Abstract
Background
The lipoprotein subfraction particle profile can be used to improve clinical assessments of cardiovascular disease risk and contribute to early detection of atherogenic dyslipidemia. Lipid alterations in gestational diabetes have been extensively studied, but the results have been inconsistent. Here, we investigated serum lipoprotein subfraction particle levels and their association with glucose metabolic status in pregnancy.
Methods
Twenty-eight pregnant women with gestational diabetes and 56 pregnant women with normal glucose tolerance matched for body mass index were enrolled in this study. We assessed fasting serum lipid concentrations and lipoprotein subfraction particle levels in participants between 24 and 28 weeks of gestation.
Results
The level of low-density lipoprotein (LDL) cholesterol was significantly lower in women with gestational diabetes than in those with normal glucose tolerance, but the triglyceride and high-density lipoprotein (HDL) cholesterol levels of the two groups were similar. Lipoprotein particle analysis showed that very-low-density lipoprotein (VLDL) particle number and the small dense LDL particle/large buoyant LDL particle (sdLDL-P/lbLDL-P) ratio were significantly higher in women with gestational diabetes than in those with normal glucose tolerance (P = 0.013 and P = 0.015, respectively). In multivariate analysis, fasting glucose was independently and positively associated with sdLDL-P/lbLDL-P ratio even after adjustment for maternal age, gestational weight gain, BMI and LDL cholesterol (standardized Beta = 0.214, P = 0.029).
Conclusions
The sdLDL-P/lbLDL-P ratio is higher in GDM compared with non-diabetic pregnant women, and positively and independently associated with fasting glucose in pregnant women.
Similar content being viewed by others
Background
Lipoproteins are comprised of multiple subfractions according to their size, density, and physicochemical properties. Determination of lipoprotein particle subfractions improves the clinical evaluation of individuals at high risk of cardiovascular diseases (CVD) and type 2 diabetes [1, 2]. Low-density lipoprotein (LDL) cholesterol is universally known as a major risk factor for CVD and type 2 diabetes, whereas LDL particle is more valuable as a risk indicator of LDL-attributable atherosclerosis [3]. Further, the small dense LDL (sdLDL) subfraction shows a positive association with coronary artery disease and is thought to be an atherogenic lipoprotein [4, 5]. Moreover, the protective effect of high-density lipoprotein (HDL) cholesterol on the incidence of type 2 diabetes is largely attributable to high HDL2 cholesterol subfractions [6].
Gestational diabetes mellitus (GDM), which is defined as impaired glucose tolerance that first appears during pregnancy, is associated with insulin resistance and beta-cell decompensation during pregnancy. There is a substantially increased risk of postpartum diabetes and CVD in GDM patients due, in part, to aberrant metabolic disturbances during pregnancy [7, 8]. In addition to dysglycemia, multiple metabolic and inflammatory factors are altered in women with GDM. Lipid alterations in GDM compared with normal pregnancy have been extensively studied, but the results have been inconsistent, especially those concerning LDL cholesterol [9]. The characterization of changes in lipoprotein particle levels in GDM may help identify lipid metabolic changes and potentially improve predictions of the risks of adverse pregnancy outcomes and postpartum metabolic diseases. Previous data obtained in American and Mediterranean populations indicated that women with GDM show remarkable changes in LDL particle distribution [10, 11]. However, the lipoprotein particle diameter differs according to ethnicity [12], and there are no published studies that describe the lipoprotein particle profile of the Chinese Han population with GDM. Thus, the objective of this study was to investigate differences in lipoprotein particle profile of GDM patients and healthy pregnant women and to examine the relationship between lipoprotein subfraction particles and the parameters of glucose metabolism during pregnancy.
