Abstract
Background
Growing evidence indicates that oxidative stress (OS) plays a pivotal role in Diabetic nephropathy (DN). In a previous study we demonstrated that ALA/LA protected HK-2 cells against high glucose-induced cytotoxicity. So we aimed to establish the glucose injury model of HK-2 cells and investigate the beneficial effects of ALA/LA on high glucose-induced excessive production of TGF-β1 and the possible mechanisms mediating the effects.
Methods
The expression of OS markers in high glucose-induced HK-2 cells treated with ALA/LA., including the antioxidant enzymes and reactive oxygen species (ROS) production, as well as the apoptosis rate were assayed by ELISA and flow cytometry. The p38/transforming growth factor β1 (TGF-β1) signal pathway were measured by real-time RT-PCR and western blot.
Results
The modeling condition of glucose toxicity on HK-2 cells was at the glucose concentration of 40.9 mM. ALA/LA can significantly increase the activities of antioxidant enzymes and decrease ROS production stimulated by high glucose. The study also found that ALA/LA caused a decrease in the apoptosis rate and TGF-β1 level of HK-2 cells under high glucose stress through the ROS/p38 pathway.
Conclusions
ALA/LA exerts protective effects in vitro through inhibition of ROS generation, down regulation of the activation of the p38MAPK pathway and the expression of TGF-β1 in HK-2 cells.
Similar content being viewed by others
Background
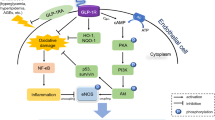
Diabetic nephropathy (DN) is a major complication in patients with either type 1 or type 2 diabetes mellitus [1, 2]. It is also the leading cause of morbidity and mortality in patients with kidney disease worldwide. Tubular injury plays a critical role in DN progression, which correlates with renal functional deterioration, a primary change associated with the disease [3]. Accumulating data indicate that excessive oxidative stress (OS) and aberrant dynamics are the primary factors responsible for tubule damage in DN [4]. Hyperglycemia is the driving force for the development of DN; it increases the production of free radicals, such as reactive oxygen species (ROS), resulting in OS [5,6,7]. Furthermore, hyperglycemia-induced ROS activates p38 mitogen-activated protein kinase(MAPK), which induces phosphorylation of transcriptional factors, altered expression of genes, and production of fibronectin in mesangial cells, resulting in DN [8, 9]. Transforming growth factor β1 (TGF-β1) exerts biological activities through MAPK cascades in certain cell lines, especially through p38 MAPK in human mesangial cells and rat renal tubular cells [10, 11]. Moreover, several authors have reported that TGF-β1 was related to p38 MAPK in DN, suggesting a role of p38 MAPK as another important mediator of the TGF-β1 signal pathway [12]. Therefore, it is inferred that the kidney injury would be improved by decreasing the activation of the ROS/p38 MAPK/TGF-β1 signal pathway.
Human kidney (HK-2) cells, a proximal tubular epithelial cell line derived from normal adult human kidney cells immortalized by transduction with human papillomavirus (HPV 16) DNA fragment [13].It has been found that these cells retain most of functional characteristics, i.e. OS, enzymes, cytochromes, consistent with in vivo system and human proximal tubular cells [14, 1]. So that HK-2 cells were used in the experiments on nephrology and nephrotoxicity.
Linoleic acid (LA; 18:2, n-6) and α-linolenic acid (ALA; 18:3, n-3) are essential fatty acids for humans and many animals and are important constituents of membranes. Recent studies have shown that dietary supplementation with n-3 polyunsaturated fatty acids (PUFAs) retards the progression of renal diseases in humans and animals [15, 16]. Adriano et al. [17] reported that dietary n-3 PUFAs have beneficial effects against OS in DM by decreasing ROS production and increasing the activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX). Our previous research also indicated that ALA was a protectant to prevent pig kidney proximal tubular cells (LLC-PK1) from high glucose-induced injuries by suppressing ROS generation and apoptosis [18]. In addition, ALA ameliorates proteinuria by down-regulating the expression of TGF-β1 and fibronectin proteins, and these favorable effects are related to the inhibition of phosphorylating activation of the p38 MAPK pathway in the renal cortex of OLETF rats [12]. However, little is known about the effect of both n-3 and n-6 PUFAs on HK-2 cells induced by high glucose. Our research investigated the relationship of ALA/LA and OS and apoptosis in the condition of high glucose and explored the possible mechanisms mediating the effects of ALA/LA.
