Abstract
Background
The relationship between body mass index (BMI) and low-density lipoprotein cholesterol (LDL-C) has not been clearly elucidated in middle-aged and older adults. This study aimed to evaluate the non-linear dose-response relationship between BMI and LDL-C in males and females.
Methods
Data was obtained from two nationally representative surveys in China—the China Health and Nutrition Survey (CHNS, 2009) and China Health and Retirement Longitudinal Study (CHARLS, 2011–2012). To evaluate the sex differences in the association between BMI and LDL-C, the generalized additive models with a smooth function for continuous BMI and smooth-factor interaction for sexes with BMI were used. Segmented regressions were fitted to calculate the slopes with different estimated breakpoints among females and males.
Results
A total of 12,273 participants (47.1% male) aged 45 to 75 years were included. The generalized additive models revealed that a non-linear relationship between BMI and LDL-C level in both sexes after adjustment for age, residence, education levels, marital status, drinking, smoking status, and cohort (CHNS or CHARLS). Slopes of the association between BMI and LDL-C association changed at BMI 20.3 kg/m2 (95% CI: 18.8 to 21.8) in females and 27.1 kg/m2 (95% CI: 25. 8 to 28.4) in males. Below these BMI breakpoints, LDL-C levels increased 1.84 (95% CI: 1.45 to 2.31) in males and 3.49 (95% CI: 1.54 to 5.45) mg/dL per kg/m2 in females. However, LDL-C levels declined − 1.50 (95% CI: − 2.92 to − 0.09) mg/dL per kg/m2 above BMI of 27.1 kg/m2 in males. The non-linear association BMI and LDL-C in males and females was varied by cohort source, age groups, and the number of metabolic syndrome criteria.
Conclusions
In the Chinese middle aged and older adults, the BMI and LDL-C relationship was inverted U-shaped with a high level of LDL-C at a BMI of 27.1 kg/m2 in males, and an approximately linear association was observed in females.
Similar content being viewed by others
Introduction
Globally, higher body mass index (BMI), a reliable overweight and obesity marker, has been a serious public health concern. It is postulated that by 2030, the prevalence of overweight and obesity will be 23 and 32%, respectively [1]. Overweight and obesity affect individuals of all ages, but especially common among the middle-aged adults. High BMI is associated with the occurrence of cardiovascular diseases (CVD) [2, 3]. It has been reported that for every 5 kg/m2 increased in BMI, the average of all-cause mortality and vascular mortality increases by 30 and 40%, respectively [4].
As one of the causes of CVD, metabolic syndrome (MS) is associated with higher BMI and dyslipidemia, which are due to elevated triglycerides (TG) and reduced high-density lipoprotein cholesterol (HDL-C), but without a clear effect on low-density lipoprotein cholesterol (LDL-C) [5]. However, Mendelian randomization and epidemiological studies have suggested that LDL-C is a risk factor for CVD [6,7,8]. Moreover, compared to HDL-C and TG, the concentration of LDL-C and its therapeutic modification are greatly associated with CVD [9,10,11].
Previous studies evaluated the linear relationship between LDL-C and BMI in limited or selected samples, however, data on potential sex differences is limited [12,13,14]. For example, a study performed in the US population suggested that LDL-C linearly associated with BMI [12]. Another cohort study involving US children also indicated that BMI is a strong determinants of lipid and lipoprotein levels [13]. One study reported that LDL-C only increased with greater BMI among younger subjects, but not among male over 50 years [14]. Notably, a recent large population-based study involving individuals form both US and Spain found that there is not always a linear relationship between LDL-C and BMI, that is, age and metabolic status play essential roles and effects on relationship [15]. However, although a visceral fat area was found to be positively correlated with serum LDL-C levels in a non-diabetic Chinese population [16], associations between BMI and LDL-C are yet to be clearly defined. Moreover, since fat distribution differs between the sexes, the effects of sex differences on the above associations should be determined in other ethnicity population.