Methods
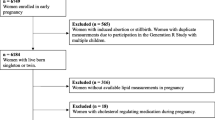
This study was conducted at Women’s Hospital Schools of Medicine Zhejiang University. Approval from the Institutional Ethics Committee at Women’s Hospital School of Medicine Zhejiang University was obtained, and all subjects provided informed consent. Twenty-eight singleton pregnant women with GDM were included in the present study. As controls, we selected 56 singleton pregnant women with normal glucose tolerance, matched for body mass index (BMI) and gestational age. GDM was diagnosed between the 24th and 28th weeks of gestation if one or more of the blood glucose levels measured during the one-step 75-g oral glucose tolerance test (OGTT) met or exceeded the following criteria: fasting, 5.1 mmol/L; 1-h, 10.0 mmol/L; and 2-h, 8.5 mmol/L. All participants resided in the Zhejiang Province and were of Han ethnicity. We excluded patients with obesity (BMI > 30 kg/m2), diabetes, thyroid disease, hypertensive disorders, renal diseases, hepatitis or other serious medical condition. In addition, any participant who had smoked or received the lipid lowering drugs, diabetic drugs during pregnancy was also excluded.
Blood samples were collected from participants at 24–28 weeks of gestation after a 12-h fast. Lipoprotein component analysis was performed using the lipoprotein subgroup particle number analysis method (SpectraCell Laboratories; Houston, TX, USA) according to a patented procedure (Patent No.: US 7,856,323 B2) [13]. The serum samples were mixed with a fluorescent dye and a gradient material and then separated in a continuous gradient (d = 1.000–1.300 g/cm3) through analytical ultracentrifugation. Then, the fluorescence of the lipoprotein particles was measured in a high-performance liquid chromatography-type flow system and normalized to a cholesterol scale using a proprietary algorithm. The lipoprotein particle profile included the quantitation of the levels of very-low-density lipoprotein particle (VLDL-P), total LDL particle (LDL-P), remnant lipoprotein particle (RLP), LDL III particle (LDLIII-P), LDL IV particle (LDLIV-P), total HDL particle (HDL-P), large buoyant HDL 2b particle (lbHDL2b-P), and non-HDL particle. Small dense LDL particle (sdLDL-P) levels were calculated as follows: sdLDL-P = LDLIII particle + LDLIV particle. Large buoyant LDL particle (lbLDL-P) number was calculated by subtracting sdLDL-P and RLP from total LDL particle. The coefficient of variation for lipoprotein particle analysis using known standards has been reported as ranging from 2% to 3%. Serum total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, insulin, lipoprotein(a) (Lp(a)), homocysteine, and high-sensitivity C-reactive protein (hsCRP) levels were determined using an Olympus AU400e chemistry immune analyzer (Tokyo, Japan). Blood glucose was measured using an Architect c16000 automated analyzer (Abbott Laboratories, Abbott Park, IL, USA). The homeostatic model assessment of insulin resistance (HOMA-IR) index was calculated as follows: HOMA-IR = [fasting glucose (mmol/L) × fasting insulin (μIU/mL)]/22.5.
Distribution of continuous variables was tested for normality using the Shapiro–Wilk test, and data showing a normal distribution were subjected to independent-sample t-tests. When the distribution was asymmetric, the two-sample Mann–Whitney U test was performed. The chi-square test was used for categorical variables. Correlation analyses between two parameters were evaluated using the Pearson correlation tests, after square root or logarithmic transformation of variables (whenever necessary). To evaluate the contribution of different variables to sdLDL-P/lbLDL-P ratio and VLDL-P, multivariate linear regression analyses were performed, using stepwise selection. Maternal age, gestational weight gain, BMI at OGTT, progesterone treatment history, FBG and LDL cholesterol were included as independent variables in the sdLDL-P/lbLDL-P ratio regression model. Maternal age, gestational weight gain, BMI at OGTT, progesterone treatment history, FINS and 2 h–OGTT were included as independent variables in the VLDL-P regression model. Two-sided p-value <0.05 was considered to indicate statistical significance. All tests were performed using the SPSS statistics version 20 (SPSS Inc., Chicago, IL, USA). The post hoc analysis was performed on the correlations to assess the appropriateness of the total sample size by determining the correlation coefficient that could be detected with 75% power using two-sided comparison with alpha = 0.05 [14].