Methods
HK-2 cell high glucose injury model
HK-2 cells were purchased from American Type Culture Collection (ATCC). HK-2 cells were grown in Dulbecco modified Eagle medium (DMEM):F12(Gibco, NY, USA) with 15 mM N-2-hydroxyethylpiperazine -N-2-ethanesulfonic acid (HEPES) (Sigma-Aldrich, MO, USA), L-glutamine (Sigma-Aldrich, MO, USA), and pyridoxine HCl (Gibco, NY, USA), supplemented with 10% (v/v) fetal calf serum (FCS, Gibco, NY, USA) at 37 °C in 95% humidified air and 5% CO2. All experiments were performed under serum-free conditions in which the cells remained viable in a nonproliferating state. Seeded cells (3 × 105 cells/mL) were treated with ALA, LA, timnodonic acid (EPA) (Nu-Chek, MN, USA), and ALA/LA (10–200 μM) for 48 h. For inhibition assays, the cells were first treated with inhibitors for 1 h before stimulation. After all treatments, the medium was collected and centrifuged. The cell-free supernatant was then stored at 80 °C for future assays. The cells remaining on the plates were rinsed with phosphate-buffered saline (PBS) and harvested.
Experimental design
In the experiments, HK-2 cells were divided into six groups: normal control group, high glucose model group, EPA treatment group, ALA treatment group, LA treatment group and mixture of ALA and LA treatment group, as shown in Fig. 1. Because of the excellent antioxidant ability of EPA [19,20,21], the EPA treatment group acts as a positive control group. The cells were harvested at the indicated time to evaluate cell viability, apoptosis and ROS.
T-SOD, GSH-PX, CAT, and NADPH assay
The activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX) were measured using enzyme-linked immunosorbent assay (ELISA) kits (Nanjing Keygen Biotech Co., Nanjing, China), according to the manufacturer’s instructions. All samples were analyzed in triplicate, and a separate standard curve was run for each ELISA plate. All samples were measured at 450 nm using a microplate reader (Bio-Rad, CA, USA).
Measurement of reactive oxygen species (ROS)production
The ROS production was detected by a commercially available kit (Beyotime Institute of Biotechnology, Haimen, China). HK-2 cells were seeded in 6-well microplates at 2 × 105 cells/well for 12 h, and then the cells were incubated with different treatments for 48 h. After incubation, cells were collected, counted and washed with PBS. Then cells (1 × 107/mL) were added into 10 μM DCFH-DA in a volume of 1 mL/group. Cells were stained at 37.C for 20 min, and washed with PBSto remove the free DCFH-DA molecules. ROS generation was assessed using flow cytometry (Becton, Dickinson and Company, NJ, USA).
Apoptosis assessment
HK-2 cells (106) were washed twice with PBS and double stained with fluorescein isothiocyanate (FITC)-conjugated Annexin V (Invitrogen, CA. USA) and PI (Invitrogen, CA. USA) according to the manufacturer’s instructions. The samples were analyzed immediately on the flow-cytometer (Becton, Dickinson and Company, NJ,USA). Cells negative for both Annexin V-FITC and PI were considered to be living cells; those positive for Annexin V-FITC and negative for PI were considered to be early apoptotic cells; and those positive for both dyes were considered to be late apoptotic or necrotic cells.
Data analysis was performed using the software WinMDI 2.9 (Invitrogen, CA, USA).
Measurement of real-time reverse transcription-polymerase chain reaction (RT-PCR) for TGF-β1 and p38mRNA
Total RNA was extracted using the method of Chomczynski and Sacchi [22], and the cDNA was synthesized by RT-PCR using 2 μg of total RNA with oligo-dT primers (Promega, WI, USA). Real-time RT-PCR was performed using a Light Cycler (Roche, Basel. Switzerland). The forward and reverse primers for TGF-β1, p38, and GADPH (Sangon, Shanghai, China) are shown in Table 1. The amplification was performed with the following time course: starting at 95 °C for 10 min, followed by denaturation at 95 °C for 15 s, then annealing at 60 °C for 60 s, and extension for 40 cycles as the last step. The detection of fluorescent products was carried out at the end of the 60 °C extension period. Each sample was tested twice. To confirm the amplification specificity, the PCR products were subjected to melting curve analysis and subsequent 1.5% agarose gel electrophoresis. For each sample, the cycle threshold (ΔCt = C TGF-β1/p38 - Ct GADPH) was calculated according to the published method [23]. The relative changes in TGF-β1/GADPH and p38/GADPH mRNA ratio between the control and experimental conditions were determined by the formula = 2-ΔΔCt, where ΔΔCt is the difference in ΔCt between the control and experimental conditions.
