Abstract
Objective
Current guidelines are debated when it comes to starting anticoagulant therapy in patients with non-valvular atrial fibrillation (NVAF) and low CHA2DS2-VASc scores (1–2 in women and 0–1 in men). However, these individuals still have a high likelihood of developing left atrial thrombus/spontaneous echo contrast (LAT/SEC) and experiencing subsequent thromboembolism. Recent research has demonstrated that lipoprotein(a) [Lp(a)] may increase the risk of thrombosis, but the relationship between Lp(a) and LAT/SEC in NVAF patients is not clearly established. Therefore, this study sought to evaluate the predictive ability of Lp(a) for LAT/SEC among NVAF patients with low CHA2DS2-VASc scores.
Methods
NVAF patients with available transesophageal echocardiography (TEE) data were evaluated. Based on the TEE results, the subjects were classified into non-LAT/SEC and LAT/SEC groups. The risk factors for LAT/SEC were examined using binary logistic regression analyses and were validated by using 1:1 propensity score matching (PSM). Subsequently, novel predictive models for LAT/SEC were developed by integrating the CHA2DS2-VASc score with the identified factors, and the accuracy of these models was tested using receiver operating characteristic (ROC) analysis.
Results
In total, 481 NVAF patients were enrolled. The LAT/SEC group displayed higher Lp(a) concentrations. It was found that enlarged left atrial diameter (LAD), high concentrations of Lp(a), and a history of coronary heart disease (CHD) were independent predictors of LAT/SEC. Lp(a) and LAD still had predictive values for LAT/SEC after adjusting for PSM. In both the highest quartile groups of Lp(a) (>266 mg/L) and LAD (>39.5 mm), the occurrence of LAT/SEC was higher than that in the corresponding lowest quartile. By incorporating Lp(a) and the LAD, the predictive value of the CHA2DS2-VASc score for LAT/SEC was significantly improved.
Conclusion
Elevated Lp(a) and enlarged LAD were independent risk factors for LAT/SEC among NVAF patients with low CHA2DS2-VASc scores. The prediction accuracy of the CHA2DS2-VASc score for LAT/SEC was significantly improved by the addition of Lp(a) and LAD. When evaluating the stroke risk in patients with NVAF, Lp(a) and LAD should be taken into account together with the CHA2DS2-VASc score.
Trial registration
Retrospectively registered.
Similar content being viewed by others
Introduction
Among individuals with non-valvular atrial fibrillation (NVAF), the occurrence of left atrial thrombus (LAT) is directly linked to stroke. Anticoagulant therapy can eliminate atrial thrombosis and reduce the risk of subsequent embolism [1]. Moreover, the presence of left atrial spontaneous echo contrast (SEC), indicating a prothrombotic condition, serves as an indication for initiating anticoagulation therapy [2].
The CHA2DS2-VASc score is an updated version of the CHADS2 score, which is commonly employed for stroke risk stratification and provides guidance for clinical anticoagulation therapy [3]. Current guidelines recommend anticoagulation medication for patients with high CHA2DS2-VASc scores; however, the necessity of anticoagulation therapy for those with low scores (women: 1-2 points; men: 0-1 point) is debatable. Nevertheless, these patients still face a significant risk of LAT/SEC and subsequent thromboembolism [4,5,6].
Lipoprotein (a) [Lp(a)] is one of the frequently found components of blood lipids [7]. Lp(a) has been considered a potential marker for thrombosis [8]. Previous studies have also shown a considerable increase in the prevalence of LAT in those with high concentrations of Lp(a) [9]. Nevertheless, the correlation between Lp(a) and LAT/SEC in those with low scores is not fully understood. Thus, this study sought to evaluate the ability of Lp(a) to predict LAT/SEC in individuals with low CHA2DS2-VASc scores, to identify patients who may have undetected thrombosis based on the current scoring system, and to provide timely oral anticoagulant treatment to enhance outcomes.