Results
The characteristics of the study population are described in Table 1. The maternal age of the GDM group was significantly higher (P = 0.005), whereas the prevalence of mothers aged ≥35 years did not significantly differ between the two groups. The history of progesterone treatment in the early gestation were found in two women with gestational diabetes compared with one control subject, which had no significant difference. Both pre-pregnancy BMI and BMI at the OGTT test were similar in the two groups. Compared with normal pregnant women, patients with GDM had higher fasting insulin (FINS) (P = 0.033), HOMA-IR (P = 0.012), and glucose values at the three time points of the OGTT (all P ≤ 0.001). Unexpectedly, GDM patients had significantly lower LDL cholesterol compared to those with normal glucose tolerance (P = 0.020). In contrast, there were no significant differences in total cholesterol, HDL cholesterol, non-HDL cholesterol, or triglyceride between the two groups. Additionally, no significant differences were found in concentrations of Lp(a) or hsCRP. Homocysteine and the triglyceride/HDL cholesterol ratio were significantly higher in the GDM group (P = 0.049 and P = 0.029, respectively).
Further analyses of lipoprotein particle numbers between two groups are presented in Table 2. As shown in Table 2, patients with GDM showed increased VLDL-P (P = 0.013) and decreased total LDL-P (P = 0.024), whereas no significant differences were found in HDL-P level. Furthermore, we studied the subfractions of LDL and HDL particle. There were no significant differences in the levels of RLP, LDLIII-P, LDLIV-P or sdLDL-P between the two groups, whereas the sdLDL-P/lbLDL-P ratio was significantly higher in GDM patients (P = 0.015). Despite the comparable lbHDL2b-P values of the two groups, the HDL2b-P/HDL-P ratio was significantly lower in the GDM group compared with the group of normal pregnant women (P = 0.0498).
The Pearson correlations between lipoprotein particle levels and glucose metabolic parameters are presented in Table 3. The number of VLDL-P was positively correlated with FINS (r = 0.293, P = 0.007), 2-h blood glucose (r = 0.286, P = 0.009) and HOMA-IR index (r = 0.308, P = 0.005) in the pooled sample. The sdLDL-P/lbLDL-P ratio was positively correlated with FBG (r = 0.297, P = 0.006), FINS (r = 0.236, P = 0.031) and HOMA-IR index (r = 0.270, P = 0.013). Multiple linear regression analysis revealed that FBG remained independently and positively associated with sdLDL-P/lbLDL-P ratio after adjusting for age, BMI, gestational weight gain, progesterone history and LDL cholesterol (Table 4). Blood glucose at 2 h of OGTT test was associated with VLDL-P number after adjusting for age, BMI, gestational weight gain, progesterone history and FINS (Table 5). The post hoc power analysis indicated that the study had a power of 75% to detect a correlation coefficient of ≥0.285 with a total sample size of 84 subjects.
Discussion
In the current study, we investigated lipid profiles, including conventional lipid measurements and lipoprotein particle levels, in women with GDM and in pregnant women with normal glucose tolerance. The results indicated that the VLDL-P number and sdLDL-P/lbLDL-P ratio of those with and without GDM significantly differed and were both correlated with glucose metabolic parameters.
There is evidence that maternal lipid levels become abnormal in the context of GDM. A recent meta-analysis reported that GDM patients had higher triglyceride and lower HDL cholesterol levels than pregnant women with normal glucose tolerance in the second trimester; yet, both total cholesterol and LDL cholesterol levels were inconsistent [9]. Our results showed a significant decrease in LDL cholesterol with GDM, which was counterintuitive but in agreement with some previous studies [10, 15, 16]. Moreover, White et al. [17] previously reported that increased LDL cholesterol levels were associated with a lower risk of type 2 diabetes. Several genetic variants that have been associated with reduced LDL cholesterol have also been associated with increased risk of diabetes [18]. These studies help explain the phenomenon of decreased LDL cholesterol during GDM with the increased risk of developing diabetes postpartum.
