Abstract
Background
Studies focusing on dietary pesticides in population-based samples are scarce and little is known about potential mixture effects. We aimed to assess associations between dietary pesticide exposure profiles and Type 2 Diabetes (T2D) among NutriNet-Santé cohort participants.
Methods
Participants completed a Food Frequency Questionnaire at baseline, assessing conventional and organic food consumption. Exposures to 25 active substances used in European Union pesticides were estimated using the Chemisches und Veterinäruntersuchungsamt Stuttgart residue database accounting for farming practices. T2D were identified through several sources.
Exposure profiles were established using Non-Negative Matrix Factorization (NMF), adapted for sparse data. Cox models adjusted for known confounders were used to estimate hazard ratios (HR) and 95% confidence interval (95% CI), for the associations between four NMF components, divided into quintiles (Q) and T2D risk.
Results
The sample comprised 33,013 participants aged 53 years old on average, including 76% of women. During follow-up (median: 5.95 years), 340 incident T2D cases were diagnosed.
Positive associations were detected between NMF component 1 (reflecting highest exposure to several synthetic pesticides) and T2D risk on the whole sample: HRQ5vsQ1 = 1.47, 95% CI (1.00, 2.18). NMF Component 3 (reflecting low exposure to several synthetic pesticides) was associated with a decrease in T2D risk, among those with high dietary quality only (high adherence to French dietary guidelines, including high plant foods consumption): HRQ5vsQ1 = 0.31, 95% CI (0.10, 0.94).
Conclusions
These findings suggest a role of dietary pesticide exposure in T2D risk, with different effects depending on which types of pesticide mixture participants are exposed to. These associations need to be confirmed in other types of studies and settings, and could have important implications for developing prevention strategies (regulation, dietary guidelines).
Trial Registration
This study is registered in ClinicalTrials.gov (NCT03335644).
Similar content being viewed by others
Introduction
Type 2 Diabetes (T2D) is a chronic disease characterized by chronic hyperglycaemia, resulting from inefficient use of insulin by the organisms’ cells. The number of people with diabetes around the world has increased from 108 million in 1980 (4.7%) to 451 million in 2017 (8.5%) [1, 2]. Furthermore, the number of deaths due to diabetes has increased by 70% worldwide between 2000 and 2019 [3]. A healthy diet – low in saturated fat, sweet products and rich in fiber – regular physical activity, maintaining a normal weight and avoiding smoking are known strategies to prevent or delay the onset of T2D [4] but environmental exposure, for instance food contaminants such as pesticides, may also play a role [5, 6].
Previous research has largely focused on organochlorine pesticides (OC) in relation with diabetes, mainly in occupational settings [7,8,9,10]. Several studies have provided information on the associations between increased T2D risk and OC exposure; for instance DDE, heptachlor, HCB, DDT, chlordane, with odds ratios ranging from 1.47 to 1.95 [9, 11]. A large proportion of these OCs have now been banned in the European Union legislation and replaced by other types of pesticides like organophosphorus (OP), neonicotinoid, and pyrethroid pesticides. Up to now, the latter have been far less investigated in relation with diabetes [12,13,14], especially through the dietary route, even though there are several mechanisms supporting potential effects [5, 15].
Moreover, in recent years, some studies have shown links between diabetes risk and organic food purchase or consumption [16], and recently a study conducted in the NutriNet-Santé cohort indicated inverse associations between a higher proportion of organic food in the diet and T2D risk [17]. These relationships could be explained by potentially lower concentrations of pesticides residues in plant organic foods, as organic agriculture regulation allows for a markedly smaller list of pesticides [18]. Consistently, organic food consumers exhibit lower urinary pesticide concentration than the non-organic consumers [19,20,21,22].
Moreover, it seems important when studying dietary exposure to pesticides, to consider exposure to mixtures, and not to compounds taken separately, as classically done in risk assessment studies.
In that context, the purpose of this work was to study the associations between dietary pesticide exposure profiles and T2D risk in a large sample of the NutriNet-Santé cohort.
Material and methods
Study population
The NutriNet-Santé study is a web-based prospective cohort of adults launched in France in May 2009 [23]. Self-administered validated questionnaires [24,25,26,27,28,29] were completed online at baseline by participants and administered every year. Dietary behaviors and specific health issues were collected through complementary questionnaires during follow-up.
Dietary intake assessment
Between June and December 2014, the participants were invited to complete a 264-item web-based self-administered semi-quantitative food frequency questionnaire (Org-FFQ) differentiating organic and conventional foods. The Org-FFQ has been extensively described in other publications [30]. It was constructed on the basis of an existing validated FFQ [31] to which a 5-point ordinal scale was added to measure the frequency of organic food consumption. Participants provided the frequency of consumption and the quantity consumed for each item, assisted by photographs showing different portion sizes [32]. For food and beverages with an existing organic version (labelled), participants answered the question “How often was the product of organic origin?” by selecting 1 of the 5 following frequency modalities: never, rarely, half-of-time, often, or always. The organic food consumption was then calculated by matching to the modalities with respective percentage of, 0, 25, 50, 75, and 100. Weighting and sensitivity analyses for the Org-FFQ have been published elsewhere [30].
All food and beverage items were combined into 33 food groups. Nutritional values were obtained from a published food composition database [33]. A global proportion (as weight) of organic food in the diet was calculated as well as the proportion of organic food for each food group.