Methods
Study population
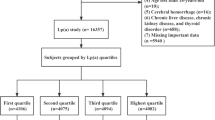
Through a systematic review of medical records, this research gathered data from 949 patients with NVAF, all of whom underwent transesophageal echocardiography (TEE) and transthoracic echocardiography (TTE) prior to left atrial appendage closure (LAAC) or radio-frequency catheter ablation (RFCA) during hospitalization between January 2019 and January 2022 in the Department of Cardiology, First Affiliated Hospital of Xi'an Jiaotong University. The following patients were disqualified based on the exclusion criteria: 1) patients with high CHA2DS2-VASc scores (women: ≥ 3 points; men: ≥ 2 points); 2) patients with valvular disease or those who have undergone surgery for valve replacement or remodeling; 3) patients with cardiomyopathy; 4) patients with congenital heart disease; 5) patients with other systemic diseases, such as hyperthyroidism, severe hepatic and renal insufficiency, systemic immune diseases, malignant tumors, and thrombophilia; 6) patients with incomplete clinical data. Eventually, 481 patients were included. All the relevant information, including demographic parameters, echocardiography results, and laboratory examination results, was gathered from the electronic medical records system. Figure 1 shows the flowchart with inclusion and exclusion criteria for the study.
CHA2DS2-VASc score
Based on the clinical information provided, the CHA2DS2-VASc score was recalculated. This scoring system assigns 1 point for each risk variable, including diabetes mellitus, vascular disease, hypertension, and congestive heart failure. Specifically, individuals aged 65–74 years received 1 point, while those aged 75 years or older received 2 points. A transient ischemic attack (TIA) or stroke received 2 points. Females were assigned an additional point. Females with scores of 1-2 and males with scores of 0-1 were classified as having low CHA2DS2-VASc scores [3].
Echocardiographic examination
All patients underwent TEE to exclude LAT or SEC prior to receiving RFCA or LAAC. The diagnostic criteria for LAT are well-defined as independent, mobile, circular, oval, or irregular shapes with uniform density that differ from the surrounding myocardial tissue density and can be detected in multiple sections of the left atrial cavity [10]. The diagnostic criteria for SEC include the observation of swirling, smoke-like, or prethrombotic states within the left atrium (LA), which must be distinguished from illusions caused by changes in near-field and high-gain artifacts [11]. TTE was conducted to collect data on cardiac chamber size and ventricular wall motion to evaluate cardiac structures and functions. Echocardiography was performed by two professional ultrasound physicians. One physician was responsible for performing the operation and making the diagnosis, while the other physician reviewed the results. Both physicians were unaware of the other clinical data of the patient before the examination.
Statistical analyses
Continuous variables were displayed as medians (interquartile ranges) or means ± standard deviations (\(\overline{{\text{X}} }\) ± SDs), depending on whether they followed a normal distribution and were compared employing Student's t tests or corrected t tests. Variables with a categorical nature were displayed as frequencies (n) and percentages (%). For comparison, Fisher's exact test or the chi-square test was performed. The risk variables for LAT/SEC were identified using multivariate logistic regression. The baseline imbalance was corrected using propensity score matching (PSM) with a caliper value of 0.01 for optimal matching. The predictive capacity of Lp(a) and other risk variables was evaluated through the receiver operating characteristic (ROC) analysis. For statistical significance, a P-value of less than 0.05 was required.
Results
481 NVAF patients with an average age of 56.81±8.88 years were included. Based on the TEE results, the subjects were classified into the LAT/SEC group (n = 88) and the non-LAT/SEC group (n = 393). LAT was found in 60 patients, including 33 patients with combined SEC; SEC alone was found in 28 patients. The incidences of LAT and SEC accounted for 12.47% and 11.43%, respectively, of the total population. 6.1% of patients in the non-LAT group underwent LAAC, and 32.1% underwent RFCA. As shown in Table 1, coronary heart disease (CHD) (9.1% vs. 2.3%) was more prevalent in the LAT/SEC group (9.1% vs. 2.3%), as was non-paroxysmal atrial fibrillation (48.9% vs. 29.5%). A higher diastolic blood pressure (83.5 (76–90) mmHg vs. 79 (73–87) mmHg, P < 0.05) and a higher Lp (a) level [281.50 (178.25–394.75) mg/L vs. 146.00 (86.50–229.00) mg/L, P < 0.01] were found in the LAT/SEC group. Additionally, activated partial thromboplastin time (APTT), NT-proBNP, and prothrombin time (PT) were also higher in this group. Furthermore, the LAT/SEC group exhibited an enlarged left atrial diameter (LAD) and a decreased left ventricular ejection fraction (LVEF). No statistical differences were observed in terms of CHA2DS2-VASc score, age, gender, smoking and alcohol consumption history, systolic blood pressure, pulse, hypertension, prior heart failure, stroke, or previous use of oral anticoagulants or other medications.
