Abstract
Background
Postoperative delirium (POD) is more prevalent among elderly patients with type 2 diabetes mellitus (T2DM). Insulin resistance (IR) can be assessed using the triglyceride-glucose (TyG) index, a novel biomarker. This study aims to investigate the predictive potential of the TyG index for POD in elderly patients with T2DM.
Materials and methods
Elderly patients (≥ 65) with T2DM who underwent non-neurosurgery and non-cardiac surgery were enrolled. Univariate and multivariate logistic regression analyses were conducted to assess the association between the TyG index and POD. Additionally, subgroup analyses were performed to compare the sex-specific differences in the predictive ability of the TyG index for POD.
Results
A total of 4566 patients were included in this retrospective cohort. The receiver operating characteristic (ROC) curve analysis determined the optimal cut-off value for the TyG index to be 8.678. In the univariate model, a TyG index > 8.678 exhibited an odds ratio (OR) of 1.668 (95% CI: 1.210–2.324, P = 0.002) for predicting POD. In the multivariate regression models, the ORs were 1.590 (95% CI: 1.133–2.252, P < 0.008), 1.661 (95% CI: 1.199–2.325, P < 0.003), and 1.603 (95% CI: 1.137–2.283, P = 0.008) for different models. Subgroup analyses demonstrated that the predictive ability of the TyG index was more pronounced in females compared to males.
Conclusion
The TyG index shows promise as a novel biomarker for predicting the occurrence of POD in elderly surgical patients with T2DM.
Similar content being viewed by others
Introduction
Diabetes has emerged as a significant metabolic disease that poses a substantial threat to human life and health. As of 2021, the global prevalence of diabetes reached 536.6 million individuals, with diabetes-related healthcare expenditure exceeding $1 trillion [1]. It is estimated that approximately half of all diabetes patients will require surgery during their lifetime [2]. However, the long-term complications associated with diabetes, including inflammation, oxidative stress, vasculopathy, and renal insufficiency, significantly elevate the risk of postoperative complications, particularly in elderly patients [2, 3]. These complications have a considerable impact on patient prognosis and quality of life. Therefore, it is imperative to focus on prevention and treatment strategies for postoperative complications in elderly diabetic patients.
Postoperative delirium (POD), which is characterized by acute disturbances in attention and awareness, is a prevalent neurological complication among elderly surgical patients [4, 5]. POD has been linked to prolonged hospital stays, increased morbidity and mortality rates, and diminished quality of life [4, 5]. Prior studies have demonstrated a higher incidence of POD in elderly patients with diabetes compared with non-diabetics, which significantly affects their prognosis [6, 7]. However, limited research has investigated the risk factors associated with POD in this specific population. Identifying independent indicators for POD in elderly diabetic surgical patients is therefore crucial, as it could serve as a basis for developing novel perioperative interventions.
Type 2 diabetes mellitus (T2DM) accounts for 90–95% of all diabetes cases [4]. Insulin resistance (IR) underlies the pathogenesis of T2DM, reflecting reduced sensitivity of the body and tissues to insulin. Chronic IR can lead to central nervous system (CNS) dysfunction due to insulin’s critical role in neurosynaptic functioning, synaptic plasticity modulation, glucose uptake, and neuronal survival [8]. Previous research has established a connection between IR and Alzheimer’s disease and other neurodegenerative disorders [8]. However, the correlation between IR and POD in elderly patients with T2DM has not been extensively explored.
The “gold standard” for identifying IR is the hyperinsulinemic euglycemic clamp, but its application during the perioperative period is not practical [9]. The triglyceride-glucose (TyG) index, calculated by fasting glucose and triglyceride, has emerged as a promising surrogate marker of IR due to its strong correlation with the hyperinsulinemic euglycemic clamp [10]. Previous studies have shown that the TyG index can independently predict the incidence and prognosis of cardiovascular and cerebrovascular diseases [10,11,12]. However, the relationship between the TyG index and POD in elderly patients with T2DM has yet to be explored.
