Abstract
Objective
This study aimed to investigate the association between the triglyceride-glucose (TyG) index in early pregnancy and the development of gestational diabetes mellitus (GDM) in the second trimester. The primary objectives were to evaluate the predictive potential of the TyG index for GDM, determine the optimal threshold value of the TyG index for GDM assessment, and compare the predictive performance of the TyG index alone versus its combination with maternal age and pre-pregnancy body mass index on GDM. Moreover, the study explored the association between the TyG index in early pregnancy and the risk of other pregnancy-related complications (PRCs), such as placental abruption and gestational hypertension.
Patients and methods
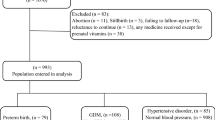
This prospective cohort study recruited 1,624 pregnant women who underwent early pregnancy antenatal counseling and comprehensive assessments with continuous monitoring until delivery. To calculate the TyG index, health indicators, including maternal triglycerides and fasting plasma glucose, were measured in early pregnancy (< 14 weeks of gestation). The predictive power of the TyG index for evaluating GDM in Chinese pregnant women was determined using multifactorial logistic regression to derive the odds ratios and 95% confidence interval (CI). Subgroup analyses were conducted, and the efficacy of the TyG index in predicting PRCs was assessed via receiver operating characteristic (ROC) curve analysis and restricted cubic spline, with the optimal cutoff value calculated.
Results
Logistic regression analyses revealed a 2.10-fold increase in the GDM risk for every 1-unit increase in the TyG index, after adjusting for covariates. The highest GDM risk was observed in the group with the highest TyG index compared with the lowest quintile group (odds ratios: 3.25; 95% CI: 2.23–4.75). Subgroup analyses indicated that exceeding the recommended range of gestational weight gain and an increased GDM risk were significantly associated (P = 0.001). Regarding predictive performance, the TyG index exhibited the highest area under the curve (AUC) value in the ROC curve for GDM (AUC: 0.641, 95% CI: 0.61–0.671). The optimal cutoff value was 8.890, with both sensitivity and specificity of 0.617.The combination of the TyG index, maternal age, and pre-pregnancy body mass index proved to be a superior predictor of GDM than the TyG index alone (AUC: 0.672 vs. 0.641, P < 0.01). After adjusting for multiple factors, the analyses indicated that the TyG index was associated with an increased risk of gestational hypertension. However, no significant association was noted between the TyG index and the risk of preeclampsia, placental abruption, intrauterine distress, or premature rupture of membranes.
Conclusion
The TyG index can effectively identify the occurrence of GDM in the second trimester, aligning with previous research. Incorporating the TyG index into routine clinical assessments of maternal health holds significant practical implications. Early identification of high-risk groups enables healthcare providers to implement timely interventions, such as increased monitoring frequency for high-risk pregnant women and personalized nutritional counseling and health education. These measures can help prevent or alleviate potential maternal and infant complications, thereby enhancing the overall health outcomes for both mothers and babies.
Similar content being viewed by others
Background
Gestational diabetes mellitus (GDM) arises from disruptions in glucose metabolism, resulting in elevated blood glucose levels during pregnancy. This condition poses significant risks for both mothers and children, including pregnancy complications, birth anomalies, and long-term health issues [1,2,3]. A meta-analysis involving 79,064 Chinese participants across 25 studies revealed a GDM prevalence of 14.8% in China [4]. Early identification and effective management of GDM are essential to safeguard the health of both mother and child. Currently, the primary diagnostic method for GDM is the oral glucose tolerance test performed between 24 and 28 weeks of gestation. However, the fetus may be exposed to intrauterine hyperglycemia before 24 weeks, highlighting the importance of early diagnosis. Delayed diagnosis between 24 and 28 weeks cannot fully reverse the adverse effects of intrauterine hyperglycemia on offspring. Additionally, most GDM patients do not exhibit obvious symptoms before diagnosis [5, 6], particularly in early pregnancy (before 14 weeks), making early detection challenging. Therefore, effective predictive methods for GDM in early pregnancy are crucial.