Risk factors for LAT/SEC
By incorporating variables that have been demonstrated to be associated with LAT formation in previous research (age, gender [12], non-paroxysmal atrial fibrillation, LVEF [13], fibrinogen [14], and D-dimer [15]), as well as risk factors identified through univariate regression analyses in the present study, multivariate regression analyses were performed and the results demonstrated that LAD, CHD history, and Lp(a) level were all predictors of LAT/SEC (Table 2).
Subjects were subsequently separated into four groups according to the quartile of LAD and Lp(a) levels. The Lp(a) level was favorably linked with LAT/SEC formation according to further multivariate logistic regression analysis (Table 3). Specifically, individuals in the highest quartile group exhibited a greater incidence of LAT/SEC compared to the first quartile group. Even after making adjustments for gender and age in Model I, as well as additional confounding factors including history of CHD, diastolic blood pressure, PT, D-dimer, fibrinogen, NT-proBNP, LAD, LVEF, and interventricular septal amplitude (IVSA) in Model II, these associations remained consistent. Similarly, regardless of whether adjustments were made for confounding factors, there was a positive correlation between an increased prevalence of LAT/SEC and left atrial enlargement. As illustrated in Fig. 2, the ROC curves showed the prediction capacity of Lp(a) and LAD for LAT/SEC. The AUC for the LAD was 0.695 (95% confidence interval [CI] 0.637-0.753, P < 0.01), while that for Lp(a) was 0.718 (95% CI 0.658-0.778, P < 0.01). According to these findings, both LAD and Lp(a) were risk factors for LAT/SEC.
However, it is necessary to point out that there may be an imbalance in the baseline characteristics of the patients, which could potentially affect the results. Therefore, PSM analysis was used for validation. Based on the ideal cut-off value, the patients were split into a high Lp(a) group (≥ 224.5 mg/L) and a control group (< 224.5 mg/L). In the high Lp(a) subgroup, there were substantially more cases of LAT/SEC (36.1% vs. 9.6%, P < 0.001). After incorporating variables that showed statistical differences between the two groups and non-paroxysmal atrial fibrillation in the PSM analysis, the confounding variables were balanced (Table 4). The high Lp(a) subgroup continued to have a significantly greater incidence of LAT/SEC (35.8% vs. 13.9%, P < 0.001). Similarly, patients were split into those with an enlarged LAD (≥ 36.5 mm) and those without (< 36.5 mm) according to the ideal cut-off value (
Table 5). After conducting the chi-square test, the expanded LAD group had a considerably greater occurrence of LAT/SEC than in the control (27.1% vs. 6.4%, P < 0.001). After PSM, the results remained consistent (17.3% vs. 5.3%, P < 0.001). Therefore, the elevation of Lp(a) and LAD can both be considered risk markers for LAT/SEC.
Lp(a) and LAD enhance the prediction accuracy of the CHA2DS2-VASc score
Given all this, enlarged LAD, increased Lp(a), and a history of CHD were predictors of LAT/SEC. Only LAD and Lp(a) were included in the new model because a history of CHD was already taken into account when calculating the CHA2DS2-VASc score. As shown in Table 6 and Fig. 3, the predictive ability for LAT/SEC of the CHA2DS2-VASc score was poor (AUC = 0.515, P > 0.05). After incorporating Lp(a) (Model A), the predictive value significantly improved (AUC difference = 0.206, Z = 4.848, P < 0.01). After combining Lp(a) and LAD (Model B), the value for LAT/SEC prediction was also improved (AUC difference = 0.245, Z = 6.259, P < 0.01). However, in comparison to Model A, Model B showed no statistically significant difference (AUC difference = 0.0394, Z = 1.595, P > 0.05).