Western blot analysis
Cells (2 × 105) were added to 10-mm dishes pre-incubated with ALA/LA (100 μM) for 12 h, and then incubated with high glucose (40.9 mM) and ALA/LA (100 μM) for 48 h. The cell lysates were prepared with ice-cold lysis buffer containing 20 mM Tris-HCl (pH 8.0); 1 mM sodium orthovanadate; 10% glycerol; 1 mM phenylmethylsulfonyl fluoride; 2 mM EDTA; 1% Triton X-100; 50 mM β-glycerolphosphate; and 10 mg/mL each of aprotinin, leupeptin, and pepstatin. A total of 25 μg proteins were determined by the BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Haimen, China), and they were resolved by SDS-PAGE using 10% gel and transferred to polyvinylidene difluoride (PVDF) (Millipore Co., MA, USA) blocked with Tris-buffered saline (TBS) containing 5% (w/v) nonfat dry milk and 0.1% (v/v) Tween-20 for more than 2 h. The membrane was then probed with primary antibodies against GADPH (1:1500), TGF-β1 (1:800), p38 (1:1000), and p-p38 (1:800) at 4 °C overnight. After washing, the membrane was incubated with HRP-conjugated goat anti-rabbit IgG (1:1000) or HRP-conjugated goat anti-mouse IgG (1:1000) at room temperature for 1 h. Immunodetection was performed with an enhanced chemiluminescence (ECL) detection kit (Cell Signaling Technology, MA, USA) and Konica X-ray film system (Konica, Tokyo, Japan).
Statistical analysis
Results are shown as mean ± standard error of three individual assays. Statistical analyses were performed following one-way analysis of variance (ANOVA) and Tukey’s test using SPSS 18.0 (SPSS, Chicago, USA). Values were considered statistically significant when P was less than 0.05 or 0.01.
Results
High glucose-induced HK-2 cells injury
The viability of the cells was determined by the MTT assay. Our previous study showed a significant reduction of cell survival after 48 h of treatment with high glucose in concentrations of 25.3, 33.1, 40.9, 48.8, 56.6, 64.6, 72.2,80, 87.8, and 95.3 mM (P < 0.05 or P < 0.01), and the dose-effect relationship was obvious (r = − 0.91,P < 0.05). The viability of HK-2 cells was reduced to 43.4% with the treatment of high glucose at a dose of 56.6 mM for 48 h [24].
ALA/LA elevated the level of SOD, GSH-PX, and CAT and reduced the level of NADPH-PX in HK-2 cells induced with high glucose
As shown in Table 2, there was a significant reduction in the level of SOD, GSH-PX, CAT, and a significant increase in NADPH-PX in the high glucose model group, as compared to those in the normal control group (P < 0.05). On the contrary, after intervention, the levels of SOD, GSH-PX and CAT in the ALA intervention group, LA intervention group, EPA intervention group, and the mixture of ALA and LA intervention group were increased, while the levels of NADPH-PX in those groups were declined.
ALA/LA decreased ROS level in HK-2 cells induced with high glucose
Compared to the normal control group, high glucose-induced intracellular ROS generation of HK-2 cells in the high glucose model group was 1.8 times higher (P < 0.01) (Fig. 2a-f). The 48 h intervention of ALA, LA, EPA, and ALA/LA caused a significant reduction in ROS generation (P < 0.01 or P < 0.05) in high glucose-induced HK-2 cells, when ALA and EPA were at doses of 10 μM, 50 μM, and 100 μM; LA was at the dose of 10 μM; ALA/LA was at the dose of 50 μM and 100 μM with ALA/LA ratios of 1:1, 1:4, and 1:8 (Fig. 2a-f). It should be noted that ALA at the dose of 100 μM showed the best effect in all groups. The production of ROS was 2.6 times decreased than the amount in the high glucose model group (P < 0.01). Results are shown in Fig. 2c and Fig. 2g.
Effects of ALA/LA on ROS level of HK-2 cells induced with high glucose (\( \overline{x} \) ±s). ROS generation of HK-2 cell treated with ALA/LA was evaluated by flow cytometery. Data are presented as mean ± SD. # P < 0.05 vs. control group. * P < 0.05, ** P < 0.01 vs. model group. N: normal control group; G: high glucose model group; A1-A4: ALA group at dose of 10, 50, 100, 200 μM; L1-L4:LA group at dose of 10, 50, 100, 200 μM; E1-E4 EPA group at dose of 10, 50, 100, 200 μM, M1-M3: mixture of ALA and LA group at dose of 50 μM with ALA/LA ratio of 1:1, 1:4, 1:8; M4-M6: mixture of ALA and LA group at dose of 100 μM with ALA/LA ratio of 1:1, 1:4, 1:8. a to f Effects of ALA, LA, EPA, ALA and LA with a different concentration on ROS generation of HK-2 cells induced with high glucose. g ROS level of HK-2 cell treated with ALA/LA (100 μM)
ALA/LA inhibited apoptosis of HK-2 cells induced with high glucose
To determine whether apoptosis was associated with OS, we also measured the apoptosis rate after intervention of free fatty acid. As shown in Fig. 3, the apoptosis rate of HK-2 cells in the high glucose model group was significantly higher than that in the normal control group (P < 0.05). Moreover, we found that the apoptosis rates were significantly inhibited after 48 h incubation with 50 μm and 100 μM ALA, 50 μM of LA, 50 μM of ALA/LA with ratios of 1:4 and 1:8, and 100 μM ALA/LA with ratios of 1:1 and 1:4 (P < 0.05 or P < 0.01). The apoptosis rate was lowest in the intervention of ALA/LA at a dose of 50 μM with a ratio of 1:4 (P < 0.01) (Fig. 3b and Fig. 3g). Interestingly, there was a significant increase in apoptosis rate of HK-2 cells with 200 μM ALA, LA, and EPA (P < 0.01) (Fig. 3d).