Insulin resistance (IR) is a critical factor in the development of GDM, heightening the susceptibility of expectant mothers and their offspring to metabolic disorders, chronic inflammation, and related conditions such as diabetes mellitus, hypertension, and cardiovascular disease [7,8,9,10]. The triglyceride-glucose (TyG) index, calculated as follows: Ln [fasting plasma glucose (FPG) (mg/dL) × fasting triglyceride (mg/dL)/2] [11], has emerged as a reliable surrogate marker for identifying IR and metabolic disorders due to its simplicity and practicality [12,13,14]. It is widely utilized in clinical settings to assess GDM risk. Various TyG indices have been established to identify women vulnerable to GDM in different regions, including southeastern and northern China [15], Mexico [16], Korea [17], Hungary [18], and Iran [19]. However, current studies on pregnant women have several methodological shortcomings, such as reliance on a single sample source, small sample sizes and subgroups, insufficient control of confounding variables, and the absence of universally accepted cutoff values for the TyG index in expectant mothers.
This study represents the first application of the TyG index to predict the incidence of GDM in northwest China (Urumqi). Its objective is to determine the optimal cutoff value of the TyG index for predicting GDM risk among pregnant women in early pregnancy while controlling for confounding variables. In contrast to previous research, it seeks to offer fresh insights into the predictive performance difference between the independent TyG index and the combination of the TyG index with age and pre-pregnancy body mass index (BMI). Utilizing logistic regression methods, the study aims to explore the feasibility of assessing GDM risk using the TyG index in early pregnancy after adjusting for potential covariates such as maternal ethnicity, pre-pregnancy BMI, age, assisted reproduction, miscarriage history, pregnancy, parity, gestational age, and gestational weight gain (GWG). Subgroup analyses were conducted based on maternal age, number of pregnancies, miscarriage history, assisted reproduction, pre-pregnancy BMI, GWG, and preterm birth. The difference in predictive performance between the independent TyG index and the combination of the TyG index with age and pre-pregnancy BMI was evaluated using the area under the receiver operating characteristic (ROC) (AUC) curve. If proven reliable, this approach can establish a comprehensive outpatient monitoring system for pregnant women with high TyG index levels in early pregnancy, facilitating timely interventions to improve pregnancy outcomes.
Materials and methods
Data source
Data from the Science and Technology Basic Resources Survey Special Project-China Maternal Nutrition and Health Scientific Survey (Northwest China Project Site) was used. This population-based prospective cohort study was conducted among pregnant women residing in Urumqi, northwest China, for an extended period. The cohort was established between August 2021 and April 2023, with pregnant women having an average age of (31.7 ± 4.0) years. Primiparous women constituted 64.1% of the sample.
The inclusion criteria for this cohort study encompassed pregnant women aged 18 years or older, with a gestational age of less than 14 weeks, engaging in prenatal care for the first time, having signed an informed consent form, and demonstrating the ability to accurately understand and independently respond to the researchers’ questions. The exclusion criteria comprised women diagnosed with severe cardiovascular and cerebrovascular diseases, liver and kidney diseases, mental disorders, intellectual disabilities, or those unable to meet the study’s requirements independently. Additionally, women with pre-GDM were excluded based on medical history inquiry and blood glucose testing, following the guidelines of the International Association of the Diabetes and Pregnancy Study Groups. Similarly, pregnant women with hypertension before pregnancy or presenting with a systolic blood pressure (SBP) of ≥ 140 mmHg and/or a diastolic blood pressure (DBP) of ≥ 90 mmHg before 20 weeks of gestation, diagnosed as hypertension complicating pregnancy according to the definition provided by the International Society for the Study of Hypertension in Pregnancy, were also excluded from participation in the study. The final study population comprised 1,624 women with singleton pregnancies (Supplementary Fig. 1).
This study received approval from the Ethics Committee of the Institute of Nutrition and Health of the Chinese Centre for Disease Control and Prevention (Approval No. 2021-008) and adhered to the guidelines of the Declaration of Helsinki. The participants were adequately informed about the study’s objectives and procedures and provided written consent.
Measurements and definitions
Data collection
During the first trimester of pregnancy (gestational age < 14 weeks), basic information questionnaires and biomarker measurements were collected. For women with regular menstrual cycles, fetal age was calculated from the first day of the last menstrual period to the current time. For those with irregular cycles, early pregnancy ultrasound examinations were conducted to estimate gestational age based on the size of the gestational sac and length of the embryonic bud. Based on this estimation, the last menstrual period can be inferred, and the fetal age can be calculated. Blood pressure was measured after a minimum 5-min rest in a sitting or lying position, using an upper arm blood pressure monitor to measure the right brachial artery blood pressure. Three measurements were taken, and the average was recorded. Blood samples were collected after a minimum 8-h fast for analysis of biomarkers including FPG (mg/dL), triglycerides (mg/dL), total cholesterol (TC, mg/dL), low-density lipoprotein cholesterol (LDL-C, mg/dL), high-density lipoprotein cholesterol (HDL-C, mg/dL), hemoglobin (g/L), uric acid (UA, μmoI/L), urine creatinine (μmoI/L), alanine aminotransferase (ALT, U/L), and aspartate aminotransferase (AST, U/L). The TyG index was also calculated. After delivery, data on pregnancy examinations and outcomes were collected.