Although three studies have investigated the relationship between LDL subfractions and GDM, there is no data concerning lipoprotein particle levels in the Chinese Han population. Rizzo et al. [11] reported an increased number but decreased average size of small dense LDL particles in Mediterranean women with GDM whose triglyceride and LDL cholesterol were similar to those of normal pregnant women. In a study conducted by Qiu et al. [10], American patients with GDM showed higher triglyceride and lower LDL cholesterol as well as smaller LDL particle size compared with pregnant women with normal glucose tolerance. Additionally, Han et al. [19] found that women had a smaller LDL peak diameter size and a higher level of the small dense LDL subfraction years before developing GDM. In this study, we used a different lipoprotein particle subfraction parameter, the sdLDL-P/lbLDL-P ratio, to reflect LDL particle mean size. Although the number of total LDL particles was decreased in the GDM group, the sdLDL-P/lbLDL-P ratio was significantly increased, which indicated a tendency toward the predominance of small dense LDL particles.
Previous studies have reported that the ratio of small dense LDL to large buoyant LDL is a potent marker for evaluating lipid metabolic status. A recent study by Lee et al. [20] determined that the sdLDL/lbLDL ratio increases during the development of impaired fasting glucose and is strongly associated with insulin resistance, suggesting atherogenic dyslipidemia from a pre-diabetic stage. A high sdLDL/lbLDL ratio has also been shown to be associated with lipid metabolic disturbance in patients with metabolic syndrome and human immunodeficiency virus (HIV)-associated lipodystrophy [21, 22]. Although a different analytic methodology was used to measure the number of lipoprotein particle in this study, the sdLDL-P/lbLDL-P ratio in pregnant women was positively correlated with fasting glucose after adjusting for age and BMI, two factors known to affect lipid metabolism. It seems plausible that alteration in LDL particle size would be linked to glucose metabolic status.
The causal relationship between abnormal lipoprotein patterns and CVD has been firmly established, and the lipoprotein profile has also been well studied in type 2 diabetes. The exacerbation of lipoprotein patterns is primarily attributed to insulin resistance in type 2 diabetes. With aggravating insulin resistance, the concentration and mean size of VLDL-P increase, whereas LDL and HDL particle sizes decrease [23]. Abnormal lipoprotein profiles, including higher small dense LDL and lower large buoyant HDL fractions, indicate poor glycemic control in overweight adolescents with type 2 diabetes [24]. A recent article by Mackey et al. [25] described the association between lipoprotein particles and the incidence of type 2 diabetes in a multicenter prospective cohort study, indicating that the number and size of VLDL-P were significantly associated with the incidence of type 2 diabetes in patients with atherosclerosis. Thus, it is justifiable to speculate aberrant lipoprotein particle profile existing at the early gestation in the subjects who developing gestational diabetes. The sdLDL-P/lbLDL-P ratio and VLDL-P number may be potential as biomarkers to improve early intervention of gestational diabetes.
Nutraceuticals have been shown a peculiar role in ameliorating dyslipidemia, also in pregnant women [26]. It was recently reported that omega-3 fatty acids and vitamin E co-supplementation had beneficial effects on glucose homeostasis parameters, serum triglycerides and VLDL cholesterol in GDM women [27]. Moreover, vitamin D and symbiotic supplementation were of some use for improve lipid profile among GDM patients [28, 29]. These data suggest that nutraceuticals and dietary intervention may modulates maternal glucose and lipid metabolism, but more randomized trials are needed to evaluate the effects of nutraceuticals [30]. In our study, as the subjects received similar dietary advice at obstetric clinic, we have not assessed nutraceutical intakes or taken this into account as a confounding variable.