Discussion
The yearly increase in morbidity from atrial fibrillation (AF) has resulted in a greater burden of thrombotic events, particularly stroke, which is attributed to increased mortality [16]. LAT and SEC are considered to be directly associated with AF-induced thromboembolism [17], and oral anticoagulants are considered to be effective treatments for eliminating left atrial thrombosis [3]. Current guidelines advocate oral anticoagulants for patients classified as "high risk" according to their CHA2DS2-VASc score (men ≥ 2; women ≥ 3). However, in patients who have low CHA2DS2-VASc scores, there is a debate regarding the use of anticoagulants. Previous studies have shown that thrombotic events still occur in NVAF patients at "low risk". According to a study conducted in Denmark involving 39,400 NVAF patients, untreated low-risk patients had an annual incidence of ischemic stroke of 0.49% [18]. Similarly, another study conducted in Taiwan revealed that, throughout the median follow-up of five years, 14.4% of men and 14.9% of women with low CHA2DS2-VASc scores suffered an ischemic stroke, and the corresponding annual stroke rates were 2.75% and 2.55%, respectively [6]. Moreover, the CHA2DS2-VASc score primarily targets stroke as the primary outcome event rather than left atrial thrombosis. Notably, low-risk patients who had not experienced a prior stroke or embolic event were classified in the high-risk group only after an embolic event occurred. This finding suggested a delay in the prediction of the CHA2DS2-VASc score.
Current studies have reported different prevalence rates of LAT in NVAF patients, with an average of 9.8% [12]. This study revealed that LAT and SEC were present in 12.47% and 11.43%, respectively, of the total study population, which might be higher than the rates reported in previous research. The disparity in findings could be attributed to potential selection bias and referral bias within the research, as well as variations in the demographics of the study populations. However, more importantly, these findings indicate that the emergence of LAT/SEC may be influenced by factors other than the CHA2DS2-VASc score. Hence, this investigation aimed to identify NVAF patients categorized as "low risk" based on conventional stroke risk assessment, yet are at elevated risk for AF-related embolic events, and to institute prompt clinical interventions to mitigate the occurrence of such events.
Lp(a) has been recognized as a possible predictor of the development of atherosclerosis and thrombosis [19,20,21]. The findings of previous study imply that Lp(a)'s pro-thrombotic impact is predominantly mediated by its apolipoprotein(a) (ApoA) component, which has a very high resemblance to plasminogen [22]. Lp(a) might bind competitively to the fibrin surface, tissue-type plasminogen activator, and cell receptors, consequently reducing the generation of plasmin and ultimately impairing fibrinolytic effects [23]. Furthermore, Lp(a) attaches to heparin as well as to heparan sulfate, thereby neutralizing their thrombosis-inhibiting effects [24]. Additionally, Lp(a) has the ability to induce the release of plasminogen activator inhibitor-1, leading to unbalanced plasma fibrinolysis and coagulation systems, and ultimately promoting thrombosis [25]. Moreover, it has also been discovered that Lp(a) promotes thrombosis by attaching to and deactivating tissue factor pathway inhibitors (TFPI) [26]. Consequently, the presence of Lp(a) disrupts the equilibrium between the coagulation and fibrinolytic systems under normal physiological circumstances, thereby facilitating thrombosis.
Most related research suggests that genetic inheritance is the primary factor affecting Lp(a) levels and holds steady for the majority of a person's lifetime [27, 28]. Due to variations in Lp(a) gene polymorphisms among different ethnic groups [7], there are inconsistencies in the cut-off points for assessing cardiovascular risk according to various guidelines and consensuses [29, 30]. This is the first study demonstrating a substantial interaction between Lp(a) levels and the frequency of LAT/SEC among NVAF patients who have low CHA2DS2-VASc scores, with an optimal predicted cut-off value of 224.5 mg/L.
In this study, except for Lp(a), the LAD was also discovered to be a predictor of LAT/SEC. Left atrial enlargement is not only a consequence of atrial fibrillation but is also considered a predictor of thrombotic events [31,32,33]. According to a study of 705 NVAF patients, the occurrence of LAT/SEC was moderately predicted by enlargement of the left atrium [34]. Zhou M. et al. indicated that among NVAF patients with a low risk of stroke, an enlarged left atrium was a risk indicator for LAT [35]. A prospective community-based survey revealed that stroke rates were higher in NVAF patients with a LAD exceeding 45 mm, and LA enlargement has been demonstrated to be a substantial indicator of both extracranial embolism events and stroke [36].
