM, Lee YF. Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells. J Biol Chem. 2019;294(9):3207–18. https://doi.org/10.1074/jbc.RA118.006682.
Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353: 104119. https://doi.org/10.1016/j.cellimm.2020.104119.
Chou YS, Yang MH. Epithelial-mesenchymal transition-related factors in solid tumor and hematological malignancy. J Chin Med Assoc. 2015;78(8):438–45. https://doi.org/10.1016/j.jcma.2015.05.002.
Beckham CJ, Olsen J, Yin PN, Wu CH, Ting HJ, Hagen FK, Scosyrev E, Messing EM, Lee YF. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192(2):583–92. https://doi.org/10.1016/j.juro.2014.02.035.
Franzen CA, Blackwell RH, Todorovic V, Greco KA, Foreman KE, Flanigan RC, Kuo PC, Gupta GN. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. 2015;4(8): e163. https://doi.org/10.1038/oncsis.2015.21.PMID:26280654;PMCID:PMC4632072.
Chen C, Zheng H, Luo Y, Kong Y, An M, Li Y, He W, Gao B, Zhao Y, Huang H, Huang J, Lin T. SUMOylation promotes extracellular vesicle-mediated transmission of lncRNA ELNAT1 and lymph node metastasis in bladder cancer. J Clin Invest. 2021;131(8): e146431. https://doi.org/10.1172/JCI146431.PMID:33661764;PMCID:PMC8262506.
Chen C, Luo Y, He W, Zhao Y, Kong Y, Liu H, Zhong G, Li Y, Li J, Huang J, Chen R, Lin T. Exosomal long noncoding RNA LNMAT2 promotes lymphatic metastasis in bladder cancer. J Clin Invest. 2020;130(1):404–21. https://doi.org/10.1172/JCI130892.PMID:31593555;PMCID:PMC6934220.
Song Q, Yu H, Cheng Y, Han J, Li K, Zhuang J, Lv Q, Yang X, Yang H. Bladder cancer-derived exosomal KRT6B promotes invasion and metastasis by inducing EMT and regulating the immune microenvironment. J Transl Med. 2022;20(1):308. https://doi.org/10.1186/s12967-022-03508-2.PMID:35794606;PMCID:PMC9258227.
Milotti E, Fredrich T, Chignola R, Rieger H. Oxygen in the tumor microenvironment: mathematical and numerical modeling. Adv Exp Med Biol. 2020;1259:53–76. https://doi.org/10.1007/978-3-030-43093-1_4.
Burger MG, Grosso A, Briquez PS, Born GME, Lunger A, Schrenk F, Todorov A, Sacchi V, Hubbell JA, Schaefer DJ, Banfi A, Di Maggio N. Robust coupling of angiogenesis and osteogenesis by VEGF-decorated matrices for bone regeneration. Acta Biomater. 2022;1(149):111–25. https://doi.org/10.1016/j.actbio.2022.07.014.
Li X, Peng X, Zhang C, Bai X, Li Y, Chen G, Guo H, He W, Zhou X, Gou X. Bladder cancer-derived small extracellular vesicles promote tumor angiogenesis by inducing HBP-related metabolic reprogramming and SerRS O-GlcNAcylation in endothelial cells. Adv Sci. 2022;9(30):e2202993. https://doi.org/10.1002/advs.202202993.
Li X, Wei Z, Yu H, Xu Y, He W, Zhou X, Gou X. Secretory autophagy-induced bladder tumour-derived extracellular vesicle secretion promotes angiogenesis by activating the TPX2-mediated phosphorylation of the AURKA-PI3K-AKT axis. Cancer Lett. 2021;28(523):10–28. https://doi.org/10.1016/j.canlet.2021.09.036.
Rashid K, Ahmad A, Meerasa SS, Khan AQ, Wu X, Liang L, Cui Y, Liu T. Cancer stem cell-derived exosome-induced metastatic cancer: an orchestra within the tumor microenvironment. Biochimie. 2023. https://doi.org/10.1016/j.biochi.2023.03.014.
Luo G, Zhang Y, Wu Z, Zhang L, Liang C, Chen X. Exosomal LINC00355 derived from cancer-associated fibroblasts promotes bladder cancer cell resistance to cisplatin by regulating miR-34b-5p/ABCB1 axis. Acta Biochim Biophys Sin. 2021;53(5):558–66. https://doi.org/10.1093/abbs/gmab023.
Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, Das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48. https://doi.org/10.1001/jamaoncol.2016.5688.
Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, Rouprêt M. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–53. https://doi.org/10.1016/j.eururo.2013.06.003.