Pesticide exposure assessment
Dietary exposure to pesticides was calculated by combining dietary intakes of each adult with pesticide residue concentration values in plant foods using contamination data from the European Union reference laboratory for pesticides, Chemisches und Veterinäruntersuchungsamt (CVUA) Stuttgart [34]. Contamination data for conventional and organic food products were both available in the database, details can be found in Supplementary Material 1. Among compounds available in this database, 25 commonly used pesticides were chosen, given both their frequency of detection above the Maximum Residue Levels (MRL) when sufficient data were available, and their frequency above Acceptable Daily Intake (ADI) otherwise, as detailed in Baudry et al. 2019 [35]. Pesticides authorized and widely used in organic farming processes (e.g. natural pyrethrins, spinosad) were also included. The 264 Org-FFQ items were broken down into 442 ingredients (comprising at least 5% of at least one food item). Animal-based ingredients were excluded, as CVUA encompassed plant-based ingredients only. Indeed, plant-based foods have notably more frequent and higher pesticides residues levels than foods of animal origin [36]. The resulting 180 plant ingredients were linked to the CVUA database and then were assigned a contamination value in organic and conventional farming modes (as the mean of corresponding data point). A description of the different steps for the decomposition and matching process is shown in Supplementary Material 2. The final 180 ingredients are listed in Supplementary Material 3.
For each ingredient/pesticide pair in conventional and organic farming, frequency of detection and frequency of quantification were determined using the formula as follows:
These frequencies were then used to determine the censoring rate in order to apply the most adapted methodology, following EFSA and WHO’s recommendations[37, 38].
Treatment of data below detection limit has been extensively described elsewhere [35, 39].
As food consumption data from NutriNet-Santé referred to edible foods (bone-free, peeled or cooked products), edibility and cooking factors were allocated to each ingredient as appropriate [40, 41]. Equal conversion factors were applied for both conventional and organic products. Cooking or peeling effects on pesticide residue levels were not considered as dilution factors were not known for all food/pesticide combinations [42]. The estimated daily intake (EDI) (in μg/kg of weight/day) under lower bound scenario was calculated for each of the selected pesticide and for each participant, following this formula:
Ei,j estimated daily exposure to pesticide j for individual i (µg/kg bw/day).
n_i number of plant foods in the diet of individual i.
Ci,k mean daily intake of plant food k by individual i (g/day).
Lk,j concentration of pesticide j in food k (mg/kg).
Bwi body weight of individual i (kg).
Lower-bound (optimistic) rather than upper-bound scenario was used for this work, as upper-bound is known to overestimate exposure levels [35, 38, 43].
Covariates
Baseline and yearly questionnaires collected sociodemographic and lifestyle characteristics such as gender, date of birth, occupation, educational level, and smoking practices. Monthly income by household unit was derived using both the household income and composition. Anthropometric measures (height, weight), physical activity (using the validated Physical Activity Questionnaire [44]) and family history of diabetes were also collected [24, 29]. Antihypertensive, lipid-lowering medications and self-declaration of hypertension or dyslipidemia were indicated at baseline through the health questionnaire. A multi-source approach was used to validate cardiovascular diseases [45].
Concerning dietary data, the simplified Programme National Nutrition Santé Guidelines Score 2 (sPNNS-GS2), indicating the level of adherence to 2017 French dietary guidelines proposed by the High Council of Public Health [46, 47] and the provegetarian score [48] were computed for adjustment. The sPNNS-GS2 includes 13 components. Component, cut-off, scoring system and ponderation are presented in Fig. 1 and Supplementary Material 4.
The provegetarian score was computed as follows [48]: 7 plant food and 5 animal food groups were defined and sex-specific quintiles adjusted for total energy intake were calculated. For each plant component, 1 to 5 points were allocated to quintile 1 to 5 and for animal food groups the scoring was reversed. The provegetarian score was obtained by summing each quintile value of vegetable food group and each reverse quintile value of animal food group thus ranging from 12 (low plant food consumption) to 60 (high plant food consumption).
Three scores assessing the quality of the diet were also computed: Comprehensive Diet Quality Index (cDQI), plant-based Diet Quality Index (pDQI) and animal-based Diet Quality Index (aDQI) [49].
Case ascertainment
Participants declared health events through a yearly health status questionnaire and a dedicated web-service at any time of the study. All medical records were compiled and examined by dedicated physicians. Physicians of participants declaring major health events were contacted to collect additional information if necessary. A medical expert committee validated these major health events.
Moreover, declared health data were merged with medico-administrative registers of the national health insurance system (Système National d’Information Inter-Régimes de l’Assurance Maladie [SNIIRAM] databases) to validate and provide information on health events. Mortality data from the French Centre for Epidemiology Medical Causes of Death database (CépiDC) were also used. Therefore, diabetes cases were identified using a multi-source approach, i.e. T2D self-reported during follow-up along with declaration of the use of T2D medication. Matching with the medico-administrative databases of the French National health insurance (SNIIRAM database) allowed us to correct potential errors. In this study, we considered as cases all T2D cases diagnosed between baseline (i.e. the date of completion of the Org-FFQ in 2014) and October 6th 2020. Prevalent cases of type 1 diabetes and T2D were removed from the analysis.
Statistical analyses
A flowchart for the study sample selection is presented in Fig. 2.
For the present study, participants who completed the Org-FFQ between June and December 2014 (N = 37,685), with no missing covariates for basal metabolic rate computation (N = 37,305), who were not detected as under- or over-reporters (N = 35,196), living in France with available data on dietary scores (N = 34,442), who were free of type 1 diabetes or T2D when they completed the Org-FFQ (N = 33,013), were selected.
The detection method for under and over-reporters was based on the comparison between energy intake and energy requirement as previously performed in other studies [30, 50].
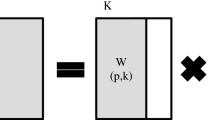
Dietary pesticide exposure profiles were analyzed using Non-Negative Matrix factorization (NMF) (detailed in Supplemental Material 5), specially adapted for non-negative data with excess zeros, developed by Lee et al. [51]. This method is frequently used in studies to identify mixtures of contaminants in the diet [52,53,54,55]. NMF procedure was performed using 25 selected pesticide exposure values (reflecting various pesticide exposure patterns) and resulted in four NMF components.
Chi2, Mantel-Haenzel, Wilcoxon and Kruskal–Wallis tests were applied as appropriate to compare sociodemographic and lifestyle characteristics, between cases and non-cases, and also across NMF-extracted component quintiles.