Oral glucose tolerance test
The oral glucose tolerance test was conducted following the Chinese GDM diagnosis guidelines (2014). Pregnant subjects in their 24th–28th week of gestation maintained a normal diet for 3 days prior to their hospital visit, consuming no less than 150 g of carbohydrates daily and fasted for at least 8 h before the oral glucose tolerance test. During the examination, they remained seated and refrained from smoking. Subjects ingested 300 mL of a water solution containing 75 g of glucose within 5 min. Venous blood samples were collected before ingestion and at 1 and 2 h after ingestion (timing starts from the initial ingestion of the glucose solution) for glucose level measurement using the glucose oxidase method. A diagnosis of GDM was made if any of the following criteria were met or exceeded: a fasting blood glucose level of 5.1 mmol/L (91.90 mg/dL), a 1-h blood glucose level of 10.0 mmol/L (180.20 mg/dL), or a 2-h blood glucose level of 8.5 mmol/L (153.17 mg/dL). These criteria are consistent with the latest guidelines from the International Association of the Diabetes and Pregnancy Study Groups [20]. The definitions of gestational hypertension (GH) and preeclampsia are referred to the International Society for the Study of Hypertension in Pregnancy. The definitions of pregnancy-related complications (PRCs) other than GDM and hypertensive disorders were referred to International Classification of Diseases-10.
Other covariates
In addition to the primary variables, the study considered several other covariates known to impact outcome measures, including ethnicity, maternal age, pre-pregnancy BMI, assisted reproduction, abortion history, gravidity, parity, GWG, and gestational week of examination. Ethnicity and age were obtained from participants’ identification cards. Pre-pregnancy BMI was calculated by dividing pre-pregnancy weight (kg) by height squared (m). Fertility status was determined using information from participants’ questionnaires and obstetric/gynecological medical records.
According to the 2009 guidelines from the Institute of Medicine in the United States [21], recommended GWG is categorized as follows: for underweight individuals (BMI < 18.5 kg/m2), a weight gain of 12.5–18 kg during pregnancy; for normal weight individuals (18.5 ≤ BMI < 25 kg/m2), a weight gain of 11.5–16 kg during pregnancy; for overweight individuals (25 ≤ BMI < 30 kg/m2), a weight gain of 7–11.5 kg during pregnancy; and for obese individuals (BMI ≥ 30 kg/m2), a weight gain of 5–9 kg during pregnancy. Based on these guidelines, weight gain during pregnancy can be classified as: i) below the recommended range; ii) within the recommended range; or iii) above the recommended range.
Statistical analysis
The normal distribution of continuous variables was assessed using the Kolmogorov–Smirnov test. Continuous variables with a normal distribution were presented as mean ± standard deviation, while those not following a normal distribution were expressed as median (25th percentile, 75th percentile). Categorical variables were presented as frequencies and percentages. Differences in continuous variables between groups were compared using a one-way analysis of variance or the Kruskal–Wallis rank-sum test, while the chi-square test was employed to compare differences in categorical variables between groups.
To investigate the association between the TyG index and GDM, a multifactorial logistic regression analysis was conducted. The TyG index was evaluated both per unit and by quintile, with the lowest quintile serving as the reference. Two adjustments were made for potential confounding factors. Model 1 adjusted for ethnicity, pre-pregnancy BMI, maternal age, assisted reproduction, miscarriage history, gravidity, parity, gestational age at delivery, and GWG. Model 2 further adjusted for gestational age at examination, SBP, DBP, TC, LDL-c, HDL-c, hemoglobin, UA, urine creatinine, ALT, and AST. Subsequently, a linear trend assessment was performed on the model after evaluating the TyG index by quintiles.
To expand the understanding of the association between the TyG index and GDM risk, subgroup analyses were conducted based on maternal age (< 30, 30–34, or ≥ 35 years), number of pregnancies (≤ 2, > 2), miscarriage history (no, yes), assisted reproduction (no, yes), pre-pregnancy BMI (underweight, normal weight, overweight, and obese), GWG (within or above recommended range), and preterm birth (no, yes). Interactions across these subgroups were evaluated using likelihood ratio tests to compare whether there were differences in effects among the subgroups.