Therefore, this study was aimed to investigate the non-linear dose-response relationship between BMI and LDL-C and determined whether there are sex differences in a Chinese adult population. We hypothesized that LDL-C levels increased with BMI only in lean individuals and sex-related differences were existed. Our findings will inform on the prevention and treatment of CVD in Asian populations.
Methods
Study participants
This study analyzed two cross-sectional nationally representative datasets from the China Health and Nutrition Survey (CHNS) and the China Health and Retirement Longitudinal Study (CHARLS). Detailed descriptions of these two studies have been published [17, 18]. Briefly, the CHNS is an ongoing open cohort study designed to establish the association between socio-economic factors and health change in China [17]. The CHNS was initially conducted in 1989, while the biomarker data were first collected in 2009. The CHARLS, a nationally representative longitudinal study involving 17,708 adults in 150 counties of 28 provinces in China, was conducted in 2011–12. This study has information on demographic characteristics, medical history, lifestyles, and laboratory data for a set of individuals [18].
The CHARLS was approved by the Biomedical Ethics Review Committee of Peking University, and the CHNS were approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill, the Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. All participants from CHNS and CHARLS provided written informed consents.
In this study, adults aged between 45 and 75 years whose data on blood biomarkers and anthropometric measures were available in both datasets were identified. Participants with missing baseline covariates or those with CVD or cancer were excluded. Finally, a total of 12,273 participants (n = 4788 from CHNS; n = 7485 from CHARLS) were included (Fig. 1).
Data collection
Data on age, sex, residence (urban or rural), education level (< high school or ≥ high school), marital status (married or others), current smoking and drinking status (yes or no), menopause status (yes or no [only available in CHALRS]) and medical history (including hypertension, diabetes, heart disease, stroke, cancer) and drug use (including hypertension medications, diabetes medications, lipid-lowering therapy [only available in CHALRS]) were obtained from the standard questionnaires and harmonized for both datasets. Blood samples were obtained and measured for serum lipids [total cholesterol (TC), TG, LDL-C, HDL-C] and blood glucose. In each survey, height, bodyweight, waist circumference (WC) and blood pressure were measured by trained investigators, according to a standardized protocol. BMI was calculated as weight in kilograms divided by the square of height in meters and categorized into underweight (< 18.5 kg/m2), normal (18.5–23.9 kg/m2), overweight (24.0–27.9 kg/m2), and obese (≥ 28.0 kg/m2). Hypertension was defined as self-reported history of hypertension, systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of anti-hypertensive drugs. Diabetes was defined as self-reported history of diabetes, fasting blood glucose ≥126 mg/dl, or as the use of anti-diabetic therapy. Hypercholesterolemia was defined as total cholesterol >240 mg/dl or use of lipid-lowering therapy. MS was defined based on the definition in “Harmonizing the metabolic syndrome 2009” which included: elevated WC (≥ 85 cm in male, ≥ 80 cm in female); elevated TG (≥ 150 mg dl); low HDL-C (< 40 mg/dl in male and < 50 mg/dl in female); elevated blood pressure (systolic ≥130 and/or diastolic ≥85 mmHg) or current treatment for hypertension; and elevated fasting glucose (fasting glucose ≥100 mg/dl or current treatment for diabetes) [19]. Cardiometabolic health was defined as the number of MS components (excluding WC due to a strong correlation between BMI and WC [r = 0.64, P < 0.001]).
Statistical analysis
Participant characteristics by sexes were separately described in CHARLS and CHNS. Differences between sexes were assessed using the t-test or Mann-Whitney U test for continuous variables, and Pearson’s χ2 test for categorical variables.