This study hypothesizes a correlation between TYG index and the incidence of POD in elderly patients with T2DM who undergo non-neurosurgery and non-cardiac surgery. The findings of this study indicated that elderly diabetic patients with higher TyG index were at greater risk of developing POD, and TyG index could be a novel predictor and intervention target for the prevention of POD in clinical practice.
Materials and methods
Study design and participants
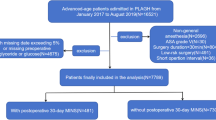
The study protocol underwent review and approval by the institutional ethics committee of Chinese PLA General Hospital (No. S2019-311–03), and the informed consent was agreed to be waived by the institutional ethics committee of Chinese PLA General Hospital because the research did not involve any clinical treatment or individual privacy information. This retrospective cohort study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. Data were collected from the first medical center of the Chinese PLA General Hospital’s perioperative retrospective database, covering the period from January 2014 to April 2019. The inclusion criteria were as follows: (1) age ≥ 65 years; (2) patients with T2DM; (3) patients who underwent non-cardiac and non-neurosurgery procedures with anesthesia. The exclusion criteria were as follows: (1) patients who only underwent digestive endoscopy; (2) missing data on fasting blood glucose and fasting triglycerides before surgery; (3) missing data for over 50% of all variables.
Outcome
The primary outcome of interest was the incidence of POD within 7 days following surgery. Cases of POD were recorded in the database of Anesthesiology department, as previously described [13]. POD was diagnosed by a neurologist based on descriptive words (mental status change, confusion, disorientation, agitation, delirium, inappropriate behaviour, inattention, hallucinations, and combative behaviour) and the postoperative drug regime (quetiapine, olanzapine, haloperidol, haloperidol, and risperidone) in the medical records according to the Diagnostic and Statistical Manual of Mental Disorders-IV criteria. The patients with presence of the above-mentioned symptoms and drugs in preoperative medical records were excluded.
Data collection and definition of variables
Relevant data were extracted from the existing database. Potential confounding factors that could influence the incidence of POD were categorized as preoperative variables and intraoperative variables. The preoperative variables of interest included patient demographics: age, sex, body mass index (BMI), smoking, alcohol use, hypertension, cardiovascular diseases, chronic kidney diseases (CKD), chronic obstructive pulmonary diseases (COPD), cerebrovascular diseases, depression and anxiety), and the latest laboratory test results before surgery (fasting blood glucose, triglycerides, glycated serum protein (GSP), hemoglobin (Hb), white blood cell (WBC) count, platelets, albumin, serum creatinine (Cre), total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, and prothrombin time (PT). The intraoperative variables included the type of surgery, type of anesthesia, duration of anesthesia, urine output, output, blood loss, administration of crystalloid and colloid solutions, dosage of dexmedetomidine, and duration of mean arterial pressure (MAP) < 60 mmHg. The TyG index was calculated by the formula:
The completeness of the data is shown in Supplementary Tables 1, and all variables had a missing percentage of less than 20%. The missing continuous variables were imputed using the median imputation method, while the missing discrete variables were imputed using the mode imputation method.
Statistical analysis
Data analysis was performed using Statistical Package for the Social Science (SPSS) version 26.0 (IBM, America) and R statistical software (R version 4.0.5, R Foundation for Statistical Computing, Australia).
Continuous variables with a normal distribution were presented as mean [standard deviation (SD)], while continuous variables with a skewed distribution were reported as median [interquartile range (IQR)]. Categorical data were summarized as counts and percentages. Student’s t-test was used to compare normally distributed continuous variables, while the Mann-Whitney U test was employed for intergroup comparisons of non-normally distributed continuous variables. The chi-squared test or Fisher’s exact test was used for inter-group comparisons of categorical variables. Moreover, the number of outcome events per variable (EPV) was taken into account, with a general minimum requirement of ten events [14]. According to the EPV method for determining sample size, our sample size was deemed sufficient for obtaining reliable estimates. In this study, the TyG index as a binary variable was used for the main focus of the research. Therefore, PASS version 15.0 was used to calculate the required sample size for a group allocation of 1:1 with α = 0.05, β = 0.20. And the POD incidences were 4.4% and 2.7% in higher TyG index group and lower TyG index group, respectively. Based on these parameters, the determined sample size of 1859 patients per group was identified.