Moreover, the possible nonlinear correlation between TyG index changes and PRCs was examined through restricted cubic spline analysis using the rms package in R software. Furthermore, to evaluate the predictive performance of the TyG index for PRCs, ROC curves were plotted, and AUC values were calculated using the pROC package in R software, with 95% confidence intervals (CI) computed using the bootstrap method. This study also compared the predictive ability of the “TyG index alone” and the “TyG index combined with maternal age and pre-pregnancy BMI” for GDM.
All statistical procedures were performed using SPSS 25.0, R 4.2.2, and MSTATA (https://www.mstata.com/). SPSS was utilized for descriptive statistics, regression analysis, and other statistical procedures, while R and MSTATA were employed for data visualization. A P-value of < 0.05 was considered statistically significant, while a P-value of < 0.01 was deemed highly statistically significant.
Results
Baseline characteristics
The study included 1,624 pregnant women with an average age of 31.7 ± 4.0 years. The majority were of Han Chinese ethnicity (62.4%), and 48.4% were experiencing their first pregnancy. Among them, 447 developed GDM, resulting in a GDM prevalence of 27.52%. As the TyG index increased, significant increases in maternal age, gravidity, parity, and DBP were observed (P < 0.05). Similarly, pre-pregnancy BMI, assisted reproduction, abortion history, SBP, FPG, triglycerides, TC, hemoglobin, UA, ALT, and AST also significantly increased with the TyG index (P < 0.01). Conversely, the duration of gestation during delivery decreased with an increasing TyG index (P < 0.05). Gestational week at examination, LDL-C, HDL-C, and GWG showed a significant trend of first increasing and then decreasing with the TyG index (P < 0.01). No significant differences were observed in ethnicity and urine creatinine across quintiles of the TyG index (Table 1).
TyG index and GDM risk
The logistic regression analysis results highlight the association between the TyG index and the GDM risk (Table 2). In Model 1, adjusted for ethnicity, pre-pregnancy BMI, maternal age, assisted reproduction, abortion history, gravidity, parity, gestational age at delivery, and GWG, each 1-unit increase in the TyG index elevated the GDM risk by 2.10-fold. The highest quintile group exhibited the most significant GDM risk compared with the lowest quintile [odds ratios: 3.25; 95% CI: 2.23–4.75]. These associations were more pronounced in Model 2, which was further adjusted for gestational age at examination, SBP, DBP, TC, LDL-C, HDL-C, hemoglobin, UA, urine creatinine, ALT, and AST. Across all models, a progressive increase in GDM risk was observed with TyG index quintiles (linear trend P < 0.001).
Subgroup analyses based on various factors were conducted to further understand the association between the TyG index and GDM risk. A significant interaction was observed between GWG and the TyG index’s association with GDM risk, particularly when GWG exceeded recommended ranges (P = 0.001) (Fig. 1).
Subgroup analysis of the association between TyG index and GDM risk. Models adjusted for ethnic, pre-pregnancy BMI, maternal age, assisted reproduction, abortion history, gravidity, parity, GWG, delivery gestations, gestational week at the examination, SBP, DBP, TC, LDL-C, HDL-C, Hb, UA, Cr, ALT, and AST
ROC curve analyses of TyG index, maternal age, pre-pregnancy BMI, and FPG in predicting GDM
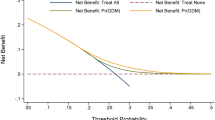
The independent predictive ability of the TyG index, FPG, maternal age, and pre-pregnancy BMI for GDM was assessed using ROC curves and AUC values. Using the DeLong method, the TyG index was found to be superior to the others (AUC: 0.641, 95% CI: 0.611–0.671), with an optimal cutoff value of 8.890 and both sensitivity and specificity of 0.617 (Fig. 2, Supplementary Table 1). Additionally, ROC curves were generated to compare the predictive ability of the combined prediction of the TyG index with maternal age and pre-pregnancy BMI for GDM. The combined prediction was found to be superior to the predictive ability of the TyG index alone (P = 0.00149) (Table 3, Fig. 3).
TyG index and risk of other PRCs
After adjusting for confounding factors, the multivariate logistic regression analysis indicated a significant 67% increase in the risk of GH and a 58% increase in the risk of preterm rupture of membranes for each 1-unit rise in the TyG index (P < 0.05). However, following combined adjustments, only the risk of GH retained significance. The restricted cubic spline plots further demonstrated an escalating risk of GH with an increasing TyG index (Supplementary Fig. 2). No association was found between the TyG index and the incidence of preeclampsia, placental abruption, and fetal distress in any of the models (Supplementary Table 2).