Effects of ALA/LA on apoptosis of HK-2 cells induced with high glucose(\( \overline{x} \) ±s). Apoptosis percents of HK-2 cell treated with ALA/LA were evaluated by flow cytometery. N: normal control group; G: high glucose model group; A, L, E: ALA/LA/EPA intervention group at dose of 50 μM; M: mixture of ALA and LA intervention group at dose of 100 μM with ALA/LA ratio of 1:4. a to f Effects of ALA, LA, EPA, ALA and LA with a different concentration on apoptosis of HK-2 cells induced with high glucose. g Apoptosis percents of HK-2 cell treated with ALA/LA (100 μM)
ALA/LA down regulated mRNA expression of the p38/TGF-β1 signal pathway in high glucose-induced HK-2 cells
As demonstrated in our study, ALA/LA treatment caused a change in ROS and apoptosis of high glucose-induced HK-2 cells. Further, we focused on the p38/TGF-β1 signal pathway and found that at the dose of 50 μM, ALA, EPA, and ALA/LA decreased the mRNA expression of TGF-β1. EPA and ALA/LA at a dose of 50 μM also inhibited the mRNA expression of p38 (Fig. 4).
Effects of ALA/LA on TGF-β1, p38 mRNA levels of HK-2 cells induced by high glucose (100 μM). Relative mRNA abundances of TGF-β1 and p38 in HK-2 cell induced by high glucose were measured. The steady-state mRNA concentrations of TGF-β1 and p38 were quantified with real-time PCR and 2-ΔΔCt values were calculated to obtain fold expression levels. GAPDH mRNA levels were similarly measured and served as a reference control for mRNA quality and quantity. Bars represent the means ± SD of relative levels. *# Means not sharing a common symbol are significantly (P < 0.05) different from each other. N: normal control group; G: high glucose model group; A, L, E: ALA/LA/EPA intervention group at dose of 100 μM; M: mixture of ALA and LA intervention group at dose of 100 μM with ALA/LA ratio of 1:4
ALA/LA inhibits the phosphorylation of p38 and TGF-β1 expression of high glucose-induced HK-2 cells
The relationship between TGF-β1 and end stage renal disease has been proved [10]. To further investigate the mechanisms underlying the high glucose-induced damage effects, we measured the TGF-β1 expression in HK-2 cells by western blot analysis (Fig. 5 and Fig. 6). There was a significant difference in TGF-β1 expression between the normal control group and the high glucose model group (P < 0.05), but after intervention of 100 μM EPA and 100 μM ALA/LA with a ratio of 1:4 for 48 h, the TGF-β1 expression significantly decreased in HK-2 cells (P < 0.05).
TGF-β1, p-p38 and p38 expression in high glucose induced HK-2cells with ALA/LA intervention (100 μM). a Protein expression of TGF-β1 and p38 in high glucose induced HK-2 cells with ALA/LA intervention by western blot. b The intensity of the bands was quantified by densitometry analysis and normalized with corresponding GADPH. Values are expressed as the mean ± SD Bars without a common superscript symbol indicate significant differences among groups at P < 0.05. N: normal control group; G: high glucose model group; A, L, E: ALA/LA/EPA intervention group at dose of 100 μM; M: mixture of ALA and LA intervention group at dose of 100 μM with ALA/LA ratio of 1:4
TGF-β1, p-p38 and p38 expression, assessed by western blot, in high glucose induced HK-2cells with ALA/LA and/or SB203580 intervention (100 μM). a Protein expression of TGF-β1 and p38 in high glucose induced HK-2cells with ALA/LA and/or SB203580 by Western blot. b The effects of SB203580 on TGF-β1, p-p38 and p38 expression in high glucose induced HK-2cells. c The effects of ALA/LA and SB203580 (the inhibitor of p38) on TGF-β1, p-p38 and p38 expression in high glucose induced HK-2cells.The intensity of the bands was quantified by densitometry analysis and normalized with corresponding GADPH. Values are expressed as the mean ± SD. Bars without a common superscript symbol indicate significant differences among groups at P < 0.05. N: normal control group; G: high glucose model group; A, L, E: ALA/LA/EPA intervention group at dose of 100 μM; M: mixture of ALA and LA intervention group at dose of 100 μM with ALA/LA ratio of 1:4
Previous studies by other groups demonstrated that TGF-β1 is activated mainly through the MAPK signal pathway [25, 26]. As shown in Fig. 5 and Fig. 6, in the high glucose model group, the expression of p38, a critical subunit of MAPK, was markedly increased compared with in the normal control group in HK-2 cells (P < 0.05), and was partly restored by ALA/LA intervention (P < 0.05). There was no significant difference in p38 expression among the different groups, whereas 100 μM ALA and EPA and 100 μM ALA/LA with a ratio of 1:4 intervention for 48 h significantly reduced the phosphorylation of p38 in HK-2 cells (P < 0.05). These results suggest that a decrease in p38 expression may play a causative role in the TGF-β1 activation in HK-2 cells. These results indicate that ALA intake may exert its kidney protection, including anti-oxidative stress effects, through the activation of TGF-β1 in a p38-dependent manner in high glucose-induced HK-2 cells.
