Abstract
Background
Depression, metabolic disturbances and inflammation have been linked to cardiovascular disease and mortality. Low levels of high-density lipoprotein cholesterol (HDL-cholesterol), a known marker of cardiovascular risk, have been observed in patients with major depression in psychiatric populations. Our main aim was to explore associations between depression, antidepressants, and metabolic and inflammatory variables in patients with type 1 diabetes (T1D). A secondary aim was to explore variables associated with HDL-cholesterol.
Methods
Cross-sectional design. T1D patients (n = 292, men 55%, age18–59 years, diabetes duration ≥1 year) were consecutively recruited from one specialist diabetes clinic. Depression was defined as ≥8 points for Hospital Anxiety and Depression Scale-Depression sub scale. Blood samples, anthropometrics, blood pressure, and data regarding medication and life style were collected from electronic health records. Non-parametric tests, multiple logistic and linear regression analyses were performed.
Results
The depression prevalence was 10 and 8% used antidepressants. Median (q1, q3) HDL-cholesterol (mmol/l) was for the depressed 1.3 (1.2, 1.5) and for the non-depressed 1.6 (1.3, 1.8), p = 0.001. HDL-cholesterol levels (per mmol/l) were negatively associated with depression (Adjusted odds ratio (AOR) 0.2, p = 0.007), and the use of antidepressants was positively associated with depression (AOR 8.1, p < 0.001). No other metabolic or inflammatory variables, or life style factors, were associated with depression when adjusted for antidepressants. Abdominal obesity was associated with antidepressants in women (AOR 4.6, p = 0.029). Decreasing HDL-cholesterol levels were associated with increasing triglyceride levels (p < 0.001), increasing high-sensitive C-reactive protein (hs-CRP) levels (p = 0.021), younger age (p < 0.001), male sex (p < 0.001), and depression (p = 0.045).
Conclusions
Lower HDL-cholesterol levels, known predictors of cardiovascular disease, were associated with depression in patients with T1D. The use of antidepressants was associated with abdominal obesity in women. Depression, low-grade inflammation measured as hs-CRP, higher triglycerides, male sex, and lower age were independently associated with lower HDL-cholesterol levels.
Similar content being viewed by others
Background
Depression is a disease with several somatic consequences both in persons with or without diabetes, such as metabolic changes, disturbances of the corticotropin releasing hormone (CRH) system, immuno-inflammatory changes, and increased cardiovascular and all-cause mortality [1,2,3,4,5,6]. In diabetes, depression is associated with increased prevalence of all diabetes complications [7]. Depression is, however, a heterogeneous disease where melancholic depression is accompanied by an activated CRH system, anxiety, loss of appetite, weight loss and insomnia, whereas atypical depression is accompanied by CRH deficiency, increased appetite, weight gain, lethargy, and hypersomnia [2]. The metabolic syndrome and inflammatory up-regulations have been linked to atypical depression [1].
LDL-cholesterol is positively, and HDL-cholesterol is inversely associated with cardiovascular disease [8,9,10,11,12]. Previous research support that high levels of LDL-cholesterol are causal in the development of cardiovascular disease [9, 11]. Low levels of HDL-cholesterol are strong predictors of atherosclerosis, cardiovascular disease and mortality [10, 12, 13]. The causal relation between HDL-cholesterol and atherosclerosis is, however, uncertain [10]. A genetic study using mendelian randomisation showed that increased plasma HDL-cholesterol levels did not lower the risk for infarction [8]. In inflammatory states it has been shown that HDL-cholesterol levels are low [14], which is important as inflammatory processes are implicated in cardiovascular disease [15]. High-sensitive C-reactive protein (hs-CRP) is a marker of inflammation that has been shown to predict incident cardiovascular disease and sudden cardiac death [15]. Inadequate glycemic control in type 1 diabetes (T1D) is associated with increased all-cause and cardiovascular mortality [16].
In psychiatric populations, major depression has been linked to lower levels of HDL-cholesterol [1, 17, 18], and low levels of HDL-cholesterol have been observed in persons who had performed suicide attempts [17, 19]. The opposite state, i.e. high levels of psychological well-being, predicted high levels of HDL-cholesterol and low levels of triglycerides in a longitudinal study [20]. HDL-cholesterol is usually high in patients with T1D [21].