Discussion
This prospective cohort study aimed to investigate the association between the TyG index during early pregnancy and the risk of PRCs, particularly GDM, among pregnant women in Northwest China. The results revealed a robust and linear correlation between the TyG index and the likelihood of GDM in singleton pregnancies, with an identified optimal cutoff value of 8.890. Additionally, a potential association between the TyG index and the risk of GH was observed. Importantly, combining the TyG index with maternal age and pre-pregnancy BMI showed superior predictive power than the TyG index alone in forecasting the onset of GDM.
GDM is typically diagnosed between the 24th and 28th weeks of pregnancy, providing a limited window for prevention and mitigation of potential adverse effects. Early identification of women at risk for GDM is crucial, as its onset is linked to IR and diminished pancreatic β-cell secretion [7]. However, standard tests for IR, such as the glucose-hyperinsulinism clamp and the homeostatic model assessment of insulin resistance (HOMA-IR), have limitations due to clinical invasiveness, complexity, and the absence of a definitive threshold.
In 2008, the TyG index emerged as an alternative to HOMA-IR, serving as a surrogate marker for detecting IR in healthy individuals [11]. The calculation of the TyG index requires only two routine biochemical parameters: triglycerides and FPG, both of which are measured during routine early pregnancy screenings, eliminating the need for additional testing or complicated experimental procedures. Subsequent research consistently demonstrated that a high TyG index is associated with an increased risk of conditions such as metabolic syndrome [13], cardiovascular disease [22, 23], and type 2 diabetes mellitus (T2DM) [24]. In a comprehensive 15-year study, Wang et al. [25] analyzed a healthy cohort and highlighted that the TyG index and the emergence of T2DM were significantly associated, pinpointing a critical inflection at a value of 8.51. Among 7,708 Koreans aged between 40 and 69 years, Lee et al. [26] found sex-specific thresholds for the TyG index in predicting T2DM (≥ 8.86 for men and ≥ 8.52 for women). Furthermore, Yoon et al. [27] highlighted that the TyG index outperformed HOMA-IR in detecting T2DM in children and adolescents (AUC: 0.839 vs. 0.645).
Research on the association between the TyG index during early pregnancy and the subsequent GDM risk has yielded diverse results. For instance, an Iranian prospective study involving 954 healthy pregnant women, after adjusting for confounding factors like maternal age, family history of diabetes, and early gestational BMI, found that those with a TyG index in the highest tertile during early pregnancy (TyG index ≥ 8.99) faced a 3.91-fold increased GDM risk compared to those in the lowest tertile (TyG index < 8.31) [19]. Similarly, a Chinese prospective cohort study in Beijing, with 352 singleton pregnancies, determined that individuals in the highest TyG index tertile during early gestation (TyG index ≥ 8.3) had a 3.54 times greater GDM risk than those in the lowest tertile (TyG index < 7.9), after accounting for covariates [28].
Conversely, a study involving 164 Latin American pregnant women in early pregnancy stages found no significant correlation between the TyG index and GDM after adjusting for confounders (RR: 1.03, 95% CI: 0.57–1.88). Although no difference in the TyG index was detected between the GDM and control groups during early pregnancy (GDM group: 8.41 ± 0.35, control group: 8.40 ± 0.39; P = 0.95), a notable increase in the TyG index was observed in the GDM group compared with that in the control group between 24 and 28 weeks of gestation (GDM group: 9.01 ± 0.30, control group: 8.73 ± 0.34; P < 0.001). The study suggested that assessing the TyG index after 12 weeks but before 24 weeks of gestation might be beneficial for the early identification of those at risk for GDM [29].
The present study aligns with the majority of prior research. After comprehensive correction of confounding factors, the results showed that compared with pregnant women in the lowest quintile (TyG index ≤ 8.273), pregnant women in the highest quintile (TyG index ≥ 9.294) in the early pregnancy period had a 3.87 times increased GDM risk. Additionally, the present study highlights the association between excessive GWG and an increased GDM risk. There is growing evidence of the dangers of excessive weight gain during pregnancy, including causing inflammation of the fetal heart and altering fetal cardiac morphology [30]. The TyG index, as a tool to identify pregnant women at elevated risk for GDM during early pregnancy could be a valuable strategy for healthcare practitioners. Early identification of high-risk individuals extends the intervention window, facilitating timely preventive actions for those more susceptible to GDM. Such measures can comprise dietary interventions, appropriate physical activities, and personalized nutritional counseling starting from the early stages of pregnancy.