Zhu CZ, Ting HN, Ng KH, Ong TA. A review on the accuracy of bladder cancer detection methods. J Cancer. 2019;10(17):4038–44. https://doi.org/10.7150/jca.28989.PMID:31417648;PMCID:PMC6692607.
Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S. Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics. 2015;15(2–3):260–71. https://doi.org/10.1002/pmic.201400234.
Katsuda T, Kosaka N, Ochiya T. The roles of extracellular vesicles in cancer biology: toward the development of novel cancer biomarkers. Proteomics. 2014;14(4–5):412–25. https://doi.org/10.1002/pmic.201300389.
Elsharkawi F, Elsabah M, Shabayek M, Khaled H. Urine and serum exosomes as novel biomarkers in detection of bladder cancer. Asian Pac J Cancer Prev. 2019;20(7):2219–24. https://doi.org/10.31557/APJCP.2019.20.7.2219.PMID:31350988;PMCID:PMC6745236.
Li C, Hou X, Zhang P, Li J, Liu X, Wang Y, Guan Q, Zhou Y. Exosome-based tumor therapy: opportunities and challenges. Curr Drug Metab. 2020;21(5):339–51. https://doi.org/10.2174/1389200221666200515103354.
Liu T, Li T, Zheng Y, Xu X, Sun R, Zhan S, Guo X, Zhao Z, Zhu W, Feng B, Wei F, Jiang N, Wang J, Chen X, Fang F, Guo H, Yang R. Evaluating adipose-derived stem cell exosomes as miRNA drug delivery systems for the treatment of bladder cancer. Cancer Med. 2022;11(19):3687–99. https://doi.org/10.1002/cam4.4745.
Jia Y, Ding X, Zhou L, Zhang L, Yang X. Mesenchymal stem cells-derived exosomal microRNA-139–5p restrains tumorigenesis in bladder cancer by targeting PRC1. Oncogene. 2021;40(2):246–61. https://doi.org/10.1038/s41388-020-01486-7.
Lee J, Park HS, Han SR, Kang YH, Mun JY, Shin DW, Oh HW, Cho YK, Lee MS, Park J. Alpha-2-macroglobulin as a novel diagnostic biomarker for human bladder cancer in urinary extracellular vesicles. Front Oncol. 2022;13(12): 976407. https://doi.org/10.3389/fonc.2022.976407.PMID:36176383;PMCID:PMC9513419.
Qiu T, Xue M, Li X, Li F, Liu S, Yao C, Chen W. Comparative evaluation of long non-coding RNA-based biomarkers in the urinary sediment and urinary exosomes for non-invasive diagnosis of bladder cancer. Mol Omics. 2022;18(10):938–47. https://doi.org/10.1039/d2mo00107a.
Lee J, McKinney KQ, Pavlopoulos AJ, Niu M, Kang JW, Oh JW, Kim KP, Hwang S. Altered proteome of extracellular vesicles derived from bladder cancer patients urine. Mol Cells. 2018;41(3):179–87. https://doi.org/10.14348/molcells.2018.2110.
Yazarlou F, Mowla SJ, Oskooei VK, Motevaseli E, Tooli LF, Afsharpad M, Nekoohesh L, Sanikhani NS, Ghafouri-Fard S, Modarressi MH. Urine exosome gene expression of cancer-testis antigens for prediction of bladder carcinoma. Cancer Manag Res. 2018;5(10):5373–81. https://doi.org/10.2147/CMAR.S180389.PMID:30464633;PMCID:PMC6225912.
Chen C, Shang A, Sun Z, Gao Y, Huang J, Ping Y, Chang W, Gu C, Sun J, Ji P, Yuan Y, Lu R, Li D. Urinary exosomal long noncoding RNA TERC as a noninvasive diagnostic and prognostic biomarker for bladder urothelial carcinoma. J Immunol Res. 2022;25(2022):9038808. https://doi.org/10.1155/2022/9038808.PMID:35127956;PMCID:PMC8811540.
Baumgart S, Meschkat P, Edelmann P, Heinzelmann J, Pryalukhin A, Bohle R, Heinzelbecker J, Stöckle M, Junker K. MicroRNAs in tumor samples and urinary extracellular vesicles as a putative diagnostic tool for muscle-invasive bladder cancer. J Cancer Res Clin Oncol. 2019;145(11):2725–36. https://doi.org/10.1007/s00432-019-03035-6.