Our study has several limitations. Firstly, because of its cross-sectional design, we were unable to determine whether abnormal lipoprotein subfractions are a cause or a consequence of impaired glucose metabolic status. Secondly, the relatively small sample size may have limited the generalization of our current findings. However, we were able to identify the correlations between lipoprotein parameters and glucose metabolic parameters. Meanwhile, the post hoc analysis showed that the study had a power of 75% to detect a correlation coefficient with r ≥ 0.285, implying that statistical power should not be a serious problem. Further cohort studies with a larger sample size and participants in their three trimesters of pregnancy and postpartum period are warranted to investigate the dynamic alterations of lipoprotein subfraction profiles in the perinatal period.
Conclusion
In conclusion, our study described the lipoprotein subfraction particle profile in pregnant women and its relationship to glucose metabolic parameters. The sdLDL-P/lbLDL-P ratio is independently and positively associated with fasting glucose in pregnant women. Our study suggests that GDM patients have a tendency toward the predominance of small dense LDL particles, which may contribute to an increased risk for atherosclerosis and CVD in the postpartum period.
Abbreviations
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular diseases
- FINS:
-
Fasting insulin
- GDM:
-
Gestational diabetes mellitus
- HDL:
-
High-density lipoprotein
- HOMA-IR:
-
The homeostatic model assessment of insulin resistance
- lbHDL2b-P:
-
Large buoyant high-density lipoprotein 2b particles
- lbLDL-P:
-
Large buoyant low-density lipoprotein particles
- LDL:
-
Low-density lipoprotein
- OGTT:
-
Oral glucose tolerance test
- RLP:
-
Remnant lipoprotein particles
- sdLDL-P:
-
Small dense low-density lipoprotein particles
- VLDL:
-
Very-low-density lipoprotein
References
Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Curr Opin Lipidol. 2010;21(4):305–11.
Stitziel NO. Human genetic insights into lipoproteins and risk of cardiometabolic disease. Curr Opin Lipidol. 2017;28(2):113–9.
Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5(2):105–13.
Diffenderfer MR, Schaefer EJ. The composition and metabolism of large and small LDL. Curr Opin Lipidol. 2014;25(3):221–6.
Zeljkovic A, Vekic J, Spasojevic-Kalimanovska V, Jelic-Ivanovic Z, Bogavac-Stanojevic N, Gulan B, et al. LDL and HDL subclasses in acute ischemic stroke: prediction of risk and short-term mortality. Atherosclerosis. 2010;210(2):548–54.
Hwang YC, Hayashi T, Fujimoto WY, Kahn SE, Leonetti DL, McNeely MJ, et al. Differential association between HDL subclasses and the development of type 2 diabetes in a prospective study of Japanese Americans. Diabetes Care. 2015;38(11):2100–5.
Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care. 2008;31(8):1668–9.
Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet. 1996;347(8996):227–30.
Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. Bjog-Int J Obstet Gy. 2015;122(5):643–51.
Qiu C, Rudra C, Austin MA, Williams MA. Association of gestational diabetes mellitus and low-density lipoprotein (LDL) particle size. Physiol Res. 2007;56(5):571–8.
Rizzo M, Berneis K, Altinova AE, Toruner FB, Akturk M, Ayvaz G, et al. Atherogenic lipoprotein phenotype and LDL size and subclasses in women with gestational diabetes. Diabetic Med. 2008;25(12):1406–11.
Frazier-Wood AC, Manichaikul A, Aslibekyan S, Borecki IB, Goff DC, Hopkins PN, et al. Genetic variants associated with VLDL, LDL and HDL particle size differ with race/ethnicity. Hum Genet. 2013;132(4):405–13.
Troup JM. Method for analyzing blood for lipoprotein components. Spectracell Laboratories, Inc. (Houston, TX, US). 2010. http://www.freepatentsonline.com/7856323.html. Accessed 21 Dec 2010.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–60.
Todoric J, Handisurya A, Leitner K, Harreiter J, Hoermann G, Kautzky-Willer A. Lipoprotein(a) is not related to markers of insulin resistance in pregnancy. Cardiovasc Diabetol. 2013;2:138.