In this cohort of patients with T1D we have previously found that depression was associated with anxiety [22], inadequate glycaemic control [23], high midnight cortisol secretion [22, 24], and with galectin-3 [25], an inflammatory biomarker which both predicts heart failure and contributes to cardiac dysfunction [26, 27]. Depression was not associated with abdominal obesity [28]. The increased levels of anxiety, increased midnight cortisol secretion, and lack of obesity in the depressed patients with T1D, indicate traits of melancholic depression [2, 22, 24, 28]. We have also shown that abdominal obesity and high LDL-cholesterol, but not HDL-cholesterol, were associated with cardiovascular complications [29]. Previous analyses in these patients with T1D, showed that the use of antidepressants was associated with abdominal obesity in the women with T1D [28], and was associated with soluble (s) CD163 [30], a biomarker involved in inflammatory processes linked to diabetic retinopathy [31].
We hypothesise that depression and/or the use of antidepressant medication might have impact on metabolic and/or inflammatory variables in patients with T1D. The main aim was to explore associations between depression, antidepressants, and metabolic and inflammatory variables in adult patients with T1D. The secondary aim was to explore variables associated with HDL-cholesterol.
Methods
Participants and setting
This study has a cross sectional design and was performed at baseline of a randomized controlled trial (ClinicalTrials.gov: NCT01714986) where Affect School with Script Analysis was tried against Basic Body Awareness Therapy for persons with diabetes, inadequate glycaemic control and psychological symptoms [32]. The patients were consecutively recruited by specialist diabetes physicians or diabetes nurses, at regular follow up visits during the period 03/25/2009 to 12/28/2009 from one secondary care hospital specialist diabetes outpatient clinic, with a catchment population of 125,000 in southern Sweden. Inclusion criteria were T1D, age 18–59 years, and diabetes duration ≥1 year. Exclusion criteria were cancer, hepatic failure, end-stage renal disease, stroke with cognitive deficiency, psychotic disorder, bipolar disorder, severe personality disorder, severe substance abuse, mental retardation or inadequate knowledge of Swedish. Two hundred and ninety-two participants, 67% of the eligible patients, provided written informed consent [23]. A questionnaire was used to assess self-reported depression. Blood samples, anthropometrics and blood pressure (BP) were collected. Data were collected from electronic health records and the Swedish National Diabetes Register (S-NDR) [16].
Self-reported depression
Self-reported depression was assessed by the Hospital Anxiety and Depression Scale-Depression subscale (HADS-D) which consists of 7 statements, with 4 response alternatives from 0 to 3. Depression was defined as HADS-D ≥ 8 points [33].
HbA1c and serum lipids
After an overnight fast, blood samples were collected. HbA1c and serum lipids were analysed with a Olympus AU clinical chemistry analyser with high specificity (Olympus AU®, Tokyo, Japan) [34]. The intra-coefficients of variation were for HbA1c < 1.2%; total cholesterol < 2.1%; HDL-cholesterol < 3.0%; LDL-cholesterol < 2,6%; and for triglycerides < 2.2%.
Serum-lipids were measured directly [35]. High HbA1c, defined as > 70 mmol/mol, correspond to the 75th percentile in these patients [23]. The analyses were performed at the department of Clinical Chemistry, Växjö Central Hospital.
Low-grade inflammation
Hs-CRP samples were collected, centrifugated, and stored at − 70 degrees Celsius until analysed with spectrophotometry on a Roche Cobas C501 instrument at the Diabetes Laboratory, Biomedical Center, Lund University, Lund [29]. The intra-coefficient of variation for the hs-CRP analysis was 1.8–3.3%.
Samples with hs-CRP ≥10 mg/l were excluded as recommended in previous research [15]. Samples stored > 1 year were not included. There were 117 missing hs-CRP measurements. A response analysis was previously performed [29]. It showed that the prevalence of abdominal obesity was lower in the 175 patients with hs-CRP measurements than in the 117 patients with missing hs-CRP measurements. Otherwise they did not differ by medians of age, diabetes duration, systolic blood pressure (SBP) or diastolic blood pressure (DBP), serum-lipids; or by prevalence of high HbA1c (> 70 mmol/mol), antihypertensive drugs (AHD), lipid lowering drugs (LLD), physical inactivity, smoking habits, or cardiovascular complications.