Discussion
In this study, we used a high glucose-induced HK-2 cell line as a model to investigate the effects of ALA/LA on DN. The results indicate that high glucose induced glucose toxicity on HK-2 cells. It showed the same effect on LLC-PK1 cells [18].Furthermore, our results showed that the viability of HK-2 cells was lowest with the treatment of high glucose at a dose of 56.6 mM for 48 h,shown in Fig. 2. However, with reference to other studies [27], and considering that a too low survival viability of HK-2 cells may interfere with the study’s results, a better condition to establish a glucose toxicity model of HK-2 cells was treatment with high glucose at dose of 40.9 mM for 48 h.
Increased ROS levels under high-glucose conditions induce OS, which can result in the development of a variety of biochemical and physiological lesions. Such cellular damage frequently impairs the metabolic function and results in cell death [13]. On the basis of these results of glucose toxicity on HK-2 cells [24] and the previous research [18], the exposure of HK-2 cells to high levels of glucose resulted in significant reduction in the cell viability. However, ALA/LA treatment was also shown to inhibit cell death, thereby suggesting that ALA/LA protects HK-2 cells against high glucose-induced cytotoxicity. Interestingly, in most cases ALA, LA and ALA/LA have these good function in low or medium concentration. Because polyunsaturated fatty acid may occur peroxidation at high dose, and it will show harmful effect in this case [28].
To combat and prevent OS, cells contain sophisticated intracellular antioxidant defense systems, including an enzymatic antioxidant system and a non-enzymatic antioxidant system. The former mainly comprises SOD, CAT, GSH-PX, and glutathione reductase (GR). The latter includes the antioxidants glutathione (GSH) as well as ascorbic acid, tocopherol, and carotenes [29]. However, there is an imbalance between the oxidant and anti-oxidant mediators prior to the development of renal lesions, and the oxidation level increases as the disease progresses, such as in DN [30]. Several studies reported that high-glucose conditions induced ROS production over antioxidant defense systems damage [31,32,33,34,35], and our study showed the same results (Table 2 and Fig. 3). We have also found that ALA/LA can protect HK-2 cells from oxidative stress induced by high glucose not only by scavenging intracellular ROS, but also by enhancing endogenous antioxidant defense systems including the antioxidant enzyme defense system and the GSH system. Comparable results have been reported by Shen et al. [36], who found that an increased level of high glucose caused slightly increased ROS generation, which correlated with a decrease in SOD activity. ALA suppressed ROS generation to a significant degree in a dose-dependent fashion and increased SOD activity significantly. In our previous work, the antioxidative capacity of ALA/LA was determined on the basis of the intracellular ROS clearance capacity in LLC-PK1 cells that had been oxidized by high glucose [18].
The antioxidant function of ALA/LA is thought to be related to the regulation of expression of the genes involved in ROS metabolism and/or ROS-dependent signaling pathways, such as the MAPK signaling pathway [37,38,39,40]. This could suggest that the ALA/LA activates the related intracellular antioxidant signaling pathways and gene expression only when oxidative stress occurs [41]. Additional studies are needed to test this hypothesis and to explain the precise mechanism of action by which antioxidant peptides in cells regulate intracellular ROS scavenging activity and antioxidant defense systems.