Huang H, Du J, Jin B, Pang L, Duan N, Huang C, Hou J, Yu W, Hao H, Li H. Combination of urine exosomal mRNAs and lncRNAs as novel diagnostic biomarkers for bladder cancer. Front Oncol. 2021;27(11): 667212. https://doi.org/10.3389/fonc.2021.667212.PMID:33987102;PMCID:PMC8111292.
Sarfi M, Abbastabar M, Khalili E. Increased expression of urinary exosomal LnCRNA TUG-1 in early bladder cancer. Gene Rep. 2020;165(12):2345–51. https://doi.org/10.1016/j.genrep.2020.101010.
Zhang S, Du L, Wang L, Jiang X, Zhan Y, Li J, Yan K, Duan W, Zhao Y, Wang L, Wang Y, Shi Y, Wang C. Evaluation of serum exosomal LncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J Cell Mol Med. 2019;23(2):1396–405. https://doi.org/10.1111/jcmm.14042.
Sabo AA, Birolo G, Naccarati A, Dragomir MP, Aneli S, Allione A, Oderda M, Allasia M, Gontero P, Sacerdote C, Vineis P, Matullo G, Pardini B. Small non-coding RNA profiling in plasma extracellular vesicles of bladder cancer patients by next-generation sequencing: expression levels of miR-126-3p and piR-5936 increase with higher histologic grades. Cancers. 2020;12(6):1507. https://doi.org/10.3390/cancers12061507.PMID:32527011;PMCID:PMC7352804.
Lee DH, Yoon H, Park S, Kim JS, Ahn YH, Kwon K, Lee D, Kim KH. Urinary Exosomal and cell-free DNA detects somatic mutation and copy number alteration in urothelial carcinoma of bladder. Sci Rep. 2018;8(1):14707. https://doi.org/10.1038/s41598-018-32900-6.PMID:30279572;PMCID:PMC6168539.
Zhou X, Kurywchak P, Wolf-Dennen K, Che SPY, Sulakhe D, D’Souza M, Xie B, Maltsev N, Gilliam TC, Wu CC, McAndrews KM, LeBleu VS, McConkey DJ, Volpert OV, Pretzsch SM, Czerniak BA, Dinney CP, Kalluri R. Unique somatic variants in DNA from urine exosomes of individuals with bladder cancer. Mol Ther Methods Clin Dev. 2021;29(22):360–76. https://doi.org/10.1016/j.omtm.2021.05.010.PMID:34514028;PMCID:PMC8408559.
Park J, Kamerer RL, Marjanovic M, Sorrells JE, You S, Barkalifa R, Selting KA, Boppart SA. Label-free optical redox ratio from urinary extracellular vesicles as a screening biomarker for bladder cancer. Am J Cancer Res. 2022;12(5):2068–83.
Igami K, Uchiumi T, Shiota M, Ueda S, Tsukahara S, Akimoto M, Eto M, Kang D. Extracellular vesicles expressing CEACAM proteins in the urine of bladder cancer patients. Cancer Sci. 2022;113(9):3120–33. https://doi.org/10.1111/cas.15438.
El-Shal AS, Shalaby SM, Abouhashem SE, Elbary EHA, Azazy S, Rashad NM, Sarhan W. Urinary exosomal microRNA-96-5p and microRNA-183-5p expression as potential biomarkers of bladder cancer. Mol Biol Rep. 2021;48(5):4361–71. https://doi.org/10.1007/s11033-021-06451-5.
Yazarlou F, Modarressi MH, Mowla SJ, Oskooei VK, Motevaseli E, Tooli LF, Nekoohesh L, Eghbali M, Ghafouri-Fard S, Afsharpad M. Urinary exosomal expression of long non-coding RNAs as diagnostic marker in bladder cancer. Cancer Manag Res. 2018;26(10):6357–65. https://doi.org/10.2147/CMAR.S186108.PMID:30568497;PMCID:PMC6267766.
Wen J, Yang T, Mallouk N, Zhang Y, Li H, Lambert C, Li G. Urinary exosomal CA9 mRNA as a novel liquid biopsy for molecular diagnosis of bladder cancer. Int J Nanomedicine. 2021;14(16):4805–11. https://doi.org/10.2147/IJN.S312322.PMID:34285483;PMCID:PMC8286733.
Abbastabar M, Sarfi M, Golestani A, Karimi A, Pourmand G, Khalili E. Tumor-derived urinary exosomal long non-coding RNAs as diagnostic biomarkers for bladder cancer. EXCLI J. 2020;4(19):301–10. https://doi.org/10.17179/excli2019-1683.PMID:32231490;PMCID:PMC7104196.