Receiver operating characteristic (ROC) curve analysis was conducted to determine the optimal cut-off value of the TyG index for predicting POD. To explore the correlation between the TyG index and POD, univariate and multivariate logistic regression analyses were performed. The TyG index was examined as a continuous variable, binary variable based on ROC analysis, and multiple categories based on the interquartile range. In multivariate logistic regression analysis, variables with a P-value < 0.05 in univariate logistic regression were included. The inclusion of these significant covariates adheres to the principles of multicollinearity in the models. Collinearity was determined using variance inflation factor, and variables were accordingly removed from the final model (Variance Inflation Factor > 5). Model 1 adjusted for preoperative factors, including age, Hb, RBC count, WBC count, HDL, CKD, depression and anxiety. Model 2 adjusted for intraoperative factors, including emergency surgery, type of surgery, duration of anesthesia, urine output, blood loss, administration of crystalloid and colloid solutions, and duration of MAP < 60 mmHg. Model 3 adjusted for variables in Model 1 and Model 2. A two-sided P-value < 0.05 was considered statistically significant for all tests, except for the P-value < 0.10 in interaction analyse.
Restricted cubic splines (RCS) with five knots (5th, 27.5th, 50th, 72.5th, and 95th centiles) were performed to present the relationship between TyG index and the incidence of POD. The subgroup analysis of multivariate logistic regression in Model 3 was performed for female and male participants, considering potential sex differences in IR. To further explore the effect of gender on the TyG index to predict POD, the interaction analyse was also conducted.
The sensitivity analysis was conducted by E-value method on the website https://www.evalue-calculator.com/evalue/ with the data in Model 3 [15]. The E-value is used to quantify the degree to which an observed association between an exposure and an outcome could be explained by potential uncontrolled and unmeasured confounding.
Specifically, the E-value reflects the minimum strength of association that an unmeasured confounder would need to have with both the exposure and outcome, above and beyond the measured confounders, to fully explain away a specific estimated effect [16]. And reported ORs of identified risk factors for POD were compared with the E-value in present study [17,18,19,20]. Additional sensitivity analysis has also been conducted using the original, non-imputed data to detect if the results of TyG index as a binary variable are affected by imputing missing values.
Results
Participants characteristics of cohorts by TyG index
A total of 4566 medical records of elderly patients were analyzed in this study (Fig. 1). The optimal cut-off value of the TyG index to predict POD was determined to be 8.678, with an area under the ROC curve (AUC) of 0.56 (Fig. 2). Table 1 presents the characteristics of the patients divided into two groups based on the critical value of the TyG index. The study included 2423 (53.1%) male participants and 2143 (46.93%) female participants. The median age of the participants was 70.0 years (interquartile range: 67.0–74.0), and the median TyG index was 8.74 (interquartile range: 8.34–9.17). The overall incidence of POD in the study participants was 3.6%. The group with TyG > 8.678 exhibited a higher incidence of POD, a higher proportion of female participants, lower prevalence of smoking and alcohol history, elevated levels of GSP and ALT, higher BMI, Hb, total cholesterol, low-density LDL, glucose, and triglyceride levels, lower levels of HDL, serum Cre, PT, and total bilirubin, and a younger age compared to the group with TyG < 8.678 (all P < 0.05). The clinical characteristics of participants by quartile of TyG index were presented in Supplementary Table 2.
ROC curve of TyG index for predicting POD in surgical elderly patients with T2DM. The optimal cut-off point was 8.678 with specificity and sensitivity of 44.6% and 65.7% (area under the curve 0.5637, 95% CI: 0.5199 to 0.6075). ROC, receiver operating characteristic; TyG: triglyceride-glucose; POD, postoperative delirium; T2DM, type 2 diabetes mellitus; CI, confidence interval
Association between the TyG index and POD incidence
The ROC curve analysis demonstrated the ability of the TyG index to predict the risk of POD (Fig. 2). And the RCS plots showed a “S-shape” association between TyG index and POD (Fig. 3). The P for non-linear relationship is 0.74, indicating the linear relationship between TyG index and POD incidence. The relationship between POD and the TyG index, grouped by the cut-off value, is presented in Table 2. In the univariate analysis (Supplementary Table 3), the odds ratio (OR) of the group with TyG > 8.678 was 1.668 (95% CI: 1.210–2.324, P = 0.002). In the multivariate logistic regression models (Supplementary Table 4), the adjusted ORs of the TyG > 8.678 group in model 1, model 2, and model 3 were 1.590 (95% CI: 1.133–2.252, P = 0.008), 1.661 (95% CI: 1.199–2.325, P = 0.003), and 1.603 (95% CI: 1.137–2.283, P = 0.008), respectively. These results demonstrate that a TyG > 8.678 is an independent risk factor for POD in elderly patients with T2DM.