To investigate the non-linear association between BMI and LDL-C among females and males, generalized additive models (GAMs) with a factor-smooth interaction between sex (factor) and BMI (smooth function) were fitted. Models were adjusted for age, residence, education levels, marital status, drinking, smoking status, and cohort sources (when pooled analysis). Thus, the multivariable model can be expressed as:
where Yi is the response of LDL-C for individual i; fBMI is the nonparametric smooth function of BMI; βsexxsex is the main effect of the sex variable (0 = females, 1 = males); \( \beta {Cov}_i^T \) is the linear predictor of covariables, and εi is the error terms, which are assumed to be normally distributed. The relationship between BMI and LDL-C was visualized via the GAMs smooth plot by sexes. The association between BMI and other serum lipids (TC, HDL-C, and TG) by sexes was additionally examined as an exploratory analysis.
Then, a multivariable adjusted generalized linear model using linear splines, with break points identified from GAMs smooth plot were separately performed in females and males. Two multivariable models were built: (1) adjusted for age, residence, education levels, marital status, drinking, smoking status, and cohort sources (when pooled analysis); (2) additionally adjusted for hypertension and diabetes. In the CHARLS cohort, use of lipid-lowering (Model 2a) and menopause status in females (Model 2b) were additionally adjusted as a sensitivity analysis.
In addition, to elucidate on age and cardiometabolic heath-based variations in the relationship between BMI and LDL-C in females and males, subgroup analyses were performed stratified by CHNS and CHARLS, age-groups (45–54, 55–64, 65–75 years) and the number of MS criteria (0, 1, 2, 3 or 4 after excluding WC criterion).
Analyses were performed using Stata 16.0 (Stata Corp LLC) and R statistical software (version 4.0.5), with the “mgcv”, “segmented” and “ggplot2” package. Two-tailed P < 0.05 was considered statistically significant.
Results
Baseline characteristics
In the current analysis, 12,273 adults (6493 females and 5780 males) aged 45–75 years were included. The characteristics of study population by sexes in CHARLS and CHNS are shown in Table 1. There were significant differences between females and males with regards to BMI, LDL-C, education level, marital status, smoking, drinking, hypercholesterolemia, hypertension medications, diastolic blood pressure, TC, TG, HDL-C, and count of MS criteria in both cohorts (P < 0.05). The distribution of BMI and LDL-C in females and males in CHARLS and CHNS are displayed in Supplementary Fig. 1. Baseline characteristics, stratified by CHARLS and CHNS, are shown in Supplementary Table 1.
GAMs analysis
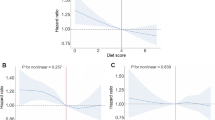
Table 2 shows the estimated regression coefficients from GAMs with factor-smooth interaction terms for sex*BMI. The interactions term sex*BMI, sex, age, residence, education, and cohort were significantly associated with LDL-C in the pooled analysis. Estimated smooth functions plot shows the non-linear association between BMI and LDL-C in females and males (P < 0.001 for smooth terms) (Fig. 2). The non-linear relationship between BMI and serum TC, HDL-C and TG by sexes is also observed (Supplementary Fig. 2).
Threshold analysis
Table 3 shows the findings from generalized linear models using linear splines. In females, BMI was linearly associated with LDL-C below a BMI of 20.33 kg/m2 (slope: 3.45, 95% CI: 1.40 to 5.49), and a gradual increase above beaks point (slope: 0.69, 95% CI: 0.34 to 0.96, P < 0.001 for slope differences) in pooled analysis (Model 1). In males, the association between BMI and LDL-C was inverse U-shaped, with a BMI break point of 27.1 kg/m2 (slope: 1.82, 95% CI: 1.44 to 2.21 for BMI < 27.1 kg/m2; slope: -1.54, 95% CI: − 2.97 to − 0.13 for BMI ≥ 27.1 kg/m2). The estimated beak points and slope between BMI and LDL-C did not significantly change with additionally adjustment for hypertension and diabetes (Model 2). The finding was robustness after adjustment with use of lipid-lowering (Model 2a) and menopause status (Model 2b) in CHARLS (Table 3). The threshold analysis also revealed that there was a cohort differences in the estimated beak points and slope between BMI and LDL-C (Table 3).