Cox proportional hazards regression models were used to assess associations between dietary pesticide exposure as profiles and T2D. NMF component scores were split into quintiles and introduced into separate models, with age as time scale, and first quintile as reference. Participants contributed person time until the date of diagnosis of diabetes, the date of last completed questionnaire, the date of death, or October 6th 2020, whichever occurred first.
Cox models were adjusted for known potential confounders including smoking status, educational level, occupation, household income, physical activity, weight, height, family history of diabetes, overall quality of the diet (measured by the PNNS-GS2 score [47]). Interactions between gender and sPNNS-GS2 (overall nutritional quality of the diet), i.e. potential modulating factors, and NMF components were tested by introducing a multiplicative interaction term into the models. Interactions with p < 0.20 were further investigated by stratified analyses.
Schoenfeld residuals were used to test the proportional hazard assumption of the Cox model.
Potential nonlinear effects of continuous exposure variables were evaluated using martingale residuals. Tests for linear trend were performed using quintiles of the NMF components as ordinal variables.
Sensitivity analyses were performed to test for consistency of findings. A model excluding early cases (within 1 year after baseline), a model excluding CVD events before or during follow-up, and a model excluding baseline metabolic abnormalities (hypertension or dyslipidaemia) were performed as sensitivity analyses. Additional models were adjusted for provegetarian score and three scores assessing quality of foods, cDQI, pDQI and aDQI (data not shown) [49]. Two-sided tests were used. Data management and statistical analyses were performed using SAS (version 9.4; SAS Institute, Inc.). The NMF analysis was performed using R’s NMF package [56].
Results
The baseline characteristics of the participants are presented in Table 1.
The sample, aged on average of 53 years old (SD 14) at baseline, was constituted of 76% of women. Monthly income of 1800€-2700€ was the most represented category. Sixty-five percent of the participants had a post-graduate diploma, and more than 50% were married. Most represented place of residence was urban units with more than 200,000 inhabitants.
Participants who developed a T2D during follow up (340 cases) were more frequently men and older subjects. Differences between cases and non-cases were observed for educational level, with less post-graduated participants in cases as well as occupation, with more retired cases compared to non-cases. Cases were more often married and more likely to be former smokers.
Participants with a BMI above 25 kg/m2 represented 82% of cases compared to 33% for non-cases. Family history of diabetes was more frequent in cases.
Considering nutritional parameters (Table 2), cases had higher energy and alcohol intakes. Organic proportion in the diet was 16% (SD 0.18) in cases, compared to 22% (SD 0.21) in non-cases (unadjusted descriptive figures). Non-cases more often followed a vegetarian or vegan diet. For information, sociodemographic and nutritional characteristics, compared across quintiles of each NMF component can be found in Supplementary Tables 1 to 8.
Regarding pesticide exposure, correlations between selected pesticides and NMF Components are shown in Table 3. NMF Component 1 was highly correlated (coefficients > 0.60) with azoxystrobin, chlorpyriphos, imazalil, malathion, profenofos and thiabendazole. High positive correlations with NMF Component 2 were observed for azoxystrobin, boscalid, cyprodinil, difenoconazole, fenhexamid, iprodione, lambda-cyhalothrin and tebuconazole. NMF Component 3 was characterized by high correlations with spinosad. High correlations for NMF Component 4 were found for acetamiprid, carbendazim, chlorpyrifos, cypermethrin, and dimethoate/omethoate. Among the active substances that are listed, some of them are no longer authorized in the EU in plant protection products as indicated in Table 3. For information, absolute values for the estimated pesticide exposure in μg/kg of weight/day are presented in Supplementary Tables 9 and 10.
Table 4 presents spearman correlations between food consumption and NMF Components: NMF Component 1 was particularly positively correlated with conventional fruit and fruit juice intakes. NMF Component 2 was positively correlated with conventional fruits and negatively with several organic food groups (potatoes, vegetables or legumes). NMF Component 3 was positively correlated with plant-based organic food groups (soup, vegetables, fruits, potatoes) while NMF Component 4 exhibited positive correlations with non-alcoholic drinks and weak correlations with organic food groups.
Cox models’ results for associations between dietary pesticide exposure and T2D risk are shown in Table 5. Median follow-up time was 5.95 years. In the extensively adjusted model (model 3), an increased risk of T2D was found for quintiles 3, 4 and 5 of NMF Component 1: HRQ5 vs Q1 = 1.47 (95% CI = 1.00, 2.18), p-trend 0.048. No significant associations were found for NMF Component 2 with HRQ5vsQ1 = 1.11, 95% CI (0.76, 1.62), nor NMF Component 4, HRQ5vsQ1 = 0.80, 95% CI (0.54, 1.18), nor Component 3, HRQ5vsQ1 = 0.88, 95% CI (0.60, 1.29). A model adjusted for fruit and vegetable intake (instead of sPNNS-GS2 score) was also performed and showed very similar results although somewhat attenuated (data not shown).
Stratifications were performed when p for interaction was < 0.20: p = 0.03 for interaction between NMF Component 1 and gender, p = 0.08 for sPNNS-GS2 tertiles and NMF Component 2, p = 0.15 for sPNNS-GS2 tertiles and NMF Component 3.
After stratification by gender (Table 6), the association remained only in women: HRQ5vsQ1 = 1.28 (95% CI = 1.00, 2.84), p-trend 0.003.
When stratifying by sPNNS-GS2 score, positive associations were found for quintiles 3–4 of NMF Component 2, in sPNNS-GS2 tertile 3 (highest adherence to French dietary guidelines). A negative association was evidenced in the same tertile for quintile 5 of NMF Component 3, HRQ5vsQ1 = 0.31 (95% CI = 0.10, 0.94).
Several sensitivity analyses were performed. Additional adjustments for provegetarian score, pDQI, cDQI or aDQI did not change results (data not shown).
After excluding T2D cases within 1 year after baseline (Table 7), similar trends were observed but association was no longer significant for NMF Component 1, HRQ5vsQ1 = 1.50 (95% CI = 0.99, 2.27). Similar magnitude was found when excluding CVD cases before or during follow-up, for NMF Component 1, HRQ5vsQ1 = 1.47, 95% CI (0.99, 2.18), p-trend 0.04.