Determining the optimal cutoff value for the TyG index to predict GDM during early pregnancy varies across studies. Li et al. [15] proposed a cutoff value of 8.55 for the TyG index in early pregnancy, with a specificity of 67.9% and a sensitivity of 53.5%. Kim et al. [17] identified an optimal cutoff value of 8.15 for the TyG index 2 years preceding the first delivery, demonstrating a sensitivity of 47.0% and specificity of 68.2%. In a meta-analysis by Liu et al. [31], the TyG index during the first prenatal visit exhibited an AUC of 0.686 (95% CI: 0.615–0.756), but no specific cutoff value was specified. The present study indicates that the TyG index, with an optimal cutoff value of 8.89, predicts GDM with an AUC of 0.641 (95% CI: 0.611–0.671). The present consensus on the optimal cutoff value for TyG index in predicting GDM ranges between 8.1 and 8.9. Furthermore, the present study suggests that combining TyG index with maternal age and pre-pregnancy BMI enhances the prediction of GDM risk. Wang et al. concluded that excessive pre-pregnancy BMI in mothers is associated with hyperglycemia and hyperlipidemia in the offspring, as well as inflammation, permanently altering organ structure, function, and homeostasis within the organism [32]. Therefore, early prediction of GDM risk in early pregnancy based on TyG index and pre-pregnancy BMI has great clinical relevance. Notably, individual variations, diverse populations, and differences in experimental techniques can introduce variability in the TyG index. Moreover, current research primarily focuses on Asian and Latin American demographics, warranting further validation through extensive, multicenter cohort studies.
The present analysis reveals a correlation between the TyG index and GH incidence, which may be attributed to hyperinsulinemia causing placental ischemia and hypoxia. Reduced nitric oxide synthesis and disruptions in lipid metabolism can impact prostaglandin E2 production, leading to increased peripheral vascular resistance and elevated blood pressure [33]. Another study supports this, indicating that IR significantly increases the risk of GH, aligning with the present study’s conclusions [34]. However, the present study did not reveal a direct association between the TyG index and conditions such as preeclampsia, placental abruption, fetal distress, or premature rupture of membranes.
Study strengths and limitations
The present study had a prospective design, which enhanced the reliability of findings by observing events over time and minimizing recall bias. Moreover, the comprehensive nature of the study provided thorough insights into the association between the TyG index during early pregnancy and GDM risk, considering various factors. Meticulous data recording methods were used, ensuring accuracy in collecting blood and urine samples during the first trimester and minimizing potential confounding effects on lipid levels. Additionally, conducting blood tests on an empty stomach within 2 h of morning collection enhances the accuracy of lipid level measurements by minimizing the impact of non-fasting conditions and freeze-thaw cycles.
Nonetheless, limitations to the study exist. First, the study did not consider potential confounders such as economic status, dietary habits, physical activity, sleep patterns, and mental health status, which could influence the study outcomes. Second, the TyG index was measured only once in early pregnancy, and fluctuations throughout pregnancy were not tracked, which might have led to overlook of potential variations in TyG index values throughout gestation. Third, while participants with certain health conditions were excluded, complete certainty about the absence of underlying diseases affecting blood glucose, lipid levels, or insulin secretion may be challenging. Lastly, the study focused on Urumqi, a more developed economic region, and generalizing the current findings to less developed regions may require additional studies with larger cohorts, diverse age ranges, and subgroup analysis by several factors to enhance the external validity of findings. Future research should include populations from less developed regions.
Future research considerations
-
1.
Addition of other biomarkers beyond the TyG index that may predict GDM risk to compare predictive capacity
-
2.
Longer-term follow-up of both mothers and infants after delivery to assess whether early pregnancy TyG index levels have any associationt on postpartum outcomes
-
3.
Analysis of lifestyle intervention measures to reduce the incidence of GDM in pregnant women with elevated TyG index in early pregnancy
Conclusion
This study highlights the TyG index’s effectiveness in identifying the development of GDM in the latter half of pregnancy, consistent with most research findings. Therefore, the TyG index serves as a valuable early screening tool and can be incorporated into routine obstetric clinical assessments, making it a powerful instrument for clinicians to evaluate the risk of diabetes in pregnant women during obstetric examinations, facilitating early and proactive interventions for high-risk pregnancies. Implementing such measures can reduce the incidence of GDM, leading to improved overall pregnancy outcomes.