Subgroup analysis
The non-linear association between BMI and LDL-C in males and females was varied by cohort source, age groups, and the number of MS criteria (Fig. 3, Fig. 4, and Fig. 5).
Discussion
In this study, we found that in the population with normal or light weight, the association between BMI and LDL-C was linearly and positively correlated, while in the overweight population, with increasing BMI, LDL-C levels gradually tended to be flat or even decreased. The trend of the above association was different between sexes, with an inverted U-shaped association in males.
Evaluating the association between BMI and CVD risk factors (i.e., LDL-C) will help prevent the occurrence and development of diseases while elucidation of the non-linear relationship between them is conducive to quantifying risk levels for people with different BMI levels. Studies have explored the linear or non-linear association between BMI and CVD risk factors, as well as differences in above associations between sexes and age subgroups [20,21,22]. For example, a study based on a Chinese cohort population revealed that high BMI is an indicator for increased risk of arterial stiffening during adulthood in males. However, this association was not established in females [21]. An American study found a U-shaped association between BMI and mortality [22].
The association between BMI and lipid metabolites was previously assessed by HDL-C or TG levels and found to be linear [23, 24], however, the dose-response relationship between BMI and LDL-C has not been conclusively determined. Several studies have reported a positive association between BMI and LDL-C [25,26,27,28], whereas other studies reported contrasting findings [29, 30]. A study performed using two cross-sectional nationally representative data from the U.S. and Spain found an inverted U-shaped association between BMI and LDL-C [15], consistent with a similar study involving with non-diabetic American Indians [30]. In addition, the association between BMI and LDL-C has been shown to differ between sex and age subgroups [28, 31]. Although the above studies revealed the non-linear association between BMI and LDL-C, they are limited to the European and American population, and most of the studies only explored the single sex. In the Chinese population, the role of serum LDL-C in different BMI levels in the elderly population remains unclear [32]. Our findings show the benefits of weight loss for people with different BMI levels in reducing the risk of occurrence of CVD.
We found that the association between BMI and LDL-C exhibits as an inverted U-shape in males, consistent with previous studies involving with U.S. and Spain populations [15, 30]. In non-obese people, TG rich very low-density lipoprotein (VLDL) were converted into cholesterol rich intermediate density lipoprotein and LDL for adipogenesis, leading to a positive correlation between BMI and LDL in lean individuals [15]. However, when lipid deposition in adipose tissue reached the maximum limit or other lipid metabolism disorders, TG may accumulated in VLDL, resulting in a decrease in LDL formation [33]. Another possible explanation is that adipose tissues store large amounts of cholesterol, thus buffering the cholesterol load of the liver [34]. Secretion of bile cholesterol in obese people increases with the accumulation of adipose cells [35]. Therefore, in obese people, normal LDL-C levels may suggest that increased adipocytes are maintaining cholesterol homeostasis. In addition, proprotein convertase subtilisin/kexin type 9 (PCSK9) is positively associated with BMI and LDL-C levels, which confirms the association between BMI and LDL-C [36, 37]. It has been reported that PCSK9 concentrations are correlated with age, gender, MS, and menopausal status, which may lead to alterations in the linear association [38]. Despite these findings, there is no direct evidence of the underlying mechanisms for these associations.