Koukkou E, Watts GF, Lowy C. Serum lipid, lipoprotein and apolipoprotein changes in gestational diabetes mellitus: a cross-sectional and prospective study. J Clin Pathol. 1996;9(8):34–7.
White J, Swerdlow DI, Preiss D, Fairhurst-Hunter Z, Keating BJ, Asselbergs FW, et al. Association of Lipid Fractions with risks for coronary artery disease and diabetes. JAMA Cardio. 2016;1(6):692–9.
Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, et al. Association between low-density lipoprotein cholesterol-lowering genetic variants and risk of type 2 diabetes a meta-analysis. Jama-J Am Med Assoc. 2016;316(13):1383–91.
Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP, et al. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Eedocr Metab. 2016;101(7):2721–7.
Lee JE, Min SH, Lee DH, Oh TJ, Kim KM, Moon JH, et al. Comprehensive assessment of lipoprotein subfraction profiles according to glucose metabolism status, and association with insulin resistance in subjects with early-stage impaired glucose metabolism. Int J Cardiol. 2016;225:327–31.
Srisawasdi P, Suwalak T, Sukasem C, Chittamma A, Pocathikorn A, Vanavanan S, et al. Small-dense LDL cholesterol/large-buoyant LDL cholesterol ratio as an excellent marker for indicating lipodystrophy in HIV-infected patients. Am J Clin Pathol. 2013;140(4):506–15.
Srisawasdi P, Vanavanan S, Rochanawutanon M, Kruthkul K, Kotani K, Kroll MH. Small-dense LDL/large-buoyant LDL ratio associates with the metabolic syndrome. Clin Biochem. 2015;48(7–8):495–502.
Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, et al. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–62.
Hanks LJ, Pelham JH, Vaid S, Casazza K, Ashraf AP. Overweight adolescents with type 2 diabetes have significantly higher lipoprotein abnormalities than those with type 1 diabetes. Diabetes Res Clin Pr. 2016;115:83–9.
Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2015;38(4):628–36.
Scicchitano P, Cameli M, Maiello M, Modesti PA, Muiesan ML, Novo S, et al. Nutraceuticals and dyslipidaemia: beyond the common therapeutics. J Funct Foods. 2014;6(1):11–32.
Taghizadeh M, Jamilian M, Mazloomi M, Sanami M, Asemi Z. A randomized-controlled clinical trial investigating the effect of omega-3 fatty acids and vitamin E co-supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes. J Clin Lipidol. 2016;10(2):386–93.
Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A. Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: a double-blind randomized controlled clinical trial. Am J Clin Nutr. 2013;98(6):1425–32.
Ahmadi S, Jamilian M, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. The effects of synbiotic supplementation on markers of insulin metabolism and lipid profiles in gestational diabetes: a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2016;116(8):1394–401.
Silva-Zolezzi I, Samuel TM, Spieldenner J. Maternal nutrition: opportunities in the prevention of gestational diabetes. Nutr Rev. 2017;75(suppl 1):32–50.
Acknowledgements
We specifically thank Fang Yuan and Aihong Wang, obstetric clinics, Women’s Hospital School of Medicine Zhejiang University, for their assistance in collecting the blood samples.
Funding
This study was supported by the National Natural Science Foundation of China (81370725), Zhejiang Provincial & Ministry of Health Research Fund for Medical Sciences (WKJ-ZJ-1722, 2016KYB170), Science and Technology Program of Zhejiang Province (2017C33003).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
YM Chen analyzed and interpreted the patients’ data, and also wrote the manuscript in collaboration with coauthors. MK Du helped with participant recruitment, acquisition of data, and oversaw the laboratory work. JY Xu provided input on the study plan and edited the manuscript. DQ Chen designed the study and critically revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee at Women’s Hospital School of Medicine Zhejiang University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Chen, Y., Du, M., Xu, J. et al. The small dense LDL particle/large buoyant LDL particle ratio is associated with glucose metabolic status in pregnancy. Lipids Health Dis 16, 244 (2017). https://doi.org/10.1186/s12944-017-0627-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-017-0627-y