The TyG index was also analyzed as a continuous variable. In the univariate analysis (Supplementary Table 3), the OR of the TyG index was 1.379 (95% CI: 1.084–1.749, P = 0.008). In the multivariate logistic regression models (Supplementary Table 5), the adjusted ORs of the TyG index in model 1, model 2, and model 3 were 1.268 (95% CI: 0.977–1.640, P = 0.072), 1.335 (95% CI: 1.047–1.698, P = 0.019), and 1.255 (95% CI: 0.962–1.631, P = 0.092), respectively. Notably, as a continuous variable, the TyG index was only significant in model 2, which included intraoperative variables.
The participants were further divided into four groups (group 1: TyG ≤ 8.338, group 2: 8.338 < TyG ≤ 8.736, group 3: 8.736 < TyG ≤ 9.171, group 4: TyG > 9.171) based on the quartiles of the TyG index levels (Supplementary Table 6). Group 1 served as the reference. In the univariate analysis (Supplementary Table 3), the incidence of POD increased with higher TyG index levels, and the OR of group 4 was 1.728 (95% CI: 1.100-2.762, P = 0.019). In the multivariate logistic regression models, the adjusted ORs of group 4 in model 1, model 2, and model 3 were 1.518 (95% CI: 0.936–2.498, P = 0.095), 1.666 (95% CI: 1.055–2.677, P = 0.031), and 1.489 (95% CI: 0.913–2.464, P = 0.115), respectively. Similar to the results obtained using the using the TyG index as a continuous variable to predict POD, only group 4 of the TyG index in model 2 showed significance in the multivariate logistic regression analysis.
Subgroup analysis for sex
The comparison between male and female participants with respect POD incidence, age, comorbidities (cerebrovascular disease, chronic kidney disease, depression, and anxiety), American Society of Anesthesiologists (ASA) grade, AST, urine output, albumin level, glucose level, emergency surgery, blood loss, duration of MAP < 60 mmHg, and platelet count revealed no significant differences (all P > 0.05) (Supplementary Table 7). However, compared to males, females had a higher prevalence of hypertension and cardiac disease, elevated BMI, TyG index, total cholesterol, LDL, HDL, and triglyceride levels, lower prevalence of COPD, GSP, ALT, Hb, WBC count, total bilirubin, and Cre levels, shorter PT and duration of anesthesia, and received lesser volumes of crystalloid and colloid solutions (all P < 0.05).
To explore the differences between males and females in the relationship between the TyG index and POD incidence, a subgroup analysis was conducted (Table 3). In male patients, the relationship between the TyG index and POD incidence was not significant in any of the models. In contrast, the adjusted OR of the TyG index as a continuous variable in females was 1.95 (95% CI: 1.28–2.96, P < 0.001), and the adjusted OR of TyG index > 8.678 in females was 2.24 (95% CI: 1.25–4.19, P = 0.01). When analyzed based on interquartile groups of the TyG index, the adjusted OR of group 4 in females was 2.68 (95% CI: 1.18–6.78, P = 0.03). The P values for interaction analyses between gender and TyG index as a continuous variable, a binary variable and a quartile variable in Model 3 were 0.067, 0.435 and 0.046, respectively. There is interaction effect between gender and TyG index to predict POD when TyG acts as a continuous and a quartile bariable. These results from the subgroup analysis indicate that the TyG index is a more effective predictor of POD incidence in females among elderly patients with T2DM.