Finally, excluding metabolic abnormalities at baseline did not substantially change the results for NMF Component 1, but statistical power seemed reduced: HRQ5vsQ1 = 1.81, 95% CI (0.93, 3.52), p-trend 0.05.
Discussion
In this large sample of French adults, we observed positive associations between NMF component 1 (reflecting higher exposure to a synthetic pesticides mixture of azoxystrobin, chlorpyriphos, imazalil, malathion, profenofos, thiabendazole) and T2D risk. After stratification on gender, these associations remained only in women. Further analysis revealed a T2D risk increase in association with NMF Component 2 and a T2D risk decrease in association with NMF Component 3, but only in the third tertile of sPNNS-GS2 score (high adherence to French dietary guidelines).
To our knowledge, the present work is the first to evaluate the associations between dietary pesticide exposure profiles and T2D risk in a large population-based sample. As a result, our findings cannot be directly compared to prior scientific literature.
However, some studies have been conducted to investigate associations between occupational, residential or domestic pesticide exposure and T2D risks [9].
Whilst research has been carried out on OCs [57, 58], now banned in the European Union, there is still little evidence on OPs, pyrethroids and neonicotinoids. However, associations between environmental exposure to pyrethroids and increased risk of all-cause and cardiovascular disease mortality and increased risk of T2D were recently found in two studies conducted in the US National Health and Nutrition Examination Survey [59, 60].
A study among wives of pesticides applicators of the Agricultural Health Study, conducted in Iowa and North Carolina (United States), did observe associations between some OPs and increased diabetes risk. However, these OPs (fonofos, phorate and parathion) were not included in our selected list [12]. Interestingly, another study among pesticide applicators of the Agricultural Health Study found a positive dose–response association between cumulative use of chlorpyrifos and incident diabetes [13]. In addition, a study in male farmers evidenced a positive correlation between malathion blood concentration, waist circumference and insulin resistance [14].
Despite different magnitudes due to the differences in exposure pathways, these results are consistent with our study where we found an association between NMF Component 1, positively correlated with malathion and chlorpyriphos, and incident T2D.
The fact that this association remains only in women after stratification on gender, could be linked to differential detoxification processes in men and women [61] or to limited power due to a low proportion of men in the cohort.
The negative associations between NMF Component 3 and T2D risk found in our study may partly be explained by the fact that this component is also negatively correlated with a few synthetic pesticides (azoxystrobin, chlorpropham, methamidophos) as well as being highly correlated with some pesticides used in organic farming (i.e. natural pyrethrins, spinosad). In addition to being less exposed to the synthetic studied pesticides, participants with high NMF Component 3 score also seemed particularly less exposed to pesticides with highly suspected toxicity such as chlorpyriphos, imazalil and malathion. The association was detected in the third tertile of sPNNS-GS2 score only (highest adherence to French dietary guidelines), where the participants have the highest consumptions of fruits and vegetables and also highest proportions of organic food in their diet. These results reflect those of a study by Kesse-Guyot et al. in 2020, in the same cohort, who also reported a negative association between high organic food score and T2D risk (HRQ5 = 0.65; 95% CI(0.43; 0.97)) [62]. It is possible, as stated in this study, that organic farming regulations lead to a lower frequency or an absence of synthetic pesticide residues in organic foods compared with conventional foods thus conferring lower T2D risk. Furthermore, the observed effects had corresponding magnitude in the two studies.
Pesticides are biologically active compounds and their mechanisms of action and cellular targets are similar to those involved in the development of metabolic syndrome and hepatic complications in mammals [63]. Therefore, they can be considered as metabolic disrupting contaminants able to influence T2D development. Mechanisms underpinning these associations could be linked to the impact of pesticide alone or to mixture on glucose and lipid metabolisms [64]. Indeed, some insecticides like imidacloprid, can stimulate cholinergic receptors, which can lead to disorders of insulin and glucagon secretions [65]. Pesticides could also affect other insulin sensitive tissues such as liver [63]. In addition, pesticides can disturb intra-cellular mechanisms in the adipose tissue, leading to excessive adipogenesis and overweight or obesity, which is an important risk factor for diabetes [66, 67]. Finally, numerous pesticides are now considered as certain or possible endocrine disruptors, able to alter estrogen or androgen actions. These effects can then lead to obesity and diabetes [68, 69].
Limitations and strengths
Some limitations of this study should be noted. Firstly, the NutriNet-Santé cohort consists of volunteers, with a higher education level and higher incomes, who are possibly more preoccupied by their health and dietary intakes than the general French population [70, 71]. Therefore, caution is needed when extrapolating our results to other populations. These characteristics can also have consequences on health outcomes with lower prevalence and incidence of diseases, than those observed in the general population. In a previous study on T2D conducted in the NutriNet-Santé cohort, T2D incidence was found lower: 186 cases per 100 000 person-years in the sample after standardization vs 289 per 100,000 in the French population [72, 73].
Secondly, this is an observational study, therefore the causality of the observed associations cannot be established and residual confounding cannot be entirely ruled out.
It is possible that risk alpha inflated with multiple comparisons. However, our analyses were hypothesis-driven, supported by available data in animal or mechanistic studies in the literature and the number of outcomes and subgroup analyses were limited.
Other limitations, inherent to the pesticide database, could be mentioned: for instance, data were not available for animal products (with generally low levels of pesticides) and the database did not contain measures for copper or sulfur-based products, widely used in organic farming. Measures were performed in Germany, but products from all over European Union were tested. Pesticide exposure was estimated based on dietary intakes, since the financial burden limits biomarker monitoring in very large cohorts. Some urinary metabolites measures were available for a limited sub-sample of the cohort (300 individuals). For information, the expected links between pesticides used in the present study and available urinary metabolites are presented in Supplementary Table 11. Means and standard deviations for urinary concentrations of several metabolites for the sub-sample, in each quintile of NMF Components are presented in Supplementary Table 12 and Supplementary Table 13. It would be very interesting to have complete biomarker data. However, this type of data would not allow to identify precisely compounds to which participants are exposed. Another source of uncertainty could origin from potential concentration or dilution effects during washing, cooking or peeling on pesticide residue levels that we were not able to account for [42].