Silvers CR, Liu YR, Wu CH, Miyamoto H, Messing EM, Lee YF. Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer. Oncotarget. 2016;7(17):23335–45. https://doi.org/10.18632/oncotarget.8024.PMID:26981774;PMCID:PMC5029630.
Wang J, Yang K, Yuan W, Gao Z. Determination of Serum Exosomal H19 as a noninvasive biomarker for bladder cancer diagnosis and prognosis. Med Sci Monit. 2018;21(24):9307–16. https://doi.org/10.12659/MSM.912018.PMID:30576305;PMCID:PMC6320644.
Silvers CR, Miyamoto H, Messing EM, Netto GJ, Lee YF. Characterization of urinary extracellular vesicle proteins in muscle-invasive bladder cancer. Oncotarget. 2017;8(53):91199–208. https://doi.org/10.18632/oncotarget.20043.PMID:29207636;PMCID:PMC5710916.
Zhan Y, Du L, Wang L, Jiang X, Zhang S, Li J, Yan K, Duan W, Zhao Y, Wang L, Wang Y, Wang C. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol Cancer. 2018;17(1):142. https://doi.org/10.1186/s12943-018-0893-y.PMID:30268126;PMCID:PMC6162963.
Strømme O, Heck KA, Brede G, Lindholm HT, Otterlei M, Arum CJ. Differentially expressed extracellular vesicle-contained microRNAs before and after transurethral resection of bladder tumors. Curr Issues Mol Biol. 2021;43(1):286–300. https://doi.org/10.3390/cimb43010024.PMID:34199766;PMCID:PMC8929081.
Wu X, Showiheen SAA, Sun AR, Crawford R, Xiao Y, Mao X, Prasadam I. Exosomes extraction and identification. Methods Mol Biol. 2019;2054:81–91. https://doi.org/10.1007/978-1-4939-9769-5_4.
Wei D, Zhan W, Gao Y, Huang L, Gong R, Wang W, Zhang R, Wu Y, Gao S, Kang T. RAB31 marks and controls an ESCRT-independent exosome pathway. Cell Res. 2021;31(2):157–77. https://doi.org/10.1038/s41422-020-00409-1.
Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Rel. 2022;347:533–43. https://doi.org/10.1016/j.jconrel.2022.05.027.
Ashrafizadeh M, Zarrabi A, Samarghandian S, Najafi M. PTEN: What we know of the function and regulation of this onco-suppressor factor in bladder cancer? Eur J Pharmacol. 2020;15(881): 173226. https://doi.org/10.1016/j.ejphar.2020.173226.
Xu T, Rao T, Yu WM, Ning JZ, Yu X, Zhu SM, Yang K, Bai T, Cheng F. Upregulation of NFKBIZ affects bladder cancer progression via the PTEN/PI3K/Akt signaling pathway. Int J Mol Med. 2021;47(6):109. https://doi.org/10.3892/ijmm.2021.4942.
Zhong XL, Wang L, Yan X, Yang XK, Xiu H, Zhao M, Wang XN, Liu JX. MiR-20a acted as a ceRNA of lncRNA PTENPL and promoted bladder cancer cell proliferation and migration by regulating PDCD4. Eur Rev Med Pharmacol Sci. 2020;24(6):2955–64. https://doi.org/10.26355/eurrev_202003_20660.
Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M, Akbari DN. A review on the role of PTENP1 in human disorders with an especial focus on tumor suppressor role of this lncRNA. Cancer Cell Int. 2022;22(1):207. https://doi.org/10.1186/s12935-022-02625-8.PMID:35655204;PMCID:PMC9161594.
Kovalenko TF, Morozova KV, Pavlyukov MS, Anufrieva KS, Bobrov MY, Gamisoniya AM, Ozolinya LA, Dobrokhotova YE, Shakhparonov MI, Patrushev LI. Methylation of the PTENP1 pseudogene as potential epigenetic marker of age-related changes in human endometrium. PLoS ONE. 2021;16(1): e0243093. https://doi.org/10.1371/journal.pone.0243093.PMID:33481830;PMCID:PMC7822536.
Qian YY, Li K, Liu QY, Liu ZS. Long non-coding RNA PTENP1 interacts with miR-193a-3p to suppress cell migration and invasion through the PTEN pathway in hepatocellular carcinoma. Oncotarget. 2017;8(64):107859–69. https://doi.org/10.18632/oncotarget.22305.PMID:29296207;PMCID:PMC5746109.