Sensitivity analysis
The E-value is established as 2.59 in present study due to the OR of TyG index > 8.678 to predict POD in Model 3. As ORs of most risk factors for POD were less than 2.59 in other reported studies, the results of present study could be considered to have good robustness.
As shown in Supplementary Table 8, OR of the group with TyG > 8.678 in the univariate analysis was 1.593 (95% CI: 1.146–2.238, P = 0.006). In the multivariate logistic regression models, the adjusted ORs of the TyG > 8.678 group in model 1, model 2, and model 3 were 1.493 (95% CI: 1.054–2.133, P = 0.025), 1.648 (95% CI: 1.763–2.331, P = 0.004), and 1.582 (95% CI: 1.108–2.279, P = 0.012), respectively. These sensitivity analysis has strengthened the the validity of results that TyG index has a effective prediction for POD in elderly patients with T2DM.
Discussion
POD is a common neurological complication among elderly surgical patients, and evidence suggests that patients with T2DM have a 1.6 times higher risk of developing POD compared to non-diabetic patients [4, 21]. Therefore, it is crucial to identify targetable risk factors or indices to reduce the incidence of POD and improve outcomes in elderly patients with T2DM. In the current study, the TyG index was found to be an independent risk factor for POD in this patient population.
IR plays a significant role in promoting surgery-induced systemic inflammation, which is a primary cause of POD [22]. Pathological mechanisms linking IR to cognitive impairment include dysregulation of the insulin signaling pathway affecting hippocampal plasticity, amyloid precursor protein metabolism, tau protein deposition, neuroinflammation, and apolipoprotein E ε4 allele expression [23]. Additionally, insulin plays a role in controlling cerebral vascular function, and reduced brain vasoreactivity during IR can lead to vascular cognitive impairment [23]. Clinical evidence supports the association between higher IR levels in elderly patients and an increased risk of postoperative cognitive impairment [9]. Conversely, interventions targeting IR, such as intranasal insulin treatment and exercise, have demonstrated cognitive function improvements in clinical and animal experiments [24,25,26,27]. Therefore, IR represents a potential pathological mechanism underlying POD in elderly patients with T2DM [28].
The TyG index is a novel biomarker that positively correlates with the degree of IR [29]. Its mechanism of indicating IR involves enhanced fat mobilization and lipid overload in the circulation [29]. Increased levels of free fatty acids and their metabolites accumulate in the liver, muscle, and pancreatic beta cells, inhibiting the insulin pathway [30, 31]. Moreover, the production of glycerol 3-phosphate leads to elevated blood sugar levels, and prolonged pathological states can result in IR [32]. While other methods exist to detect IR, such as the frequently sampled intravenous glucose tolerance test, oral glucose tolerance test, mixed meal tolerance test, insulin suppression test, and homeostatic model assessment (HOMA), the TyG index is more suitable for clinical application [33]. Previous measurements often require additional blood collection to determine insulin or C-peptide levels, which are not part of routine preoperative biochemical examinations. In contrast, the TyG index can be conveniently calculated using fasting plasma glucose and triglyceride levels, which are commonly tested preoperatively. Therefore, the TyG index serves as an ideal clinical marker for assessing perioperative IR in surgical patients.
Previous studies have shown that the TyG index can predict major adverse cardiovascular events in patients with T2DM [34, 35]. It has also been associated with an increased risk of long-term dementia and cognitive impairment following cerebral vessel disease in T2DM patients [36]. Furthermore, the TyG index has demonstrated better predictive performance than traditional indices such as HOMA-IR in neural disorders [37]. However, the relationship between the TyG index and POD in T2DM patients remains unclear. The present study is the first to reveal a significant association between a TyG index > 8.678 and increased incidence of POD in elderly patients with T2DM, suggesting it could serve as a new biochemical threshold for preoperative interventions in this population. According to the RCS curve, the relationship between TyG index and POD showed a “S-shape”. In the range of TyG index values below 9, the incidence of postoperative delirium (POD) increases as the TyG index increases. However, in the range of TyG index values above 9, there is no significant change in the incidence of POD. What’s more, the interaction effect of gender on TyG index was significant when TyG index was analyzed as a continuous variable and a quartile variable. This may be the reason for the better predictive ability of the TyG index for POD when acts as a binary variable.