The LAT/SEC group had a larger LAD than the non-LAT/SEC group did among the NVAF patients (39.0 (35.0-43.0) vs. 34.0 (31.0-39.0), P < 0.001). Additionally, for every 1 mm increase in LAD, the risk of LAT/SEC increased by 7.2%, indicating a positive correlation between LAD and LAT/SEC. The CHA2DS2-VASc score's ability to predict LAT/SEC was significantly improved by the addition of Lp(a) and LAD (AUC difference = 0.245, Z = 6.259, P < 0.01). Therefore, a plasma Lp(a) level ≥224.5 mg/L and a LAD ≥36.5 mm indicate a heightened likelihood of LAT formation, and TEE should be performed to detect intra-atrial thrombosis when assessing thrombosis risk.
Study strengths and limitations
The risk factors for LAT/SEC among NVAF patients with low CHA2DS2-VASc scores have been the subject of only a small amount of research to date. This research is the first to identify the predictive utility of increased Lp(a) levels for LAT/SEC, with a threshold of 224.5 mg/L. Consistent with the findings of previous research, this study also found that left atrial dilatation is a predictor of LAT/SEC. The predictive capacity of the CHA2DS2-VASc score for LAT/SEC was significantly improved by incorporating Lp(a) and LAD. These findings suggest that when assessing stroke risk in NVAF patients, Lp(a) and LAD should be taken into account together with the CHA2DS2-VASc score. This comprehensive approach might help recognize patients who are at potential risk of thrombosis and enable the timely administration of anticoagulation therapy to improve the long-term prognosis.
However, there were several limitations that should be addressed. First, only individuals who underwent TEE examination during their hospitalization were included in this single-center study. This may have led to some selection bias. Second, the findings of this research could be influenced by the variability in referrals. The majority of patients treated at our facility were referred by subordinate hospitals, which may involve additional complexity and greater clinical complications. As a result, this could lead to a higher rate of positive outcomes. Notably, Lp(a) levels vary among different ethnicities. This study focused primarily on the Chinese Han population, so the findings may not be generalizable to other races. In addition, due to the relatively limited clinical data collected, this study used only the LAD to evaluate left atrial enlargement. Other indicators, such as left atrial appendage morphology and left atrium volume index, were not included in the systematic evaluation of left atrium function. Furthermore, a prospective study may be needed to verify the conclusions of this study due to its retrospective nature.
Conclusion
Elevated Lp(a) and enlarged LAD were independent risk factors for LAT/SEC among NVAF patients with low CHA2DS2-VASc scores. The prediction accuracy of the CHA2DS2-VASc score for LAT/SEC was significantly improved by the addition of Lp(a) and LAD. When evaluating the stroke risk in patients with NVAF, Lp(a) and LAD should be taken into account together with the CHA2DS2-VASc score.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AF:
-
Atrial Fibrillation
- ApoA:
-
Apolipoprotein(a)
- APTT:
-
Activated Partial Thrombin Time
- AUC:
-
Area Under the Curve
- CHD:
-
Coronary Heart Disease
- CI:
-
Confidence Interval
- IVSA:
-
Interventricular Septal Amplitude
- LA:
-
Left Atrium
- LAAC:
-
Left Atrial Appendage Closure
- LAD:
-
Left Atrial Diameter
- LAT:
-
Left Atrial Thrombosis
- Lp(a):
-
Lipoprotein(a)
- LVEF:
-
Left Ventricular Ejection Fraction
- NVAF:
-
Non-valvular Atrial Fibrillation
- OR:
-
Odds Ratio
- PSM:
-
Propensity Score Matching
- PT:
-
Prothrombin Time
- RFCA:
-
Radio-Frequency Catheter Ablation
- ROC:
-
Receiver Operating Characteristic
- SEC:
-
Spontaneous Echo Contrast
- TEE:
-
Transesophageal Echocardiography
- TFPI:
-
Tissue Factor Pathway Inhibitors
- TIA:
-
Transient Ischemic Attack
- TTE:
-
Transthoracic Echocardiography
References
Jame S, Barnes G. Stroke and thromboembolism prevention in atrial fibrillation. Heart. 2020;106:10–7.