Anthropometrics and blood pressure
Waist circumference (WC), weight, length and BP were measured according to standard procedures by a nurse. Abdominal obesity was defined as WC ≥1.02 m for men, and as WC ≥0.88 m for women [36]. BMI (kg/m2) was calculated and was divided into four weight classes: obesity (≥30), overweight (25 to < 30), normal weight (18.5 to < 25), and underweight (< 18.5) [37].
Clinical psychiatric diagnoses
Clinical psychiatric diagnoses made prior to recruitment were collected from the medical records and were dichotomized as having or not having a clinical psychiatric diagnosis. Patients with single or recurrent episodes of depression, anxiety, stress related syndromes, and former alcohol substance abuse, but under control at the time for inclusion, were included.
Episodes of hypoglycemia
A severe episode of hypoglycemia was defined as needing help from another person. Episodes during the last 6 months prior to recruitment were registered.
Smoking and physical activity
Smokers were defined as having smoked any amount of tobacco during the last year.
Physical activity, which was defined as at least 30 min of moderate activities, was divided into four levels performed weekly: less than once, 1–2 times, 3–5 times, > 5 times, as registered in the S-NDR [16]. Physical activity was also dichotomized into physical inactivity, which was defined as less than 30 min of moderate activities once a week, and physical activity which represents all other levels of physical activity.
Medication
Antidepressants were SSRIs, SNRIs and/or specific serotonergic antidepressants (N06AB, N06AX16, N06AX11).
LLD were HMG CoA-reductase inhibitors (statins) (C10AA). Indications for LLD were total cholesterol > 4.5 mmol/l (> 1.74 mg/dl) and/or LDL-cholesterol > 2.5 mmol/l (> 97 mg/dl) according to the Swedish national guidelines in 2009 [38].
AHD were calcium antagonists (ATC codes C08CA01–02); angiotensin-converting enzyme (ACE) inhibitors (ATC codes C09AA-BA); angiotensin II antagonists (ATC codes C09CA-DA); diuretics (ATC code C03A); selective beta-adrenoreceptor antagonists (ATC code C07AB). Indications for AHD were SBP > 130 mmHg and/or DBP > 80 mmHg according to the Swedish national guidelines in 2009 [38].
Diabetes specific treatment was divided into two groups: Insulin only (multiple daily insulin injections or continuous subcutaneous insulin infusion), or insulin combined with oral antidiabetic drugs (OAD) (ATC code A10BA02). The indications for OAD prescription in addition to insulin were obesity and insulin resistance.
Statistical analysis
Analysis of data distribution using histograms revealed that age, diabetes duration, hs-CRP, triglycerides, BMI, and WC were not normally distributed and non-parametric analyses were performed. The Mann-Whitney U test was used to compare median values, which were presented as median (quartile (q)1, q3; min-max). Fisher’s exact test and Linear-by-Linear Association (both two-tailed) were used to analyse categorical data.
Crude odds ratios (CORs) for two dependent variables, depression and the use of antidepressants, were calculated by using simple logistic regression analyses. Variables with p-values ≤0.10 for the CORs, and gender and age independent of p-value, were entered into multiple logistic regression analyses (Backward: Wald) [23,24,25, 28,29,30, 39], with depression and use of antidepressants as dependent variables. These analyses were performed for all and gender specified. The Hosmer and Lemeshow test for goodness-of-fit and Nagelkerke R2 were used to evaluate each multiple logistic regression analysis model.
Simple linear regression analyses were performed with HDL-cholesterol as dependent variable. Variables with p-values ≤0.10 were entered in multiple linear regression analysis (Backward) [29, 40], with HDL-cholesterol as dependent variable.
Confidence intervals (CIs) of 95% were used. p < 0.05 was considered statistically significant. SPSS® version 18 (IBM, Chicago, Illinois, USA) was used for all statistical analyses.
Results
Baseline data, comparisons between depressed and non-depressed, and between users and non-users of antidepressants
In this cross-sectional study of patients with T1D (n = 292, men 55%, age 18–59 years), 30 depressed patients were compared to 262 non-depressed patients, and 23 users of antidepressants were compared to 269 non-users (Table 1).