Surgical patients with T2DM are prone to hyperglycemia due to IR, and intensive glucose control during the perioperative period has been shown to reduce the incidence of POD in diabetic patients [38]. However, strict glucose control to lower glucose levels may increase the risk of hypoglycemia, and the optimal standard for perioperative glucose levels remains unknown. Consequently, managing glucose levels alone is insufficient to address the risk of POD in patients with diabetes, and it is proposed that the TyG index should be screened in clinical practice to assess the risk of POD in elderly patients with T2DM.
Interestingly, this study found that the TyG index demonstrated better predictive performance for POD in females compared to males. This finding may be attributed to the fact that females are more likely to develop insulin resistance after the age of fifty [39], and estrogens have a potential protective effect on the body’s insulin sensitivity [40]. Consistent with current study, females had been observed to have significantly higher average TyG index and BMI values, as well as markedly higher incidences of hypertension and cardiac disease compared to males. These findings suggest that estrogen deficiency may promote insulin resistance in elderly women. However, there was no statistically significant difference in the incidence of POD between males and females. This may be due to the presence of different risk factors for POD between the sexes. Further research is warranted to explore potential differences in cognitive function between males and females. Some studies have reported a significant association between high TyG index in females and subclinical atherosclerosis [41]. In the subgroup analysis stratified by sex, a significant relationship was found between the TyG index and POD in females across all three models, which included TyG index as two categories, four categories, and continuous variables. This highlights the influence of gender on the relationship between insulin resistance and the prediction of POD.
Study strengths and limitations
Firstly, the study included 4566 elderly patients aged 65 or above with T2DM, which is more targeted on high-risk individuals for POD. Secondly, the study used univariate and multivariate logistic regression analyses to thoroughly evaluate the association between the TyG index and POD. Thirdly, composed of clinical routine indicators, the TyG index is easy to implement and promote for the prevention of POD.
Despite the valuable insights gained from present study, there are several limitations that should be acknowledged. Firstly, the current study was retrospective in nature, and the assessment of POD relied on medical and nursing records instead of standardized scales like the 3-minute Diagnostic Interview for Confusion Assessment Method (3D-CAM). This might have led to an measurement bias of POD incidence compared to the actual situation. Further more, it is possible that unaccounted confounding factors may have influenced the observed association between TyG and POD in our study and the cross-sectional design of the study suggests that the associations observed in the data do not necessarily imply causality. Secondly, given the AUC value, it appears that the effectiveness of the TyG index as a predictive tool might still be constrained. Due to the multitude of etiologies and risk factors for delirium, as well as its varied clinical presentations, it is difficult to explain the occurrence and progression of delirium with a single pathological mechanism. What’s more, our study population consists of patients with T2DM, who are already at a high risk for POD and are susceptible to multiple high-risk factors for POD. Thirdly, the impact of the duration of diabetes onset and the use of diabetes medications had not been analyzed, as extracting relevant data from medical records was proved to be challenging.
Conclusion
The TyG index holds promise as a potential biomarker for predicting POD in elderly surgical patients with T2DM, with a stronger predictive performance observed in females compared to males. Furthermore, a comprehensive consideration of blood glucose and triglyceride levels provides a new perspective and strategy for anesthesiologists in the preoperative management and prevention of POD in elderly patients with T2DM.
Data availability
No datasets were generated or analysed during the current study.
Change history
30 April 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12944-024-02120-1
Abbreviations
- POD:
-
Postoperative delirium
- T2DM:
-
type 2 diabetes mellitus
- IR:
-
Insulin resistance
- TyG:
-
Triglyceride-glucose
- ROC:
-
Receiver operating characteristic
- OR:
-
Odds ratio
- CNS:
-
Central nervous system
- COPD:
-
Chronic obstructive pulmonary disease
- CKD:
-
Chronic kidney disease
- ASA:
-
American Society of Anesthesiologists
- E.N.T.:
-
Otolaryngology head, and neck surgery
- GSP:
-
Glycated serum protein
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- Hb:
-
Hemoglobin
- WBC:
-
White blood cell
- PT:
-
Prothrombin time
- Cre:
-
Creatinine
- LDL:
-
Low density lipoprotein
- HDL:
-
High density lipoprotein
- MAP:
-
Mean artery pressure
- SPSS:
-
Statistical Package for the Social Science
- SD:
-
standard deviation
- IQR:
-
interquartile range
- HOMA:
-
homeostatic model assessment
- 3D-CAM:
-
3-minute Diagnostic Interview for Confusion Assessment Method
- RCS:
-
restricted cubic spline
References
Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119.