We also evaluated the association between BMI and LDL-C stratified by sexes. The inverted U-shaped associations are more pronounced in males than in females. Most studies on the above-mentioned associations involved in a single gender, especially in males [14, 26, 28, 29] . Moreover, after eliminating the confounding factor of body fat percentage, the associations between physical fitness levels and plasma lipid profiles between male and female genders were found to be different [39]. Sex-specific hormones can lead to sex differences in lipid metabolism [40]. LDL-C levels are correlated with menopausal status, that is, postmenopausal females have higher concentrations of LDL-C [41,42,43]. For example, PCSK9 concentrations were significantly higher in females than in males [38]. Thus, differences in hormonal status between males and females may lead to different associations between BMI and LDL-C. Some studies have shown that BMI and LDL-C exhibit a positive linear relationship in females [31]. Quantitatively, in a previous study, LDL-C levels among females with BMI between 27.1 kg/m2 and 30.0 kg/m2 increased by 17 mg/dl compared to those whose BMI was between 21.1 kg/m2 and 23.0 kg/m2 [31]. We found that LDL-C levels increased with BMI. Moreover, for the first time, we show that there were one turning point in the association between BMI and LDL-C, that is, the slope began to flatten near the edge of obesity, and gradually increased again when BMI reached 27.1 kg/m2. The sex-specific BMI and LDL-C associations may be due genetic vulnerability and hormonal status [24, 28, 29]. Females store more lipids and have higher percent body-fat, less visceral white adipose tissue, and more subcutaneous adipose tissue than males. Furthermore, females have a higher rate of TG synthesis compared to males [30]. Although we postulated that hormonal status and sex-specific effects in lipid metabolism may be responsible for above association, specific mechanisms have not been established.
We confirmed supported that MS status plays a role in association between BMI and LDL-C, that is, with aggravation of metabolic impairment, the turning point of the association curve between BMI and LDL-C gradually appeared earlier. In the extreme group with three or four MS components, the association trend in males was weakened, while it was more unstable in females, in tandem with findings from a previous US population study [15]. We further investigated dose-response association between BMI and LDL-C among males and females, respectively. MS, which are associated with increased BMI, are caused by abnormal functions of the adipose tissue. Another cause may be that, in the regulation of homeostasis mechanism, lipid transport cholesterol may reduce the risk of diabetes and lead to high levels of LDL-C. However, when the homeostatic mechanism breaks, abnormal cholesterol synthesis and transport may also break the linear relationship between BMI and LDL-C.
Comparisons with previous studies
Previous study majorly involved European and American populations, exploring the linear relationship between BMI and LDL-C [15]. This study explored the non-linear association between BMI and LDL-C in the Asian population, and further explored the association between different genders and MS status subgroups. A clear dose-response for Asian populations by reducing body weight and reducing LDL-C levels were estimated.
Study strengths and limitations
This study has several strengths. First, two large population-based nationally representative surveys in China were used to examine the association between BMI and LDL-C levels. Second, we addressed sex differences in the non-linear association between BMI and LDL-C by estimating the factor-smooth interaction between sex (factor) and BMI (smooth function) in the generalized additive models. Third, both datasets used standardized methods to collect exposure, outcome, and confounder. However, there are some limitations. First, this study is a cross-sectional study design, therefore, it did not establish the causal relationship between BMI and LDL-C. Thus, longitudinal studies should be performed to confirm the findings in this study using generalized additive mixed model. Second, the data of obesity-related indices, such as percent body fat and visceral fat area, was not available in the current study. Third, although the association between BMI and LDL-C was reevaluated by a series of sensitivity analyses, residual confounders still existed, such as hormone treatment. Lastly, cardiometabolic health was defined according to the severity of MS in the current analysis. Further prospective cohort studies are warranted to explore the role of cardiometabolic health on the association between BMI and LDL-C among males and females using more accurate quantitative indicators, such as coronary artery calcium score or carotid intima-media thickness.
Conclusions
In people with normal or light weight, the association between BMI and LDL-C is linearly and positively correlated in both sexes, in the overweight people, with increasing BMI, the level of LDL-C tends to be flat or decreased in males. Future studies are warranted to determine the longitudinal association between BMI and LDL-C level.
Availability of data and materials
The original datasets are publicly available for CHARLS (http://charls.pku.edu.cn/) and CHNS (https://www.cpc.unc.edu/projects/china).
References
Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–7. https://doi.org/10.1038/ijo.2008.102.
Kivimaki M, Kuosma E, Ferrie JE, Luukkonen R, Nyberg ST, Alfredsson L, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2(6):e277–85. https://doi.org/10.1016/S2468-2667(17)30074-9.
Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, et al. Association of Body Mass Index with Lifetime Risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280–7. https://doi.org/10.1001/jamacardio.2018.0022.
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083–96. https://doi.org/10.1016/S0140-6736(09)60318-4.
Grundy SM. Atherogenic dyslipidemia associated with metabolic syndrome and insulin resistance. Clin Cornerstone. 2006;8(Suppl 1):S21–7. https://doi.org/10.1016/S1098-3597(06)80005-0.
Verbeek R, Hoogeveen RM, Langsted A, Stiekema LCA, Verweij SL, Hovingh GK, et al. Cardiovascular disease risk associated with elevated lipoprotein(a) attenuates at low low-density lipoprotein cholesterol levels in a primary prevention setting. Eur Heart J. 2018;39(27):2589–96. https://doi.org/10.1093/eurheartj/ehy334.
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013;40(1):195–211. https://doi.org/10.1016/j.pop.2012.11.003.
Abdullah SM, Defina LF, Leonard D, Barlow CE, Radford NB, Willis BL, et al. Long-term Association of low-Density Lipoprotein Cholesterol with Cardiovascular Mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation. 2018;138(21):2315–25. https://doi.org/10.1161/CIRCULATIONAHA.118.034273.
Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial Mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552–61. https://doi.org/10.1016/j.jacc.2015.02.020.
Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375(9726):1634–9. https://doi.org/10.1016/S0140-6736(10)60545-4.
Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J. 2015;36(9):539–50. https://doi.org/10.1093/eurheartj/eht571.
McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism. 2004;53(4):495–9. https://doi.org/10.1016/j.metabol.2003.10.032.
DeStefano F, Berg RL, Griese GG Jr. Determinants of serum lipid and lipoprotein concentrations in children. Epidemiology. 1995;6(4):446–9. https://doi.org/10.1097/00001648-199507000-00024.
Maki KC, Kritsch K, Foley S, Soneru I, Davidson MH. Age-dependence of the relationship between adiposity and serum low density lipoprotein cholesterol in men. J Am Coll Nutr. 1997;16(6):578–83.
Laclaustra M, Lopez-Garcia E, Civeira F, Garcia-Esquinas E, Graciani A, Guallar-Castillon P, et al. LDL cholesterol rises with BMI only in lean individuals: cross-sectional U.S. and Spanish representative data. Diabetes Care. 2018;41(10):2195–201. https://doi.org/10.2337/dc18-0372.
Luo Y, Ma X, Shen Y, Hao Y, Hu Y, Xiao Y, et al. Positive relationship between serum low-density lipoprotein cholesterol levels and visceral fat in a Chinese nondiabetic population. PLoS One. 2014;9(11):e112715. https://doi.org/10.1371/journal.pone.0112715.
Popkin BM, Du S, Zhai F, Zhang B. Cohort profile: the China health and nutrition survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol. 2010;39(6):1435–40. https://doi.org/10.1093/ije/dyp322.
Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. 2014;43(1):61–8. https://doi.org/10.1093/ije/dys203.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; world heart federation; international atherosclerosis society; and International Association for the Study of obesity. Circulation. 2009;120(16):1640–5. https://doi.org/10.1161/CIRCULATIONAHA.109.192644.
Zhang L, Li JL, Zhang LL, Guo LL, Li H, Li D. Association and interaction analysis of body mass index and triglycerides level with blood pressure in elderly individuals in China. Biomed Res Int. 2018;2018:8934534–1. https://doi.org/10.1155/2018/8934534.
Liao YY, Chu C, Wang Y, Zheng WL, Ma Q, Hu JW, et al. Sex differences in impact of long-term burden and trends of body mass index and blood pressure from childhood to adulthood on arterial stiffness in adults: a 30-year cohort study. Atherosclerosis. 2020;313:118–25. https://doi.org/10.1016/j.atherosclerosis.2020.10.003.