Availability of data and materials
The datasets utilized and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Change history
04 May 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12944-024-02110-3
Abbreviations
- GDM:
-
Gestational diabetes mellitus
- IR:
-
Insulin resistance
- TyG:
-
Triglyceride-glucose
- BMI:
-
Body mass index
- ROC:
-
Receiver operating characteristic
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- UA:
-
Uric acid
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- PRCs:
-
Pregnancy-related complications
- GWG:
-
Gestational weight gain
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- HOMA-IR:
-
Homeostatic model assessment of insulin resistance
- GH:
-
Gestational hypertension
- T2DM:
-
Type 2 diabetes mellitus
References
Moon JH, Jang HC. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes Metab J. 2022;46(1):3–14.
Malaza N, Masete M, Adam S, Dias S, Nyawo T, Pheiffer C. A systematic review to compare adverse pregnancy outcomes in women with pregestational diabetes and gestational diabetes. Int J Environ Res Public Health. 2022;19(17):10846.
Juan J, Yang H. Prevalence, prevention, and lifestyle intervention of gestational diabetes mellitus in China. Int J Environ Res Public Health. 2020;17(24):9517.
Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. 2019;10(1):154–62.
Ohara R, Obata-Yasuoka M, Abe K, Yagi H, Hamada H, Yoshikawa H. Effect of hyperemesis gravidarum on gestational diabetes mellitus screening. Int J Gynaecol Obstet. 2016;132(2):156–8.
Bayraktar B, Balıkoğlu M, Bayraktar MG, Kanmaz AG. The effects of hyperemesis gravidarum on the oral glucose tolerance test values and gestational diabetes. Prague Med Rep. 2021;122(4):285–93.
Ellerbrock J, Spaanderman B, Drongelen JV, Mulder E, Lopes van Balen V, Schiffer V, Jorissen L, Alers RJ, Leenen J, Ghossein-Doha C, et al. Role of beta cell function and insulin resistance in the development of gestational diabetes mellitus. Nutrients. 2022;14(12):2444.
Bucher M, Montaniel KRC, Myatt L, Weintraub S, Tavori H, Maloyan A. Dyslipidemia, insulin resistance, and impairment of placental metabolism in the offspring of obese mothers. J Dev Orig Health Dis. 2021;12(5):738–47.
Piao C, Wang X, Peng S, Guo X, Zhao H, He L, Zeng Y, Zhang F, Zhu K, Wang Y. IL-34 causes inflammation and beta cell apoptosis and dysfunction in gestational diabetes mellitus. Endocr Connect. 2019;8(11):1503–12.
Liang W, Sun FF. Does gestational diabetes mellitus increase the risk of cardiovascular disease? A Mendelian randomization study. J Endocrinol Invest. 2023. https://doi.org/10.1007/s40618-023-02233-x.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Li X, Li G, Cheng T, Liu J, Song G, Ma H. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study: TyG index and incident diabetes. Lipids Health Dis. 2020;19(1):236.
Son DH, Lee HS, Lee YJ, Lee JH, Han JH. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 2022;32(3):596–604.
Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride Glucose (TyG) Index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72(5):986–8.
Li H, Miao C, Liu W, Gao H, Li W, Wu Z, Cao H, Zhu Y. First-trimester triglyceride-glucose index and risk of pregnancy-related complications: a prospective birth cohort study in southeast China. Diabetes Metab Syndr Obes. 2022;15:3705–15.
Sánchez-García A, Rodríguez-Gutiérrez R, Saldívar-Rodríguez D, Guzmán-López A, Castillo-Castro C, Mancillas-Adame L, Santos-Santillana K, González-Nava V, González-González JG. Diagnostic accuracy of the triglyceride-glucose index for gestational diabetes screening: a practical approach. Gynecol Endocrinol. 2020;36(12):1112–5.
Kim JA, Kim J, Roh E, Hong SH, Lee YB, Baik SH, Choi KM, Noh E, Hwang SY, Cho GJ, et al. Triglyceride and glucose index and the risk of gestational diabetes mellitus: a nationwide population-based cohort study. Diabetes Res Clin Pract. 2021;171:108533.
Nádasdi Á, Gál V, Masszi T, Somogyi A, Firneisz G. PNPLA3 rs738409 risk genotype decouples TyG index from HOMA2-IR and intrahepatic lipid content. Cardiovasc Diabetol. 2023;22(1):64.
Pazhohan A, Rezaee Moradali M, Pazhohan N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med. 2019;32(7):1167–75.
Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva A, Hod M, Kitzmiler JL, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–82.
Institute of M, National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press (US). Copyright © 2009, National Academy of Sciences; 2009.
Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–73.
Liu D, Yang K, Gu H, Li Z, Wang Y, Wang Y. Predictive effect of triglyceride-glucose index on clinical events in patients with acute ischemic stroke and type 2 diabetes mellitus. Cardiovasc Diabetol. 2022;21(1):280.
Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, Li L, Zhang L, Ren Y, Zhao Y, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc Diabetol. 2017;16(1):30.
Wang Z, Zhao L, He S. Triglyceride-glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: a 15-year prospective study. Endocrine. 2021;72(1):124–31.
Lee JW, Lim NK, Park HY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. 2018;18(1):33.
Yoon JS, Lee HJ, Jeong HR, Shim YS, Kang MJ, Hwang IT. Triglyceride glucose index is superior biomarker for predicting type 2 diabetes mellitus in children and adolescents. Endocr J. 2022;69(5):559–65.
Liu PJ, Liu Y, Ma L, Yao AM, Chen XY, Hou YX, Wu LP, Xia LY. The predictive ability of two triglyceride-associated indices for gestational diabetes mellitus and large for gestational age infant among Chinese pregnancies: a preliminary cohort study. Diabetes Metab Syndr Obes. 2020;13:2025–35.
Sánchez-García A, Rodríguez-Gutiérrez R, Saldívar-Rodríguez D, Guzmán-López A, Mancillas-Adame L, González-Nava V, Santos-Santillana K, González-González JG. Early triglyceride and glucose index as a risk marker for gestational diabetes mellitus. Int J Gynaecol Obstet. 2020;151(1):117–23.
Kandadi MR, Hua Y, Zhu M, Turdi S, Nathanielsz PW, Ford SP, Nair S, Ren J. Influence of gestational overfeeding on myocardial proinflammatory mediators in fetal sheep heart. J Nutr Biochem. 2013;24(11):1982–90.
Liu Y, Chi R, Jiang Y, Chen B, Chen Y, Chen Z. Triglyceride glycemic index as a biomarker for gestational diabetes mellitus: a systemic review and meta-analysis. Endocr Connect. 2021;10(11):1420–7.
Wang L, O’Kane AM, Zhang Y, Ren J. Maternal obesity and offspring health: adapting metabolic changes through autophagy and mitophagy. Obes Rev. 2023;24(7):e13567.
Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C. Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol. 1838;2018:9.
Lin J, Jin H, Chen L. Associations between insulin resistance and adverse pregnancy outcomes in women with gestational diabetes mellitus: a retrospective study. BMC Pregnancy Childbirth. 2021;21(1):526.
Acknowledgements
The authors would like to express their sincere gratitude to all the participants, staff members, and fellow researchers who have made invaluable contributions to this study. Additionally, the authors would like to extend their thanks to Dr. Hongxin Zhao (China) for providing technical assistance and for his invaluable help in analyzing and visualizing the data. We appreciate the linguistic assistance provided by Zhiyun during the preparation of this manuscript.
Funding
This research was supported by the Special Project of Scientific and Technological Basic Resources Survey of the Ministry of Science and Technology of China (Grant No. 2019FY101002) and the 14-th Five-Year Plan Distinctive Program of Public Health and Preventive Medicine in Higher Education Institutions of Xinjiang Uygur Autonomous Region. The authors would like to express their sincere gratitude to the funding agencies for their financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. Hong Ding and Yufeng Guo provided the research idea and design. Yufeng Guo, Junwen Lu, Mailiman Bahani, Yuxia Zhang, Chengyao Liu, Xiaolan Liu, and Xiaoli Wang collected the data. Yufeng Guo, Junwen Lu, Mailiman Bahani, Lijun Zhou, and Fangshen Li analyzed the data, compiled the data and tables, and interpreted the experimental results. Yufeng Guo and Junwen Lu wrote the manuscript. Yufeng Guo, Guifeng Ding, Lei Wang, and Huanmei Zhang revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The human subjects involved in this study met the guidelines outlined in the Helsinki Declaration. The study participants voluntarily provided written informed consent, indicating their willingness to participate. In addition, all research protocols have been reviewed and approved by the Ethics Committee of the Institute of Nutrition and Health of the China Center for Disease Control and Prevention, approval number 2021-008.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the authors requested to add an additional funding source to the funding section.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, Y., Lu, J., Bahani, M. et al. Triglyceride-glucose index in early pregnancy predicts the risk of gestational diabetes: a prospective cohort study. Lipids Health Dis 23, 87 (2024). https://doi.org/10.1186/s12944-024-02076-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02076-2