Drayton DJ, Birch RJ, D’Souza-Ferrer C, Ayres M, Howell SJ, Ajjan RA. Diabetes mellitus and perioperative outcomes: a scoping review of the literature. Br J Anaesth. 2022;128(5):817–28.
Grandl G, Wolfrum C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin Immunopathol. 2018;40(2):215–24.
Jin Z, Hu J, Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125(4):492–504.
Ballweg T, White M, Parker M, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126(2):458–66.
Feinkohl I, Winterer G, Pischon T. Diabetes is associated with risk of postoperative cognitive dysfunction: a meta-analysis. Diabetes Metab Res Rev. 2017;33(5).
Liu K, Song Y, Yuan Y, et al. Type 2 diabetes Mellitus with tight glucose control and poor Pre-injury Stair climbing Capacity May Predict Postoperative Delirium: a secondary analysis. Brain Sci. 2022;12(7):951.
Scherer T, Sakamoto K, Buettner C. Brain insulin signalling in metabolic homeostasis and disease. Nat Rev Endocrinol. 2021;17(8):468–83.
He X, Long G, Quan C, Zhang B, Chen J, Ouyang W. Insulin resistance predicts postoperative cognitive dysfunction in Elderly gastrointestinal patients. Front Aging Neurosci. 2019;11:197.
Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108.
Zhao Y, Sun H, Zhang W, et al. Elevated triglyceride-glucose index predicts risk of incident ischaemic stroke: the rural Chinese cohort study. Diabetes Metab. 2021;47(4):101246.
Su WY, Chen SC, Huang YT, et al. Comparison of the effects of Fasting glucose, Hemoglobin A1c, and triglyceride-glucose Index on Cardiovascular events in type 2 diabetes Mellitus. Nutrients. 2019;11(11):2838.
Song Y, Luo Y, Zhang F, et al. Systemic immune-inflammation index predicts postoperative delirium in elderly patients after surgery: a retrospective cohort study. BMC Geriatr. 2022;22(1):730.
Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9.
Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and Macrovascular Disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. 2018;320(15):1570–82.
VanderWeele TJ, Ding P. Sensitivity analysis in Observational Research: introducing the E-Value. Ann Intern Med. 2017;167(4):268–74.
Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477–91. https://doi.org/10.1097/ALN.0000000000002729. PMID: 31166241; PMCID: PMC6692220.
Mevorach L, Forookhi A, Farcomeni A, Romagnoli S, Bilotta F. Perioperative risk factors associated with increased incidence of postoperative delirium: systematic review, meta-analysis, and Grading of recommendations Assessment, Development, and evaluation system report of clinical literature. Br J Anaesth. 2023;130(2):e254–62.
Lindroth H, Bratzke L, Twadell S, et al. Predicting postoperative delirium severity in older adults: the role of surgical risk and executive function. Int J Geriatr Psychiatry. 2019;34(7):1018–28.
Sadeghirad B, Dodsworth BT, Schmutz Gelsomino N, et al. Perioperative factors Associated with postoperative delirium in patients undergoing noncardiac surgery: an individual Patient Data Meta-Analysis. JAMA Netw Open. 2023;6(10):e2337239.
Chen H, Mo L, Hu H, Ou Y, Luo J. Risk factors of postoperative delirium after cardiac surgery: a meta-analysis. J Cardiothorac Surg. 2021;16(1):113.
Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46.
Ma L, Wang J, Li Y. Insulin resistance and cognitive dysfunction. Clin Chim Acta. 2015;444:18–23.