Some strengths of this study can also be advanced. First of all, this work proposes to study several compounds at the same time, via the approach by NMF profiles, unlike classical studies in the field where molecules are evaluated separately which neglects potential synergistic effects whereas mixtures are present even at very low doses [15, 68, 69, 74].
Moreover, a wide range of covariates were taken into account for adjustment in the Cox regression models, including major potential confounders such as diet quality indicators and lifestyle factors. In spite of limited number of diabetes cases, the sample size still allowed to compute additional stratified and sensitivity models in order to improve comprehension of these results and reduce confounding bias.
Conclusion
We observed a positive association between NMF Component 1, highly correlated to a mixture of synthetic pesticides such as azoxystrobin, chlorpyriphos, imazalil, malathion, profenofos, thiabendazole, and T2D risk. Another important finding was the negative association between lower synthetic pesticide exposure profile (through NMF component 3) and diabetes risk, specifically in those with a healthy diet. A positive association for NMF Component 2 was also found after stratification on French dietary guidelines only for those with a healthy diet (highest adherence to French dietary guidelines). Some published experimental studies provide basic knowledge explaining, at least partly, these observations.
These associations should be examined in other prospective studies, in diverse settings, to complement these observational studies in order to validate estimated dietary pesticide exposure. The pesticide mixtures found in this study could be administrated to animals in order to better understand underlying mechanisms. If confirmed by other studies, these findings may help to understand the role of dietary pesticide exposure in major chronic diseases’ incidence. These results would have important implications for developing prevention strategies for the whole population, through regulation or dietary guidelines.
Availability of data and materials
Researchers from public institutions can submit a collaboration request including information on the institution and a brief description of the project to collaboration@etude-nutrinet-sante.fr. All requests will be reviewed by the steering committee of the NutriNet-Santé study. A financial contribution may be requested. If the collaboration is accepted, a data access agreement will be necessary and appropriate authorizations from the competent administrative authorities may be needed. In accordance with existing regulations, no personal data will be accessible.
Abbreviations
- ADI:
-
Acceptable Daily Intake
- BMI:
-
Body Mass Index
- CépiDC:
-
French Centre for Epidemiology Medical Causes of Death database
- CI:
-
Confidence Interval
- CNIL:
-
Commission Nationale de l’Informatique et des Libertés
- CVUA:
-
Chemisches und Veterinäruntersuchungsamt
- DDE:
-
Dichlorodiphenyldichloroethylene
- DDT:
-
Dichlorodiphenyltrichloroethane
- EDI:
-
Estimated Daily Intake
- EFSA:
-
European Food and Safety Authority
- FFQ:
-
Food Frequency Questionnaire
- HCB:
-
Hexachlorobenzene
- HR:
-
Hazard Ratio
- IPAQ:
-
International Physical Activity Questionnaire
- IRB INSERM:
-
Institutional Review Board of the French Institute for Health and Medical Research
- NMF:
-
Non-negative Matrix Factorization
- OC:
-
Organochlorine
- OP:
-
Organophosphorous
- PNNS:
-
Programme National Nutrition Santé
- Q:
-
Quintile
- SD:
-
Standard Deviation
- SNIIRAM:
-
Système National d’Information Inter-Régimes de l’Assurance Maladie
- T2D:
-
Type 2 diabetes
- WHO:
-
World Health Organization
References
World Health Organization. Diabetes [Internet]. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. Cited 2 Feb 2021.
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81 Elsevier.
World Health Organization. WHO reveals leading causes of death and disability worldwide: 2000-2019 [Internet]. 2020. Available from: https://www.who.int/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019. Cited 2 Feb 2021.
Schwingshackl L, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Schwedhelm C, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol Springer. 2017;32:363–75.
Rezg R, Mornagui B, El-Fazaa S, Gharbi N. Organophosphorus pesticides as food chain contaminants and type 2 diabetes: a review. Trends Food Sci Technol. 2010;21:345–57.
Longnecker M, Daniels J. Environmental contaminants as etiologic factors for diabetes. Environ Health Perspect. 2001;109:871–6.
Lind PM, Lind L. Endocrine-disrupting chemicals and risk of diabetes: an evidence-based review. Diabetologia. 2018;61:1495–502.
Han X, Zhang F, Meng L, Xu Y, Li Y, Li A, et al. Exposure to organochlorine pesticides and the risk of type 2 diabetes in the population of East China. Ecotoxicol Environ Saf. 2020;190:110125.
Evangelou E, Ntritsos G, Chondrogiorgi M, Kavvoura FK, Hernández AF, Ntzani EE, et al. Exposure to pesticides and diabetes: A systematic review and meta-analysis. Environ Int. 2016;91:60–8.
Juntarawijit C, Juntarawijit Y. Association between diabetes and pesticides: a case-control study among Thai farmers. Environ Health Prev Med. 2018;23:3.
Jaacks LM, Staimez LR. Association of persistent organic pollutants and non-persistent pesticides with diabetes and diabetes-related health outcomes in Asia: A systematic review. Environ Int. 2015;76:57–70.
Starling AP, Umbach DM, Kamel F, Long S, Sandler DP, Hoppin JA. Pesticide use and incident diabetes among wives of farmers in the Agricultural Health Study. Occup Environ Med. 2014;71:629–35 BMJ Publishing Group Ltd.
Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP. Incident Diabetes and Pesticide Exposure among Licensed Pesticide Applicators: Agricultural Health Study, 1993–2003. Am J Epidemiol Oxford Academic. 2008;167:1235–46.
Raafat N, Abass MA, Salem HM. Malathion exposure and insulin resistance among a group of farmers in Al-Sharkia governorate. Clin Biochem. 2012;45:1591–5.
Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol Cell Endocrinol. 2012;361:106–15.
Sun Y, Liu B, Du Y, Snetselaar LG, Sun Q, Hu FB, et al. Inverse association between organic food purchase and diabetes mellitus in US adults. Nutrients. 2018;10:1877 Multidisciplinary Digital Publishing Institute.
European Food Safety Authority. The European Union report on pesticide residues in food. EFSA J. 2017;(15):e04791.
Monitoring data on pesticide residues in food. results on organic versus conventionally produced food. EFSA Support Publ. 2018;15:1397E.
Oates L, Cohen M, Braun L, Schembri A, Taskova R. Reduction in urinary organophosphate pesticide metabolites in adults after a week-long organic diet. Environ Res. 2014;132:105–11.
Baudry J, Debrauwer L, Durand G, Limon G, Delcambre A, Vidal R, et al. Urinary pesticide concentrations in French adults with low and high organic food consumption: results from the general population-based NutriNet-Santé. J Expo Sci Environ Epidemiol. 2018. Available from: http://www.nature.com/articles/s41370-018-0062-9. Cited 23 Nov 2018.
Bradman A, Quirós-Alcalá L, Castorina R, Aguilar Schall R, Camacho J, Holland NT, et al. Effect of Organic Diet Intervention on Pesticide Exposures in Young Children Living in Low-Income Urban and Agricultural Communities. Environ Health Perspect. 2015;123:1086–93.
Curl CL, Beresford SAA, Fenske RA, Fitzpatrick AL, Lu C, Nettleton JA, et al. Estimating Pesticide Exposure from Dietary Intake and Organic Food Choices: The Multi-Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 2015;123:475–83.
Hercberg S, Castetbon K, Czernichow S, Malon A, Mejean C, Kesse E, et al. The Nutrinet-Santé Study: a web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health. 2010;10:242.
Touvier M, Méjean C, Kesse-Guyot E, Pollet C, Malon A, Castetbon K, et al. Comparison between web-based and paper versions of a self-administered anthropometric questionnaire. Eur J Epidemiol. 2010;25:287–96.
Touvier M, Kesse-Guyot E, Méjean C, Pollet C, Malon A, Castetbon K, et al. Comparison between an interactive web-based self-administered 24 h dietary record and an interview by a dietitian for large-scale epidemiological studies. Br J Nutr. 2011;105:1055–64.
Vergnaud A-C, Touvier M, Méjean C, Kesse-Guyot E, Pollet C, Malon A, et al. Agreement between web-based and paper versions of a socio-demographic questionnaire in the NutriNet-Santé study. Int J Public Health. 2011;56:407–17.
Lassale C, Castetbon K, Laporte F, Camilleri GM, Deschamps V, Vernay M, et al. Validation of a Web-based, self-administered, non-consecutive-day dietary record tool against urinary biomarkers. Br J Nutr Cambridge University Press. 2015;113:953–62.
Lassale C, Castetbon K, Laporte F, Deschamps V, Vernay M, Camilleri GM, et al. Correlations between Fruit, Vegetables, Fish, Vitamins, and Fatty Acids Estimated by Web-Based Nonconsecutive Dietary Records and Respective Biomarkers of Nutritional Status. J Acad Nutr Diet. 2016;116:427-438.e5.
Lassale C, Péneau S, Touvier M, Julia C, Galan P, Hercberg S, et al. Validity of web-based self-reported weight and height: results of the Nutrinet-Santé study. J Med Internet Res. 2013;15:e152.
Baudry J, Méjean C, Allès B, Péneau S, Touvier M, Hercberg S, et al. Contribution of Organic Food to the Diet in a Large Sample of French Adults (the NutriNet-Santé Cohort Study). Nutrients. 2015;7:8615–32.
Kesse-Guyot E, Castetbon K, Touvier M, Hercberg S, Galan P. Relative validity and reproducibility of a food frequency questionnaire designed for French adults. Ann Nutr Metab. 2010;57:153–62.
Le Moullec N, Deheeger M, Hercberg S, Preziosi P, Monteiro P, Valeix P, et al. Validation du manuel-photos utilisé pour l’enquête alimentaire de l’étude SU.VI.MAX. Cah nutr diét. 1996;31:158–64 Paris: Masson.
Etude NutriNet-Santé. Table de Composition des Aliments de l’étude NutriNet-Santé (Nutrinet-Santé Study Food Composition Database). 2013.
Untersuchungsämter Baden-Württemberg. Chemisches und Veterinäruntersuchungsamt Stuttgart (Startseite) [Internet]. Available from: http://www.cvuas.de/pub/default.asp?subid=1. Cited 29 May 2019.
Baudry J, Pointereau P, Seconda L, Vidal R, Taupier-Letage B, Langevin B, et al. Improvement of diet sustainability with increased level of organic food in the diet: findings from the BioNutriNet cohort. Am J Clin Nutr. 2019;109:1173–88.
European Food Safety Authority. The European Union Report on Pesticide Residues in Food. EFSA J. 2016;(14):e04611.
GEMS/Food Euro. Second workshop on reliable evaluation of low-level contamination of food. Report on a workshop in the frame of GEMS/Food-Euro. Geneva: WHO; 1995.
European Food Safety Authority. Management of left-censored data in dietary exposure assessment of chemical substances. EFSA J. 2010;8:1557.
Rebouillat P, Vidal R, Cravedi J-P, Taupier-Letage B, Debrauwer L, Gamet-Payrastre L, et al. Estimated dietary pesticide exposure from plant-based foods using NMF-derived profiles in a large sample of French adults. Eur J Nutr. 2020. Available from: https://doi.org/10.1007/s00394-020-02344-8. Cited 31 Jul 2020.
Bognár A. Tables on weight yield of food and retention factors of food constituents for the calculation of nutrient composition of cooked foods (dishes). Karlsruhe: BFE; 2002.