The median HDL-cholesterol was lower in the depressed than in the non-depressed patients (p = 0.011). The prevalence of high HbA1c (> 70 mmol/mol) (P = 0.017), use of antidepressants (p < 0.001), and cardiovascular complications (p = 0.012) were higher in the depressed than in the non-depressed. The prevalence of abdominal obesity (p = 0.037), high HbA1c (> 70 mmol/mol) (p = 0.029), and cardiovascular complications (p < 0.001) were higher in the users of antidepressants.
Gender sub analyses
In the 16 depressed men median (q1, q3) HDL-cholesterol (mmol/l) was 1.4 (1.1, 1.6) and in the 146 non-depressed men 1.5 (1.2, 1.7), p = 0.20. The prevalence of antidepressants was 5 (31%) in the depressed men, and 6 (4%) in the 146 non-depressed men, p = 0.002. Medians did not differ between the depressed and the non-depressed men for total cholesterol, triglycerides, LDL-cholesterol, WC, BMI, hs-CRP, SBP or DBP, all p-values ≥0.33. The prevalence did not differ between the depressed and the non-depressed men for high HbA1c, the use of LLD or AHD, all p-values ≥0.54. Eleven men used antidepressants. The prevalence of abdominal obesity was in the 11 men using antidepressants 1 (9%) compared to 12 (8%) in the 148 non-users, p > 0.99.
In the 14 depressed women median (q1, q3) HDL-cholesterol (mmol/l) was 1.3 (1.3, 1.6), and in the 116 non-depressed women 1.6 (1.4, 1.9), p = 0.008. The prevalence of high HbA1c was 9 (64%) in the depressed women, and 32 (28%) in the non-depressed, p = 0.012. The prevalence of use of antidepressants was 5 (36%) in the depressed women, and 7 (6%) in the non-depressed, p = 0.004. Medians did not differ between depressed and non-depressed women for total cholesterol, triglycerides, LDL-cholesterol, WC, BMI, hs-CRP, SBP or DBP, all p-values ≥0.19. The prevalence did not differ between depressed and non-depressed women regarding the use of LLD or AHD, both p -values ≥0.53. The prevalence of abdominal obesity was 7 (58%) in the 12 women using antidepressants, and 29 (25%) in the 115 non-users, p = 0.037.
Associations with depression
HDL-cholesterol levels were negatively associated with depression (per mmol/l) (AOR 0.2, p = 0.007) (Table 2).
High HbA1c was associated with depression when the use of antidepressants was not included (AOR 2.5), but not when the use of antidepressants was included (AOR 2.1). Higher age was associated with depression (per year) (AOR 1.05). Depression and the use of antidepressants were associated for both men (AOR 10.6) and women (AOR 5.8) (Table 2).
Association with the use of antidepressants
In women the association between abdominal obesity and antidepressants was significant (AOR 4.6) (Table 3).
Associations with HDL-cholesterol
Depression (p = 0.045), triglycerides (p = < 0.001) and hs-CRP (p = 0.021) were negatively associated with HDL-cholesterol levels, whereas female sex (p < 0.001), age (p = 0.005) and total cholesterol (p < 0.001) were positively associated with HDL-cholesterol levels in 171 patients (Table 4).
Discussion
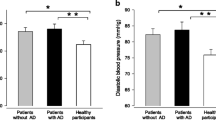
The main findings of this study of 292 T1D patients were that self-reported depression was associated with lower HDL-cholesterol levels, and that the use of antidepressants was associated with abdominal obesity in the women (Fig. 1). Depression, low-grade inflammation, higher triglycerides, male sex, and lower age were independently associated with lower HDL-cholesterol levels.