Erichsen JM, Calva CB, Reagan LP, Fadel JR. Intranasal insulin and orexins to treat age-related cognitive decline. Physiol Behav. 2021;234:113370.
Kullmann S, Goj T, Veit R, et al. Exercise restores brain insulin sensitivity in sedentary adults who are overweight and obese. JCI Insight. 2022;7(18):e161498.
Hallschmid M. Intranasal insulin for Alzheimer’s Disease. CNS Drugs. 2021;35(1):21–37.
Ott V, Benedict C, Schultes B, Born J, Hallschmid M. Intranasal administration of insulin to the brain impacts cognitive function and peripheral metabolism. Diabetes Obes Metab. 2012;14(3):214–21.
Lee YC, Lee JW, Kwon YJ. Comparison of the triglyceride glucose (TyG) index, triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio, and metabolic score for insulin resistance (METS-IR) associated with periodontitis in Korean adults. Ther Adv Chronic Dis. 2022;13:20406223221122671.
Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68.
Chen L, Chen XW, Huang X, Song BL, Wang Y, Wang Y. Regulation of glucose and lipid metabolism in health and disease. Sci China Life Sci. 2019;62(11):1420–58.
Yazıcı D, Sezer H. Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol. 2017;960:277–304.
Nakamura MT, Yudell BE, Loor JJ. Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res. 2014;53:124–44.
Park SY, Gautier JF, Chon S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab J. 2021;45(5):641–54.
Gentreau M, Reynes C, Sabatier R, et al. Glucometabolic Changes Are Associated with Structural Gray Matter alterations in Prodromal Dementia. J Alzheimers Dis. 2022;89(4):1293–302.
Hong S, Han K, Park CY. The insulin resistance by triglyceride glucose index and risk for dementia: population-based study. Alzheimers Res Ther. 2021;13(1):9.
Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring HU. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev. 2016;96(4):1169–209.
Wang M, Mei L, Jin A, et al. Association between triglyceride glucose index and atherosclerotic plaques and Burden: findings from a community-based study. Cardiovasc Diabetol. 2022;21(1):204.
Jiang J, Li S, Zhao Y, et al. Intensive glucose control during the perioperative period for diabetic patients undergoing surgery: an updated systematic review and meta-analysis. J Clin Anesth. 2021;75:110504.
Yang K, Liu W. Triglyceride and glucose index and sex differences in relation to major adverse Cardiovascular events in hypertensive patients without diabetes. Front Endocrinol (Lausanne). 2021;12:761397.
De Paoli M, Zakharia A, Werstuck GH. The role of estrogen in insulin resistance: a review of clinical and preclinical data. Am J Pathol. 2021;191(9):1490–8.
Lu YW, Chang CC, Chou RH, et al. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol. 2021;20(1):206.
Acknowledgements
We would like to thank Yuting Zhou and Wei Wei of Hangzhou Le9 Healthcare Technology Co., Ltd., for help in the clinical data collection of this study.
Funding
This work was supported by the Capital Health Research and Development of Special Fund (2022-4-5025), the National Key Research and Development Program of China (2018YFC2001901), and the National Natural Science Foundation of China (No. 82171180, 82171464).
Author information
Authors and Affiliations
Contributions
YLM and WDM conceptualized and designed the study. YLM and WDM obtained funding. MS, YXS, PL, LJS and JBC collected and interpreted the data. FQZ, LBM, and SYL analyzed the data and prepared and reviewed the figures. MS and ML wrote the original draft. HKY provided critical revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was conducted following the Declaration of Helsinki. The study was approved by the Ethics Committee Board of the First Medical Center of Chinese PLA General Hospital (No. S2019-311–03). Patient informed consent was agreed to be waived by the institutional ethics committee of Chinese PLA General Hospital because the study was retrospective, and all data were anonymized before analysis.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the authors noticed that Figure 2 is incorrect.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, M., Liu, M., Zhang, F. et al. Triglyceride-glucose index predicts postoperative delirium in elderly patients with type 2 diabetes mellitus: a retrospective cohort study. Lipids Health Dis 23, 107 (2024). https://doi.org/10.1186/s12944-024-02084-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02084-2