Bergström L. Nutrient losses and gains in the preparation of foods. Rapport 32/94. Upps Livsmedelsverket Natl Food Adm [Internet]. 1994; Available from: http://www.fao.org/uploads/media/Bergstroem_1994_32_Livsmedelsverket_nutrient_losses_and_gains.pdf
Yigit N, Velioglu YS. Effects of processing and storage on pesticide residues in foods. Crit Rev Food Sci Nutr [Internet]. 2019; Available from: https://doi.org/10.1080/10408398.2019.1702501. Cited 8 Jan 2020
Barański M, Średnicka-Tober D, Volakakis N, Seal C, Sanderson R, Stewart GB, et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: a systematic literature review and meta-analyses. Br J Nutr. 2014;112(5):794–811.
Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–62.
Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Méjean C, Andrianasolo RM, et al. Ultra-processed food intake and risk of cardiovascular disease: prospective cohort study (NutriNet-Santé). BMJ. 2019;365:l1451.
High Council for Public Health. French Nutrition and Health Programme’s dietary guidelines for adults for the period 2017–2021. 2017;7.
Chaltiel D, Adjibade M, Deschamps V, Touvier M, Hercberg S, Julia C, et al. Programme National Nutrition Santé – guidelines score 2 (PNNS-GS2): development and validation of a diet quality score reflecting the 2017 French dietary guidelines. Br J Nutr. 2019;122(3):331–42.
Martínez-González MA, Sánchez-Tainta A, Corella D, Salas-Salvadó J, Ros E, Arós F, et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(Suppl 1):320S-S328.
Keaver L, Ruan M, Chen F, Du M, Ding C, Wang J, et al. Plant- and animal-based diet quality and mortality among US adults: a cohort study. Br J Nutr. 2020;18:1–11. Cambridge University Press.
Ferrari P, Slimani N, Ciampi A, Trichopoulou A, Naska A, Lauria C, et al. Evaluation of under- and overreporting of energy intake in the 24-hour diet recalls in the European Prospective Investigation into Cancer and Nutrition (EPIC). Public Health Nutr Cambridge University Press. 2002;5:1329–45.
Seung D, Lee L. Algorithms for non-negative matrix factorization. Adv Neural Inf Process Syst. 2001;13:556–62.
Béchaux C, Zetlaoui M, Tressou J, Leblanc J-C, Héraud F, Crépet A. Identification of pesticide mixtures and connection between combined exposure and diet. Food Chem Toxicol. 2013;59:191–8.
Traoré T, Béchaux C, Sirot V, Crépet A. To which chemical mixtures is the French population exposed? Mixture identification from the second French Total Diet Study. Food Chem Toxicol. 2016;98:179–88.
Traoré T, Forhan A, Sirot V, Kadawathagedara M, Heude B, Hulin M, et al. To which mixtures are French pregnant women mainly exposed? A combination of the second French total diet study with the EDEN and ELFE cohort studies. Food Chem Toxicol. 2018;111:310–28.
Mancini FR, Frenoy P, Fiolet T, Fagherazzi G, Crépet A. Identification of chemical mixtures to which women are exposed through the diet: Results from the French E3N cohort. Environ Int. 2021;152:106467.
Gaujoux R, Seoighe C. A flexible R package for nonnegative matrix factorization. BMC Bioinformatics. 2010;11:367.
Lee D-H, Steffes MW, Sjödin A, Jones RS, Needham LL, Jacobs DR. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PLoS ONE. 2011;6:e15977.
Tang M, Chen K, Yang F, Liu W. Exposure to Organochlorine Pollutants and Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLOS One. 2014;9:e85556 Public Library of Science.
Park J, Park SK, Choi Y-H. Environmental pyrethroid exposure and diabetes in US adults. Environ Res. 2019;172:399–407.
Bao W, Liu B, Simonsen DW, Lehmler H-J. Association Between Exposure to Pyrethroid Insecticides and Risk of All-Cause and Cause-Specific Mortality in the General US Adult Population. JAMA Intern Med. 2020;180:367.
Liang X, Feswick A, Simmons D, Martyniuk CJ. Environmental toxicology and omics: A question of sex. J Proteomics. 2018;172:152–64.
Kesse-Guyot E, Rebouillat P, Payrastre L, Allès B, Fezeu LK, Druesne-Pecollo N, et al. Prospective association between organic food consumption and the risk of type 2 diabetes: findings from the NutriNet-Santé cohort study. Int J Behav Nutr Phys Act. 2020;17:136.
Rives C, Fougerat A, Ellero-Simatos S, Loiseau N, Guillou H, Gamet-Payrastre L, et al. Oxidative Stress in NAFLD: Role of Nutrients and Food Contaminants. Biomolecules. 2020;10:1702 Multidisciplinary Digital Publishing Institute.
Xiao X, Clark JM, Park Y. Potential contribution of insecticide exposure and development of obesity and type 2 diabetes. Food Chem Toxicol. 2017;105:456–74.
Kim J, Park Y, Yoon KS, Clark JM, Park Y. Imidacloprid, a neonicotinoid insecticide, induces insulin resistance. J Toxicol Sci. 2013;38:655–60.
Park Y, Kim Y, Kim J, Yoon KS, Clark J, Lee J, et al. Imidacloprid, a neonicotinoid insecticide, potentiates adipogenesis in 3T3-L1 adipocytes. J Agric Food Chem ACS Publications. 2013;61:255–9.
Egusquiza RJ, Blumberg B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology [Internet]. 2020;161. Available from: https://doi.org/10.1210/endocr/bqaa024. Cited 28 Apr 2021
Alonso-Magdalena P, Quesada I, Nadal A. Endocrine disruptors in the etiology of type 2 diabetes mellitus. Nat Rev Endocrinol Nature Publishing Group. 2011;7:346–53.
Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015;36:E1-150.
Andreeva VA, Salanave B, Castetbon K, Deschamps V, Vernay M, Kesse-Guyot E, et al. Comparison of the sociodemographic characteristics of the large NutriNet-Santé e-cohort with French Census data: the issue of volunteer bias revisited. J Epidemiol Community Health. 2015;69:893–8 BMJ Publishing Group Ltd.