Lower HDL-cholesterol levels were clearly associated with depression in this study. To our knowledge, we are the first to demonstrate an independent association between low HDL-cholesterol and depression in T1D patients. This is intriguing as HDL-cholesterol levels generally are high in patients with T1D [21]. The association between low HDL-cholesterol levels and depression is however in concordance with results in psychiatric populations [1, 17,18,19]. Lower HDL-cholesterol in depressed patients with T1D may be an important finding as low HDL-cholesterol is a strong predictor of atherosclerosis, cardiovascular disease and mortality [10, 12, 13]. Nevertheless, important evidence from a genetic study using mendelian randomization showed that low HDL-cholesterol levels were not the cause of cardiovascular disease [8]. The depressive state with associated disturbances of the CRF system, metabolic and immuno-inflammatory changes, seems to be an important trigger to increased cardiovascular disease and mortality [16, 26, 27, 41, 42]. HDL-cholesterol levels did not differ between users and non-users of antidepressants medication, which is in accordance with previous research [17]. We found that depression and low-grade inflammation were independently associated with low HDL-levels. Low-grade inflammation has previously been associated with abdominal obesity, cardiovascular disease and mortality [4, 15, 29]. We did not, however, find any association between low-grade inflammation measured as hs-CRP and depression, which is in accordance with previous research where the results were adjusted for BMI [43].
Strengths of the study are that we systematically explored the associations between depression, antidepressant medication, metabolic and inflammatory variables, life style factors, and medication targeting hypertension, dyslipidemia, obesity and insulin resistance. We also systematically explored gender differences. The association between low levels of HDL-cholesterol and depression was strong, despite the limited number of depressed patients. One weakness was that self-reported depression is not equivalent with a clinical diagnosis. We therefore explored the associations between self-reported depression and clinical psychiatric diagnoses, and the use of antidepressants. We found that both these associations were strong. Another weakness was the limited number of patients using antidepressants, particularly when gender sub analyses were performed.
This study does not provide any explanation to why low HDL-cholesterol levels were associated with depression. In previous research we found no association between the depression associated galectin-3 and HDL-cholesterol [25]. With this new finding of lower HDL-cholesterol levels linked to depression in T1D patients, we have altogether demonstrated four biological links to depression, increased midnight cortisol secretion, impaired glycemic control, increased galectin-3 levels, and decreased HDL-cholesterol levels [22,23,24,25]. These factors have in in previous research been described as either predictors of risk, or causal in the development of diabetes complications, cardiovascular and all-cause mortality [10, 12, 13, 16, 26, 27, 41, 42].
We suggest in future research of the impact of HDL-cholesterol levels on cardiovascular disease, that depression should be included in the analyses, which has to our knowledge not been done systematically previously. We also suggest comparisons between different types of depression, for example atypical and melancholic depression [2]. It would also be of interest to study whether HDL-cholesterol levels increase after recovery from depression. The mechanisms behind the negative association between depression and HDL is another subject for exploration. We also suggest surveys of this subject in patients with the metabolic syndrome or type 2 diabetes (T2D), and in larger samples of T1D patients.
As inflammatory processes previously have been demonstrated in depression [1, 3, 5, 25], and as HDL-cholesterol levels have recently been shown to decrease with inflammation [14], we plan further exploration of inflammatory markers such as proinflammatory cytokines in patients with T1D and depression [5]. We also advocate comparative research on antidepressants regarding their impact on metabolism, immunology, the CRH system, diabetes complications, and mortality, considering potential gender differences.
Conclusions
Lower HDL-cholesterol levels, known predictors of cardiovascular disease, were associated with depression in patients with T1D. Abdominal obesity was associated with the use of antidepressants in women. Depression, low-grade inflammation measured as hs-CRP, higher triglycerides, male sex, and lower age were independently associated with lower HDL-cholesterol levels. The detrimental effects previously linked to low HDL-cholesterol levels might be caused by metabolic, endocrinological or immunological changes involved in the depressive state, or in inflammatory disorders.
Abbreviations
- AHD:
-
Antihypertensive drugs
- AOR:
-
Adjusted odds ratio
- BMI:
-
Body Mass Index
- CI:
-
Confidence interval
- COR:
-
Crude odds ratio
- DBP:
-
Diastolic blood pressure
- HADS-D:
-
Hospital Anxiety and Depression-Depression subscale
- HDL-cholesterol:
-
High-density lipoprotein cholesterol
- Hs-CRP:
-
High-sensitivity C-reactive protein
- LDL-cholesterol:
-
Low-density lipoprotein-cholesterol
- LLD:
-
Lipid lowering drugs
- OAD:
-
Oral antidiabetic drugs
- SBP:
-
Systolic blood pressure
- S-NDR:
-
Swedish National Diabetes Registry
- T1D:
-
Type 1 diabetes
- T2D:
-
Type 2 diabetes
- WC:
-
Waist circumference
References
Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129.