Kim YG, Choi J-I, Kim M-N, Cho D-H, Oh S-K, Kook H, Park H-S, Lee KN, Baek Y-S, Roh S-Y. Non-vitamin K antagonist oral anticoagulants versus warfarin for the prevention of spontaneous echo-contrast and thrombus in patients with atrial fibrillation or flutter undergoing cardioversion: a trans-esophageal echocardiography study. PLoS One. 2018;13:e0191648.
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan G-A, Dilaveris PE. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498.
Liu X-B, Jia Z-X, Xia S-J, He L, Lu S-X, Guo X-Y, Li S-N, Liu N, Jiang C-X, Sang C-H. The thromboembolism risk of low-risk atrial fibrillation patients with different clinical characteristics. Zhonghua xin xue Guan Bing za zhi. 2020;48:735–9.
Chao T-F, Liu C-J, Wang K-L, Lin Y-J, Chang S-L, Lo L-W, Hu Y-F, Tuan T-C, Chen T-J, Lip GY. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65:635–42.
Hayden DT, Hannon N, Callaly E, D NC, Horgan G, Kyne L, Duggan J, Dolan E, O'Rourke K, Williams D, et al: Rates and Determinants of 5-Year Outcomes After Atrial Fibrillation-Related Stroke: A Population Study. Stroke. 2015; 46:3488-3493.
Ruscica M, Sirtori CR, Corsini A, Watts GF, Sahebkar A. Lipoprotein (a): Knowns, unknowns and uncertainties. Pharmacol Res. 2021;173:105812.
Song J, Zhang X, Wei M, Bo Y, Zhou X, Tang B. Association between lipoprotein (a) and thromboembolism in patients with non-valvular atrial fibrillation: a cross-sectional study. Lipids Health Dis. 2022;21:78.
Igarashi Y, Kasai H, Yamashita F, Sato T, Inuzuka H, Ojima K, Aizawa Y. Lipoprotein (a), left atrial appendage function and thromboembolic risk in patients with chronic nonvalvular atrial fibrillation. Japan Circ J. 2000;64:93–8.
Koca V, Bozat T, Akkaya V, Sarikamis C, Turk T, Vural H, Ozdemir A. Left atrial thrombus detection with multiplane transesophageal echocardiography: an echocardiographic study with surgical verification. J Heart Valve Dis. 1999;8:63–6.
Black IW, Hopkins AP, Lee LC, Walsh WF. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J Am Coll Cardiol. 1991;18:398–404.
Di Minno MND, Ambrosino P, Russo AD, Casella M, Tremoli E, Tondo C. Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. Thromb Haemost. 2016;115:663–77.
Whiteside H, Nagabandi A, Brown K, Ayyala DN, Sharma G. Prevalence and clinical characteristics associated with left atrial thrombus detection: Apixaban. J Am Coll Cardiol. 2019;73:417–417.
Chen J, Zhou M, Wang H, Zheng Z, Rong W, He B, Zhao L. Risk factors for left atrial thrombus or spontaneous echo contrast in non-valvular atrial fibrillation patients with low CHA2DS2-VASc score. J Thromb Thromb. 2022;53:523–31.
Almorad A, Ohanyan A, Bentea GP, Wielandts J-Y, El Haddad M, Lycke M, O’Neill L, Morissens M, De Keyzer E, Nguyen T. D-dimer blood concentrations to exclude left atrial thrombus in patients with atrial fibrillation. Heart. 2021;107:195–200.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–492.
Zhan Y, Joza J, Al Rawahi M, Barbosa RS, Samuel M, Bernier M, Huynh T, Thanassoulis G, Essebag V. Assessment and management of the left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2018;34:252–61.
Lip GY, Skjøth F, Rasmussen LH, Larsen TB. Oral anticoagulation, aspirin, or no therapy in patients with nonvalvular AF with 0 or 1 stroke risk factor based on the CHA2DS2-VASc score. J Am Coll Cardiol. 2015;65:1385–94.
Di Fusco SA, Maggioni AP, Scicchitano P, Zuin M, D’Elia E, Colivicchi F. Lipoprotein (a), Inflammation, and Atherosclerosis. J Clin Med. 2023;12:2529.
Duarte Lau F, Giugliano RP. Lipoprotein(a) and its significance in cardiovascular disease: A review. JAMA Cardiology. 2022;7:760–9.