Xiong T, Li J, Chen F, Zhang F. PCAT-1: a novel oncogenic long non-coding RNA in human cancers. Int J Biol Sci. 2019;15(4):847–56. https://doi.org/10.7150/ijbs.30970.PMID:30906215;PMCID:PMC6429018.
Goyal B, Yadav SRM, Awasthee N, Gupta S, Kunnumakkara AB, Gupta SC. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer. 2021;1875(2): 188502. https://doi.org/10.1016/j.bbcan.2021.188502.
Rao X, Cao H, Yu Q, Ou X, Deng R, Huang J. NEAT1/MALAT1/XIST/PKD–Hsa-Mir-101-3p–DLGAP5 axis as a novel diagnostic and prognostic biomarker associated with immune cell infiltration in bladder cancer. Front Genet. 2022;8(13): 892535. https://doi.org/10.3389/fgene.2022.892535.PMID:35873473;PMCID:PMC9305813.
Zhou LJ, Yang DW, Ou LN, Guo XR, Wu BL. Circulating expression level of LncRNA Malat1 in diabetic kidney disease patients and its clinical significance. J Diabetes Res. 2020;1(2020):4729019. https://doi.org/10.1155/2020/4729019.PMID:32832561;PMCID:PMC7421584.
Gong X, Zhu Y, Chang H, Li Y, Ma F. Long noncoding RNA MALAT1 promotes cardiomyocyte apoptosis after myocardial infarction via targeting miR-144-3p. 2019. Biosci Rep. https://doi.org/10.1042/BSR20191103.
Baumgart S, Hölters S, Ohlmann CH, Bohle R, Stöckle M, Ostenfeld MS, Dyrskjøt L, Junker K, Heinzelmann J. Exosomes of invasive urothelial carcinoma cells are characterized by a specific miRNA expression signature. Oncotarget. 2017;8(35):58278–91. https://doi.org/10.18632/oncotarget.17619.PMID:28938555;PMCID:PMC5601651.
Hiltbrunner S, Mints M, Eldh M, Rosenblatt R, Holmström B, Alamdari F, Johansson M, Veerman RE, Winqvist O, Sherif A, Gabrielsson S. Urinary exosomes from bladder cancer patients show a residual cancer phenotype despite complete pathological downstaging. Sci Rep. 2020;10(1):5960. https://doi.org/10.1038/s41598-020-62753-x.PMID:32249794;PMCID:PMC7136268.
Eldh M, Mints M, Hiltbrunner S, Ladjevardi S, Alamdari F, Johansson M, Jakubczyk T, Veerman RE, Winqvist O, Sherif A, Gabrielsson S. Proteomic profiling of tissue exosomes indicates continuous release of malignant exosomes in urinary bladder cancer patients, even with pathologically undetectable tumour. Cancers. 2021;13(13):3242. https://doi.org/10.3390/cancers13133242.PMID:34209558;PMCID:PMC8267924.
Jingushi K, Kawashima A, Saito T, Kanazawa T, Motooka D, Kimura T, Mita M, Yamamoto A, Uemura T, Yamamichi G, Okada K, Tomiyama E, Koh Y, Matsushita M, Kato T, Hatano K, Uemura M, Tsujikawa K, Wada H, Nonomura N. Circulating extracellular vesicles carrying Firmicutes reflective of the local immune status may predict clinical response to pembrolizumab in urothelial carcinoma patients. Cancer Immunol Immunother. 2022;71(12):2999–3011. https://doi.org/10.1007/s00262-022-03213-5.
Luo H, Xu C, Ge B, Wang T. CASC1 expression in bladder cancer is regulated by exosomal miRNA-150: a comprehensive pan-cancer and bioinformatics study. Comput Math Methods Med. 2022;5(2022):8100325. https://doi.org/10.1155/2022/8100325.PMID:35836922;PMCID:PMC9276518.
Acknowledgements
Authors would like to thank National Natural Science Foundation of China for the financial support.
Funding
This work was supported by National Natural Science Foundation of China (81974092).
Author information
Authors and Affiliations
Contributions
All authors participated in the conception and design of the study. YY and SW conceived and drafted the manuscript. LM searched the involved studies. YL and YS participated in the data process, analysis and interpretation. Shaogang Wang and Yi Sun supervised the project and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Literature search flowchart.
Additional file 2: Table S1
. Overview of exosomes and their contents identified in bladder cancer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Miao, L., Lu, Y. et al. Exosome, the glass slipper for Cinderella of cancer—bladder cancer?. J Nanobiotechnol 21, 368 (2023). https://doi.org/10.1186/s12951-023-02130-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02130-8