Wang Z, Dong B, Hu J, Adegbija O, Arnold LW. Exploring the non-linear association between BMI and mortality in adults with and without diabetes: the US National Health Interview Survey. Diabet Med. 2016;33(12):1691–9. https://doi.org/10.1111/dme.13111.
Stępień A, Stępień M, Wlazeł RN, Paradowski M, Banach M, Rysz J. Assessment of the relationship between lipid parameters and obesity indices in non-diabetic obese patients: a preliminary report. Med Sci Monit. 2014;20:2683–8. https://doi.org/10.12659/MSM.890845.
Shamai L, Lurix E, Shen M, Novaro GM, Szomstein S, Rosenthal R, et al. Association of body mass index and lipid profiles: evaluation of a broad spectrum of body mass index patients including the morbidly obese. Obes Surg. 2011;21(1):42–7. https://doi.org/10.1007/s11695-010-0170-7.
Terry RB, Wood PD, Haskell WL, Stefanick ML, Krauss RM. Regional adiposity patterns in relation to lipids, lipoprotein cholesterol, and lipoprotein subfraction mass in men. J Clin Endocrinol Metab. 1989;68(1):191–9. https://doi.org/10.1210/jcem-68-1-191.
Seidell JC, Cigolini M, Deslypere JP, Charzewska J, Ellsinger BM, Cruz A. Body fat distribution in relation to serum lipids and blood pressure in 38-year-old European men: the European fat distribution study. Atherosclerosis. 1991;86(2-3):251–60. https://doi.org/10.1016/0021-9150(91)90221-N.
Beckles GL, Miller GJ, Alexis SD, Price SG, Kirkwood BR, Carson DC, et al. Obesity in women in an urban Trinidadian community. Prevalence and associated characteristics. Int J Obes. 1985;9:127–35.
Denke MA, Sempos CT, Grundy SM. Excess body weight. An underrecognized contributor to high blood cholesterol levels in white American men. Arch Intern Med. 1993;153(9):1093–103. https://doi.org/10.1001/archinte.1993.00410090045006.
Zamboni M, Armellini F, Cominacini L, Turcato E, Todesco T, Bissoli L, et al. Obesity and regional body-fat distribution in men: separate and joint relationships to glucose tolerance and plasma lipoproteins. Am J Clin Nutr. 1994;60(5):682–7. https://doi.org/10.1093/ajcn/60.5.682.
Hu D, Hannah J, Gray RS, Jablonski KA, Henderson JA, Robbins DC, et al. Effects of obesity and body fat distribution on lipids and lipoproteins in nondiabetic American Indians: the strong heart study. Obes Res. 2000;8(6):411–21. https://doi.org/10.1038/oby.2000.51.
Denke MA, Sempos CT, Grundy SM. Excess body weight. An under-recognized contributor to dyslipidemia in white American women. Arch Intern Med. 1994;154(4):401–10. https://doi.org/10.1001/archinte.1994.00420040061010.
Li J, Cao Y, Xiao C. Subgroup analysis of the influence of body mass index on the association between serum lipids and cognitive function in Chinese population. Lipids Health Dis. 2020;19(1):130. https://doi.org/10.1186/s12944-020-01314-7.
Laclaustra M, Corella D, Ordovas JM. Metabolic syndrome pathophysiology: the role of adipose tissue. Nutr Metab Cardiovasc Dis. 2007;17(2):125–39. https://doi.org/10.1016/j.numecd.2006.10.005.
Le Lay S, Ferré P, Dugail I. Adipocyte cholesterol balance in obesity. Biochem Soc Trans. 2004;32(1):103–6. https://doi.org/10.1042/bst0320103.
Grundy SM, Barnett JP. Metabolic and health complications of obesity. Dis Mon. 1990;36:641–731.
Mba CM, Mbacham W, Sobngwi E, Mbanya JC. Is PCSK9 associated with plasma lipid levels in a sub-Saharan African population of patients with obesity and type 2 diabetes? Diabetes Metab Syndr Obes. 2019;12:2791–7. https://doi.org/10.2147/DMSO.S234243.