Andreeva VA, Deschamps V, Salanave B, Castetbon K, Verdot C, Kesse-Guyot E, et al. Comparison of Dietary Intakes Between a Large Online Cohort Study (Etude NutriNet-Santé) and a Nationally Representative Cross-Sectional Study (Etude Nationale Nutrition Santé) in France: Addressing the Issue of Generalizability in E-Epidemiology. Am J Epidemiol. 2016;184:660–9.
Santé Publique France. L’état de santé de la population en France. Rapport 2017 [Internet]. 2017. Available from: https://www.santepubliquefrance.fr/docs/l-etat-de-sante-de-la-population-en-france.-rapport-2017.
Srour B, Fezeu LK, Kesse-Guyot E, Allès B, Debras C, Druesne-Pecollo N, et al. Ultraprocessed Food Consumption and Risk of Type 2 Diabetes Among Participants of the NutriNet-Santé Prospective Cohort. JAMA Intern Med American Medical Association. 2020;180:283–91.
Lukowicz C, Ellero-Simatos S, Régnier M, Polizzi A, Lasserre F, Montagner A, et al. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ Health Perspect. 2018;126:067007.
Acknowledgements
We especially thank Cédric Agaesse (manager), Alexandre De-Sa and Rebecca Lutchia (dietitians); Thi Hong Van Duong, Younes Esseddik (IT manager), Régis Gatibelza, Jagatjit Mohinder and Aladi Timera (computer scientists); Julien Allegre, Nathalie Arnault, Laurent Bourhis, Nicolas Dechamp and Fabien Szabo de Edelenyi, PhD (manager) (data-manager/statisticians); Sandrine Kamdem (health event validator); Maria Gomes (Nutrinaute support) for their technical contribution to the NutriNet-Santé study and Nathalie Druesne-Pecollo, PhD (operational manager).
We also thank the CVUAS for the pesticide residue database and Noémie Soton for her contribution to the data management of the CVUA database.
We warmly thank all of the dedicated and conscientious volunteers involved in the NutriNet-Santé cohort.
Funding
The NutriNet-Santé study is supported by the following public institutions: French Ministry of Health (DGS), Santé Publique France, the National Institute for Health and Medical Research (INSERM), the French National Research Institute for Agriculture, Food and Environment (INRAE), the National Conservatory of Arts and Crafts (CNAM) and the Sorbonne Paris Nord University. The BioNutriNet project was supported by the French National Research Agency (ANR) in the context of the 2013 Programme de Recherche Systèmes Alimentaires Durables (ANR-13-ALID-0001). This study is also supported by the Medical Research Foundation (FRM n° ENV202109013962). Pauline Rebouillat is supported by a doctoral fellowship from Sorbonne Paris Nord University.
Author information
Authors and Affiliations
Contributions
RV, DL, JB, SH, MT, LF and EK-G conducted the research. PR performed statistical analyses and drafted the manuscript. All authors critically helped in the interpretation of results, revised the manuscript and provided relevant intellectual input. They all read and approved the final manuscript. EK-G supervised the study, had primary responsibility for the final content, she is the guarantor.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The NutriNet-Santé study is conducted in accordance with the Declaration of Helsinki, and all procedures were approved by the Institutional Review Board of the French Institute for Health and Medical Research (IRB Inserm 0000388FWA00005831) and the Commission Nationale de l’Informatique et des Libertés (CNIL 908,450 and 909,216). All participants provided their informed consent with an electronic signature, and this study is registered in ClinicalTrials.gov (NCT03335644).
Consent for publication
Not applicable.
Competing Interests
None declared.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Material1. Description of the Chemischesund Veterinäruntersuchungsamtpesticide exposure database. Supplementary Material 2. Flowchart fordecomposition of ingredients and matching. Supplementary Material 3. 180 ingredients afterdecomposition. Supplementary Material 4. Details on thecomputation of the simplified Programme National Nutrition Santé Guideline Score2 (sPNNS-GS2 score). Supplementary Material 5. Details of the Non-Negative Matrix Factorization (NMF) procedure. Supplementary Table 1. Characteristics of quintiles forNMF Component 1, NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 2. Nutritional characteristics of the participants across quintiles of NMFcomponent 1, NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 3. Characteristics of quintiles for NMF Component 2, NutriNet-Santé Study, 2014(N=33,013). Supplementary Table 4 . Nutritional characteristics of the participants across quintiles of NMFcomponent 2, NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 5. Characteristics of quintiles for NMF Component 3, NutriNet-Santé Study, 2014(N=33,013). Supplementary Table 6 . Nutritional characteristics of the participants across quintiles of NMFcomponent 3, NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 7. Characteristics of quintiles for NMF Component 4, NutriNet-Santé Study, 2014(N=33,013). Supplementary Table 8 . Nutritional characteristics of the participants across quintiles of NMFcomponent 4, NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 9. Estimated dietary pesticide exposure across NMF Components 1 and 2 quintiles(in μg/kg of weight/day), NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 10. Estimated dietary pesticide exposure across NMF Components 3 and 4 quintiles(in μg/kg of weight/day), NutriNet-Santé Study, 2014 (N=33,013). Supplementary Table 11. Expected links between metabolites and parent compounds. Supplementary Table 12. Urinaryconcentrations (µg/g creatinine) for parent compounds and metabolites in NMFComponents 1 and 2 quintiles, NutriNet-Santé Study (N=296). Supplementary Table 13. Urinaryconcentrations (µg/g creatinine) for parent compounds and metabolites in NMFComponents 3 and 4 quintiles, NutriNet-Santé Study (N=296).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rebouillat, P., Vidal, R., Cravedi, JP. et al. Prospective association between dietary pesticide exposure profiles and type 2 diabetes risk in the NutriNet-Santé cohort. Environ Health 21, 57 (2022). https://doi.org/10.1186/s12940-022-00862-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-022-00862-y