Previous studies suggested that ROS are generated as an early signal in human tubular cells, which subsequently develop apoptotic changes under high-glucose media, implicating ROS as potential mediators of glucose-induced apoptosis [42, 43]. In addition, based on recent reports regarding the relationship between ROS and MAPKs in apoptosis, it has been well established that p38 activation contributes to a positive feedback loop, such as the ROS/p38 MAPK cascade for cell apoptosis [44]. We investigated the apoptotic effect of ALA/LA and the underlying molecular mechanism for glucose toxicity on HK-2 cells. The data showed that the production of both ROS and p38 is involved in high glucose-induced apoptosis and that these effects were recovered by ALA/LA treatment, which was a ROS scavenger (Fig. 3 and Fig. 4). Furthermore, the results confirmed that ALA/LA treatment reduced high glucose-induced activation of p38, and SB203580 (p38 inhibitor) (Fig. 5 and Fig. 6) partially reduced ROS production. Taken together, these results demonstrate that ALAL/LA decreased the apoptosis induced by high glucose through the ROS/p38 signal pathway.
Diabetic nephropathy is characterized histologically by glomerular basement membrane thickening and mesangial matrix expansion, resulting in glomerulosclerosis [45]. TGF-β1 is known as a key mediator of the sclerosing process in diseased glomeruli and is related to glomerulosclerosis and interstitial fibrosis in various renal diseases [46, 47]. Several authors have described that elevated TGF-β1 production was induced by exposure to high glucose in the diabetic kidney [48, 49]. Our data showed that the mRNA and protein level of TGF-β1significantly increased in HK-2 cells in the condition of glucose toxicity compared with the control group. However, ALA/LA attenuated the increased expression of TGF-β1 in HK-2 cells in the condition of glucose toxicity. Thus, our aim was also to investigate the beneficial effects of ALA/LA on high glucose-induced excessive production of TGF-β1 and the possible mechanisms mediating the effects. It is well known that ROS and MAPK are closely related to TGF-β1. First, it was presence of excessive ROS in the kidney that can directly promote the activation of multiple OS-related signaling pathways, including the p38MAPK pathways, as well as the overexpression of downstream various fibrogenic cytokines containing TGF-β1. Consequently, it is possible to improve renal fibrosis and delay the progress of DN by reducing OS and regulating OS-related signaling pathway activities in the kidney [50, 51]. Our study show that high glucose triggered ROS overproduction and high expression of p38 and TGF-β1 in HK-2 cells. Moreover, after intervention with 100 μM ALA and 100 μM ALA/LA with a ratio of 1:4, ROS generation, the mRNA level of TGF-β1 and p38 MAPK, and the protein level of TGF-β1 and phosphorylated p38 MAPK were decreased. In conclusion, high glucose induces OS and apoptosis of HK-2 cells. The therapeutic actions of anti-oxidants in vitro are directly related to controlling the activities of intracellular signaling pathways associated with OS, in which the p38MAPK pathways occupy the key positions in DN at the early stage. The potential mechanisms by which ALA/LA exerts protective effects against in glucose toxicity in HK-2 cells in vitro are through inhibition of ROS generation, down regulating the activation of the p38MAPK pathway, and the expression of TGF-β1. Thus, our study provided new insights into the role of ALA/LA in high glucose-induced OS in HK-2 cells through the ROS/p38MAPK /TGF-β1 signal pathway.
Conclusions
The results of the present study concluded that high glucose induced OS and glucose toxicity of HK-2 cells.. While ALA/LA exerts protective effects in vitro through inhibition of ROS generation, down regulation of the activation of the p38MAPK pathway and the expression of TGF-β1 in HK-2 cells.
Abbreviations
- ALA:
-
α-linolenic acid
- CAT:
-
Catalase
- DN:
-
Diabetic nephropathy
- EPA:
-
Timnodonic acid
- GSH-PX:
-
Glutathione peroxidase
- HK-2:
-
Human tubular epithelial cell
- LA:
-
Linoleic acid
- MAPKs:
-
Mitogen -activated protein kinase
- NADPH-PX:
-
Nicotinamide adenine dinucleotide phosphate oxidase
- OS:
-
Oxidative Stress
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxie pismutese
- TGF-β1 :
-
Transforming growth factor-β1
References
Hauschke M, Roušarová E, Flídr P, Čapek J, Libra A, Rouš T. Neutrophil gelatinase-associated lipocalin production negatively correlates with HK-2 cell impairment: evaluation of NGAL as a marker of toxicity in HK-2. Toxicol in Vitro. 2017;39:52–7.
Van DC, Berl T. Pathogenesis of diabetic nephropathy. Rev Endocr Metab Disord. 2004;5:237–48.
Li X, Xu XX, Zhang F, Wang M, Xu Y, Tang D, Wang J H, Qin Y, Liu Y, Tang CY, He LY, Grek A, Zhou ZG, Liu FY, Dong Z, Sun L. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Bio2017; 11: 297–311.
Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy. Brit J Pharmacol. 2014;171:1917–42.
Elmas O, Erbas O, Yigitturk G. The efficacy of Aesculus Hippocastanum seeds on diabetic nephropathy in a streptozotocin-induced diabetic rat model. Biomed Pharmacother. 2016;83:392–6.
Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–52.
Dabhi B, Kinnari N. Mistry. Oxidative stress and its association with TNF-α-308 G/C and IL-1α-889 C/T gene polymorphisms in patients with diabetes and diabetic nephropathy. Gene. 2015;562:197–202.
Kang SW, Adler SG, Lapage J, Natarajan R. p38 MAPK and MAPK kinase 3/6mRNA and activities are increased in early diabetic glomeruli. Kidney Int. 2001;60:543–52.
Wang J, Huang H, Liu P, Tang F, Qin J, Huang W, Chen F, Guo F, Liu W, Yang B. Inhibition of phosphorylation of p38MAPK involved in the protection of nephropathy by emodin in diabetic rats. Eur J Pharmacol. 2006;553:297–303.
Chin BY, Mohsenin A, Li SX, Choi AM, Choi ME. Stimulation of pro-α1 (I) collagen by TGFβ1 in mesangial cells: role of the p38 MAPK pathway. Am J Renal Physiol. 2001;280:495–504.
Hills CE, Squires PE. The role of TGF-b and epithelial-to mesenchymal transition in diabetic nephropathy. Cytokine Growth Factor Rev2011. 22:131–9.
Lee SJ, Kang JG, Ryu OH, Kim CS, Ihm SH, Choi MG, Yoo HJ, Kim DS, Kimb TW. Effects of α-lipoic acid on transforming growth factor β1–p38 mitogen-activated protein kinase–fibronectin pathway in diabetic nephropathy. Metabolism. 2009;58:616–23.
Ryan MJ, Johnson G, Kirk J, Fuerstenberg SM, Zager RA, Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45:48–57.
Arbillaga L, Azqueta A, Ezpeleta O, Cerain ALD, Oxidative DNA. Damage induced by Ochratoxin a in the HK-2 human kidney cell line: evidence of the relationship with cytotoxicity. Mutagenesis. 2007;22:35–42.
Lauretani F, Maggio M, Pizzarelli F, Michelassi S, Ruggiero C, Ceda GP, Bandinelli S, Ferrucci L. omega-3 and renal function in older adults. Curr Pharm Des 2009; 15: 4149–4156.
Goksu Erol AY, Avci G, Sevimli A, Ulutas E, Ozdemir M. The protective effects of omega-3 fatty acids and sesame oil against cyclosporine A–induced nephrotoxicity. Drug Chem Toxicol. 2013;36:241–8.
Assis AMD, Rech A, Longoni A, Morrone MDS, Pasquali MADB, Perry MLS, Souza DO, Moreira JCF. Dietary n-3 polyunsaturated fatty acids revert renal responses induced by a combination of 2 protocols that increase the amounts of advanced glycation end product in rats. Nutr Res. 2015;35:512–22.
Jiang MX, Zheng JF, Wei Y, Lv GL, Xu Q, Zhou YN, Zhai CK. Protective effect of ALA on high glucose induced cellular injury of LLC-PK1 cell. J Hygiene Res. 2012;41:191–4.
Dasilva G, Pazos M, Egido EG, Gallardo JM, Rodríguez I, Cela R, Medina I. Healthy effect of different proportions of marine ω-3 PUFAs EPA and DHA supplementation in Wistar rats: Lipidomic biomarkers of oxidative stress and inflammation. J. Nutr. Biochemist. 2015;26:1385–92.
Wang D, Zhang LY, Wen M, Du L, Gao X, Xue CH, Xu J, Wang YM. Enhanced neuroprotective effect of DHA and EPA-enriched phospholipids against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced oxidative stress in mice brain. J. Func. Foods. 2016;25:385–96.
Ramaiyan B, Bettadahalli S, Talahalli RR. Dietary omega-3 but not omega-6 fatty acids down-regulate maternal dyslipidemia induced oxidative stress: a three generation study in rats. Biochem Bioph Res Co. 2016;477:887–94.
Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidium thiocyanate phenol chloroform extraction. Anal. Biochemist. 1987;162:156–60.
Liu J, Yeo HC, Doniger SJ, Ames BN. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal. Biochemist. 1997;245:161–6.
Jiang MX, Zhang HF, Ye BL, Zhai CK. Glucose toxicity on HK-2 cells and the intervention of ALA/LA. Acta nutrimenta Sinica. 2017;39:41–4.
Fujita H, Omori S, Ishikura K, Hida M, Awazu M. ERK and p38 mediate high glucose induced hypertrophy and TGF-β expression in renal tubular cells. Am J Phys. 2004;286:120–6.
Gruden G, Zonca S, Hayward A, Thomas S, Maestrini S, Gnudi L, Viberti GC. Mechanical stretch-induced fibronectin and transforming growth factor–beta1 production in human mesangial cells is p38 MAPK-dependent. Diabetes. 2000;49:655–61.