Di Fusco SA, Arca M, Scicchitano P, Alonzo A, Perone F, Gulizia MM, Gabrielli D, Oliva F, Imperoli G, Colivicchi F. Lipoprotein (a): a risk factor for atherosclerosis and an emerging therapeutic target. Heart. 2023;109:18–25.
Jawi MM, Frohlich J, Chan SY. Lipoprotein(a) the insurgent: A new insight into the structure, function, metabolism, pathogenicity, and medications affecting lipoprotein(a) molecule. J Lipids. 2020;2020:3491764.
Barre D. The molecular nature and consequences of lipoprotein (a)’s association with platelets. Protein Peptide Lett. 2007;14:839–42.
Jang AY, Han SH, Sohn IS, Oh PC, Koh KK. Lipoprotein(a) and cardiovascular diseases - revisited. Circ J 2020;84:867–74.
Vučković B, Đerić M. Lipoprotein (a): a link between thrombogenesis and atherogenesis. Medicinski Pregled. 2007;60:37–41.
Caplice NM, Panetta C, Peterson TE, Kleppe LS, Mueske CS, Kostner GM, Broze GJ Jr, Simari RD. Lipoprotein (a) binds and inactivates tissue factor pathway inhibitor: a novel link between lipoproteins and thrombosis. Blood. 2001;98:2980–7.
Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of lipoprotein (a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13:374–92.
Lu W, Cheng Y-C, Chen K, Wang H, Gerhard GS, Still CD, Chu X, Yang R, Parihar A, O’Connell JR. Evidence for several independent genetic variants affecting lipoprotein (a) cholesterol levels. Hum Mol Genet. 2015;24:2390–400.
Zhang E, Liu T, Li Z, Zhao J, Li G. High CHA2DS2− VASc score predicts left atrial thrombus or spontaneous echo contrast detected by transesophageal echocardiography. Int J Cardiol. 2015;184:540–2.
Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clinical epidemiology. 2014;6:213.
Al Missary AMA. Echocardiographic Predictors of Non Rheumatic Atrial Fibrillation. Diyala J Med. 2011;1:33–6.
Higashiyama A, Kokubo Y, Watanabe M, Nakao YM, Okamura T, Okayama A, Miyamoto Y. Echocardiographic parameters and the risk of incident atrial fibrillation: the Suita study. J Epidemiol. 2020;30:183–7.
Chen YC, Voskoboinik A, La Gerche A, Marwick TH, McMullen JR. Prevention of pathological atrial remodeling and atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol. 2021;77:2846–64.
Lin W-D, Xue Y-M, Liu F-Z, Fang X-H, Zhan X-Z, Liao H-T, Tse G, Wu S-L. Left atrial enlargement and non-paroxysmal atrial fibrillation as risk factors for left atrial thrombus/spontaneous Echo contrast in patients with atrial fibrillation and low CHA2DS2-VASc score. J Geriatr Cardiol. 2020;17:155.
Zhou M, Chen J, Wang H, Xi S, Gan T, Zhao L: Independent risk factors of atrial thrombosis in patients with nonvalvular atrial fibrillation and low CHA 2 DS 2-VASc scores. Nan Fang yi ke da xue xue bao= Journal of Southern Medical University. 2021; 41:1243-1249.
Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, Chun Y-H, Tsuji H, Wada H, Hasegawa K. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. 2016;6:1–8.
Funding
This study was supported by the Nature Science Foundation of China (81873513, 81600574, and 30871042).
Author information
Authors and Affiliations
Contributions
K.K and G.T conceptualized the idea of this study; K.K conducted the data analysis and wrote the manuscript; C.L, T.Z, and W.X helped with the analysis through constructive discussions; and all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was conducted according to the principles expressed in the Declaration of Helsinki. The Research Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University was consulted, and the need for informed consent was waived due to the observational nature of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Kamila Kamili takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kamili, K., Zheng, T., Luo, C. et al. Predictive value of lipoprotein(a) for left atrial thrombus or spontaneous echo contrast in non-valvular atrial fibrillation patients with low CHA2DS2-VASc scores: a cross-sectional study. Lipids Health Dis 23, 22 (2024). https://doi.org/10.1186/s12944-023-01990-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-023-01990-1