Zenti MG, Lupo MG, De Martin S, Altomari A, Galvan S, Aventaggiato M, et al. Impact of bariatric surgery-induced weight loss on circulating PCSK9 levels in obese patients. Nutr Metab Cardiovasc Dis. 2020;30(12):2372–8. https://doi.org/10.1016/j.numecd.2020.07.013.
Jeenduang N. Circulating PCSK9 concentrations are increased in postmenopausal women with the metabolic syndrome. Clin Chim Acta. 2019;494:151–6. https://doi.org/10.1016/j.cca.2019.04.067.
Tanaka HYM, Tanaka M, Sasaki J, Shindo M. Abnormally lower HDL-cholesterol levels in relation to physical fitness. Circulation. 1989;80:511.
Neufang S, Specht K, Hausmann M, Güntürkün O, Herpertz-Dahlmann B, Fink GR, et al. Sex differences and the impact of steroid hormones on the developing human brain. Cereb Cortex. 2009;19(2):464–73. https://doi.org/10.1093/cercor/bhn100.
Perry AC, Applegate EB, Allison ML, Miller PC, Signorile JF. Relation between anthropometric measures of fat distribution and cardiovascular risk factors in overweight pre- and postmenopausal women. Am J Clin Nutr. 1997;66(4):829–36. https://doi.org/10.1093/ajcn/66.4.829.
Gordon T, Kannel WB, Hjortland MC, McNamara PM. Menopause and coronary heart disease. The Framingham Study. Ann Intern Med. 1978;89(2):157–61. https://doi.org/10.7326/0003-4819-89-2-157.
Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–6. https://doi.org/10.1056/NEJM198909073211004.
Acknowledgements
We thank the CHNS and CHARLS research team and field team for collecting the data and making the data publicly accessible.
This analysis uses data or information from the Harmonized CHARLS data set and Codebook, Version C as of April 2018 developed by the Gateway to Global Aging Data. The development of the Harmonized CHARLS was funded by the National Institute on Ageing (R01 AG030153, RC2 AG036619, and R03 AG043052). For more information, please refer to www.g2aging.org.
This research uses data from China Health and Nutrition Survey (CHNS). We thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention, Carolina Population Center (P2C HD050924,T32 HD007168), the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, R24 HD050924 and R01-HD38700) and the NIH Fogarty International Center (D43 TW009077, D43 TW007709) for financial support for the CHNS data collection and analysis files from 1989 to 2015 and future surveys, and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009 and Beijing Municipal Center for Disease Prevention and Control since 2011.
We additionally thank for Home for Researchers (www.home-for-researchers.com) polishing the language.
Funding
This study was supported by the National Natural Science Foundation of China (8210120410), Golden Seed Program of Beijing Chaoyang Hospital (CYJZ202101) and Scientific Research Seed Fund of Peking University First Hospital (2021SF36).
Author information
Authors and Affiliations
Contributions
HBL contributed to the study planning and design, data collection and manuscript writing, revision, and interpretation. JHM contributed to the manuscript writing, revision, and interpretation. DQZ, XL, and XHG contributed to the data analysis, revision, and interpretation. WY and CDL contributed to the study planning, manuscript revision and interpretation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The CHARLS was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052–11015), and the CHNS were approved by the Institutional Review Boards at the University of North Carolina at Chapel Hill, the Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention. Written informed consents were obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Baseline Characteristics by CHARLS and CHNS. Figure S1. The distribution of BMI and LDL-C levels by sexes and cohort. Figure S2. The association of BMI and TC, HDL-C, and TG levels by sexes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Ma, J., Zheng, D. et al. Sex differences in the non-linear association between BMI and LDL cholesterol in middle-aged and older adults: findings from two nationally representative surveys in China. Lipids Health Dis 20, 162 (2021). https://doi.org/10.1186/s12944-021-01591-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-021-01591-w