Zhang W, Wang R, Han SF, Bu L, Wang SW, Ma H, Jia GL. α-Linolenic acid attenuates high glucose-induced apoptosis in cultured human umbilical vein endothelial cells via PI3K/Akt/eNOS pathway. Nutrition. 2007;23:762–70.
Neil B, Jillian EL, Alma RH, Julie AC, Flavia F, Peter JC, Michael HLG, Irene CG. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutat Res-Fund Mol M. 2003;530:27–33.
Wang L, Ding L, Yu Z, Zhang T, Ma S, Liu J, Intracellular ROS. Scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res Int. 2016;90:33–41.
Tabur S, Korkmaz H, Eren MA, Oğuz E, Sabuncu T, Aksoy N. Urotensin-II level and its association with oxidative stress in early diabetic nephropathy. J Diabetes Complicat. 2015;29:115–9.
Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313.
King GL, Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–8.
Newsholme P, Haber EP, Hirabara SM, Rebelato ELO, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583:9–24.
Kim KY, SN Y, Lee SY, Chun SS, Choi YL, Park YM, Chatterjee B, Ahn SC. Salinomycin-induced apoptosis of human prostate cancer cells due to accumulated reactive oxygen species and mitochondrial membrane depolarization. Biochem Biophys Res Commun. 2011;413:80–6.
Zhang DX, Gutterman DD. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H2023–31.
Nordberg J, Arner ESJ. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Bio Med. 2001;31:1287–312.
Zhang W, Liu HTMAPK. Signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18.
Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256–69.
Shen J, Shen S, Das UN, Xu G. Effect of essential fatty acids on glucose induced cytotoxicity to retinal vascular endothelial cells. Lipids Health Dis. 2012; 11: http: //dx. doi. Org/https://doi.org/10.1186/1476-511X-11-90.
Hancock JT, Desikan R, Neill SJ. Role of reactive oxygen species in cell signalling pathways. Biochem Soct. 2001;29:345–50.
Kensler TW, Wakabayash N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicology. 2007;47:89–116.
Li JX, Shen YQ, Cai BZ, Zhao J, Bai X, YJ L, Li XQ. Arsenic trioxide induces the apoptosis in vascular smooth muscle cells via increasing intracellular calcium and ROS formation. Mol Biol Rep. 2010;37:1569–76.
Parka SG, Kima SH, Kimb KY, Yua SN, Choia HD, Kima YW, Nama HW, Seoc YK, Ahna SC. Toyocamycin induces apoptosis via the crosstalk between reactive oxygen species and p38/ERK MAPKs signaling pathway in human prostate cancer PC-3 cells. Pharmacol Rep. 2017;69:90–6.
Park MH, Heo SJ, Kim KN, Ahn G, Park PJ, Moon SH, Jeon BT, Lee SH. 6,6′-Bieckol protects insulinoma cells against high glucose-induced glucotoxicity by reducing oxidative stress and apoptosis. Fitoterapia. 2015;106:135–40.
Kreisberg JI, Garoni JA, Radnik R, Ayo SH. High glucose and TGF-β1 stimulate fibronectin gene expression through a cAMP response element. Kidney Int. 1994;46:1019–24.
August P, Suthanthiran M. Transforming growth factor β signaling, vascular remodeling, and hypertension. N Engl J Med. 2006;354:2721–3.
Hayashida T, Poncelet AC, Hubchak SC, Schnaper HW. TGF-β1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney Int. 1999;56:1710–20.
Border WA, Noble NA. Cytokines in kidney disease: the role of transforming growth factor β. Am J Kidney Dis. 1993;22:105–13.
Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN. Reversibility of established diabetic glomerulopathy by anti–TGF-β antibodies in db/db mice. Biochem Biophys Res Commun. 2003;300:16–22.
Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176–84.
Sedeek M, Nasrallah R, Touyz RM, Hebert RLNADPH. Oxidases, reactive oxygen species, and the kidney: friend and foe. J Am Soc Nephrol. 2013;24:1512.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 81372986).
Funding
Financial assistance for this study was provided by the National Natural Science Foundation of China (No. 81372986).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
CKZ designed the study and interpreted the data. MXJ, FHZ, BLY and YC carried out the study. MXJ collated the data, performed all the statistical analyses, interpreted the data and wrote the manuscript. LJZ interpreted the data and correct the English. All authors read and approved the findings of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Jiang, M., Zhang, H., Zhai, L. et al. ALA/LA ameliorates glucose toxicity on HK-2 cells by attenuating oxidative stress and apoptosis through the ROS/p38/TGF-β1 pathway. Lipids Health Dis 16, 216 (2017). https://doi.org/10.1186/s12944-017-0611-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-017-0611-6