Gold P, Chrousos G. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–75.
Korczak DJ, Pereira S, Koulajian K, Matejcek A, Giacca A. Type 1 diabetes mellitus and major depressive disorder: evidence for a biological link. Diabetologia. 2011;54:2483–93.
Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28:1339–45.
Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31.
Enko D, Brandmayr W, Halwachs-Baumann G, Schnedl WJ, Meinitzer A, Kriegshäuser G. Prospective plasma lipid profiling in individuals with and without depression. Lipids Health Dis. 2018;17:149.
de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30.
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–80.
Trialists CT. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78.
Rader DJ, Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–25.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–72.
Pease A, Earnest A, Ranasinha S, Nanayakkara N, Liew D, Wischer N, et al. Burden of cardiovascular risk factors and disease among patients with type 1 diabetes: results of the Australian National Diabetes Audit (ANDA). Cardiovasc Diabetol. 2018;17:77.
Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. Arterioscler Thromb Vasc Biol. 1997;17:107–13.
Feingold KR, Grunfeld C. Effect of inflammation on HDL structure and function. Curr Opin Lipidol. 2016;27:521–30.
Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9.
Lind M, Svensson A-M, Kosiborod M, Gudbjörnsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–82.
Maes M, Smith R, Christophe A, Vandoolaeghe E, Gastel AV, Neels H, et al. Lower serum high-density lipoprotein cholesterol (HDL-C) in major depression and in depressed men with serious suicidal attempts: relationship with immune-inflammatory markers. Acta Psychiatr Scand. 1997;95:212–21.
Lehto SM, Hintikka J, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, et al. Low HDL cholesterol associates with major depression in a sample with a 7-year history of depressive symptoms. Prog Neuro-Psychopharmacol Biol Psychiatry. 2008;32:1557–61.
Zhang J, McKeown RE, Hussey JR, Thompson SJ, Woods JR, Ainsworth BE. Low HDL cholesterol is associated with suicide attempt among young healthy women: the third National Health and nutrition examination survey. J Affect Disord. 2005;89:25–33.
Radler BT, Rigotti A, Ryff CD. Persistently high psychological well-being predicts better HDL cholesterol and triglyceride levels: findings from the midlife in the U.S. (MIDUS) longitudinal study. Lipids Health Dis. 2018;17(1).
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia. 2013;56:1462–70.
Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO. Depression differed by midnight cortisol secretion, alexithymia and anxiety between diabetes types: a cross sectional comparison. BMC Psychiatry. 2017;17:335.
Melin EO, Thunander M, Svensson R, Landin-Olsson M, Thulesius HO. Depression, obesity and smoking were independently associated with inadequate glycemic control in patients with type 1 diabetes. Eur J Endocrinol. 2013;168:861–9.
Melin EO, Thunander M, Landin-Olsson M, Hillman M, Thulesius HO. Depression, smoking, physical inactivity and season independently associated with midnight salivary cortisol in type 1 diabetes. BMC Endocr Disord. 2014;14:75.
Melin EO, Dereke J, Thunander M, Hillman M. Depression in type 1 diabetes was associated with high levels of circulating galectin-3. Endocr Connect. 2018;7:819–28.
Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–56.
Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8.
Melin EO, Svensson R, Thunander M, Hillman M, Thulesius HO, Landin-Olsson M. Gender, alexithymia and physical inactivity associated with abdominal obesity in type 1 diabetes mellitus: a cross sectional study at a secondary care hospital diabetes clinic. BMC Obes. 2017;4:21.
Melin EO, Thulesius HO, Hillman M, Landin-Olsson M, Thunander M. Abdominal obesity in type 1 diabetes associated with gender, cardiovascular risk factors and complications, and difficulties achieving treatment targets: a cross sectional study at a secondary care diabetes clinic. BMC Obes. 2018;5:15.
Melin EO, Dereke J, Thunander M, Hillman M. Soluble CD163 was linked to galectin-3, diabetic retinopathy and antidepressants in type 1 diabetes. Endocr Connect. 2018;7:819–28.
Kobayashi Y, Yoshida S, Nakama T, Zhou Y, Ishikawa K, Arita R, et al. Overexpression of CD163 in vitreous and fibrovascular membranes of patients with proliferative diabetic retinopathy: possible involvement of periostin. Br J Ophthalmol. 2015;99:451–6.
Melin EO, Svensson R, Gustavsson S-Å, Winberg A, Denward-Olah E, Landin-Olsson M, et al. Affect school and script analysis versus basic body awareness therapy in the treatment of psychological symptoms in patients with diabetes and high HbA1c concentrations: two study protocols for two randomized controlled trials. Trials. 2016;17:221.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983:361–70.
Lasnier E, Mario N, Boque M-C, You S-N, Vaubourdolle M. Evaluation of the clinical chemistry analyser Olympus AU400. Clin Chem Lab Med. 2000;38:1043–9.
Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384:607–17.
Tanamas SK, Permatahati V, Ng WL, Backholer K, Wolfe R, Shaw JE, et al. Estimating the proportion of metabolic health outcomes attributable to obesity: a cross-sectional exploration of body mass index and waist circumference combinations. BMC Obes. 2016;3:4.
Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. Jama. 2005;293:1861–7.
The National Board of Health and Welfare. Swedish National Guidelines for Diabetes. https://www.socialstyrelsen.se/nationellariktlinjerfordiabetesvard. Data collected 23rd of March, 2009.
Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17.
Zhu J, Chen Z. Research on the multiple linear regression in non-invasive blood glucose measurement. Biomed Mater Eng. 2015;26:447–53.
Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1:291.
Reynolds RM, Labad J, Strachan MWJ, Braun A, Fowkes FGR, Lee AJ, et al. Elevated fasting plasma cortisol is associated with ischemic heart disease and its risk factors in people with type 2 diabetes: the Edinburgh type 2 diabetes study. J Clin Endocrinol Metab. 2010;95:1602–8.
Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–44.
Acknowledgements
The authors are grateful to Anna Lindgren, PhD at the Department of Mathematical Statistics, Lund University, Lund, Sweden, for her statistical skills.
Funding
This research was supported by the Research and Development Fund of Region Kronoberg, Växjö, Sweden, and by the Research Council of South Eastern Sweden (FORSS), Linköping, Sweden. The funding sources were not involved in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Availability of data and materials
The data set analysed during the current study is not available publicly as individual privacy could be compromised, and we have no permission from the Regional Ethical Board to share the research data publicly. The data set is available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
EOM, HOT, MH, RS, ML-O and MT participated as investigators and reviewed, edited, and approved of the manuscript. All authors contributed to the study design, implementation and analysis. EOM was the initiator of this study, wrote the statistical methods and the manuscript, and is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Ethics declarations
Authors’ information
EOM is PhD, medical doctor, specialist in Paediatrics and Family Medicine. EOM works in Primary Care and at the Department of Research and Development, Region Kronoberg, Växjö, and is affiliated to the Department of Clinical Sciences, Lund University, Lund. HOT is PhD, assistant professor, medical doctor, specialist in Family Medicine, and works in Primary Care and at the Department of Research and Development, Region Kronoberg, Växjö and is affiliated to the Department of Clinical Sciences, Lund University, Malmö. Magnus Hillman is PhD and works at the Diabetes Research Laboratory, Lund University, Lund. RS is psychologist and works at the Department of Psychology, Linnaeus University, Växjö. ML-O is PhD, medical doctor, professor of Endocrinology at the Department of Clinical Sciences, Lund University, Lund, and works at Skane University Hospital. MT is PhD, medical doctor, specialist in Endocrinology and Internal Medicine, and works at the Central Hospital, Växjö, and at the Department of Research and Development, Region Kronoberg, Växjö, and is affiliated to the Department of Clinical Sciences, Lund University, Lund. All Sweden.
Ethics approval and consent to participate
The study was approved by the Regional Ethical Review Board of Linköping University, Linköping (Registration no. M120–07, T89–08). All participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Melin, E.O., Thulesius, H.O., Hillman, M. et al. Lower HDL-cholesterol, a known marker of cardiovascular risk, was associated with depression in type 1 diabetes: a cross sectional study. Lipids Health Dis 18, 65 (2019). https://doi.org/10.1186/s12944-019-1009-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-019-1009-4