Abstract
Background
The association between exposure to secondhand smoke (SHS) during pregnancy and a child’s neurodevelopment has not been established yet. We explored the association between prenatal exposure to SHS and neurodevelopment at 24 months of age considering genetic polymorphism and breastfeeding in 720 mothers and their offspring enrolled in the Korean multicenter birth cohort study (Mothers and Children Environmental Health, MOCEH).
Methods
We quantified urine cotinine concentrations in mothers once from 12th to 20th gestational weeks and excluded those whose urine cotinine levels exceeded 42.7 ng/ml to represent SHS exposure in early pregnancy. Mental developmental index (MDI) and psychomotor developmental index (PDI) values were measured using the Korean version of the Bayley Scales of Infant Development II (K-BSID-II) at 24 months of age. A general linear model was used to assess the relationship between maternal urinary cotinine level and neurodevelopment.
Results
MDI scores were inversely associated with cotinine [β = − 2.73; 95% confidence interval (CI): − 5.32 to − 0.15] in children whose mothers had early pregnancy urinary cotinine levels >1.90 ng/ml. No association was evident in children whose mothers had cotinine levels ≤1.90 ng/ml. This negative association was more pronounced in children whose mothers had both Glutathione S-transferases mu 1 (GSTM1) and theta 1 (GSTT1) null type [β = − 5.78; 95% CI: -10.69 to − 0.87], but not in children whose mothers had any present type of GSTM1/GSTT1 [β = − 1.64; 95% CI: -4.79 to 1.52]. The association was no longer significant when children received breast milk exclusively for up to 6 months [β = − 0.24; 95% CI: -4.69 to 4.20] compared to others [β = − 3.75; 95% CI: -7.51 to 0.00]. No significant association was found for PDI.
Conclusions
Maternal exposure to SHS during pregnancy may result in delayed MDI in early childhood. This effect might be modified by genetic polymorphism and breastfeeding behavior.
Similar content being viewed by others
Background
Exposure to active smoking and secondhand smoke (SHS) causes health concern. About 40% of children, 35% of women, and 33% of men are exposed to SHS in their daily lives [1]. World Health Organization (WHO) has recently reported that environmental risks including SHS take lives of 1.7 million children under 5 years of age every year [2]. Harmful exposure to these environmental risks could begin in the mother’s womb and affect fetal development. Therefore, more attention should be paid to pregnant women and infants who are susceptible to SHS exposure.
It is well known that SHS exposure brings about almost the same adverse health outcomes as active smoking [3]. Smoking during pregnancy is a well-known risk factor for adverse birth outcomes such as spontaneous abortion [4], low birth weight, and preterm birth [5] that, in turn might affect children’s development. Tobacco smoke contains over 7000 chemicals including nicotine, polycyclic aromatic hydrocarbons (PAHs), aromatic amines, and carbon monoxide. Placental passage of these environmental toxicants might affect prenatal nervous system development. Although effects of prenatal exposure to SHS on early neurodevelopment vary among studies, they remain significant issues. Lower development scores in cognition, language, and fine motor scales [6, 7], gross motor scores [8], and MDI scores [9] have been reported in children with prenatal SHS exposure. Therefore, SHS exposure should be considered a modifiable risk factor for delayed neurodevelopment and cognitive impairment in children. As cotinine is a predominant metabolite of nicotine, it is considered a biomarker of exposure to SHS [10]. Cotinine assays provide an objective quantitative measure that is more reliable than smoking history or counting the number of cigarettes smoked per day. Measure of cotinine in hair, blood, and urine permits the assessment of SHS exposure or active smoking.
Metabolic gene polymorphisms might modify the effect of toxins on the outcome of pregnancy and development afterwards. Glutathione S-transferases mu 1 (GSTM1) and theta 1 (GSTT1) are major detoxification phase II enzymes that provide critical defense against xenobiotics. Numerous smoke-derived chemicals such as PAHs and aromatic amines can be detoxified by GSTM1. Metabolites of 1,3-butadiene and ethylene oxide present in tobacco smoke are detoxified by GSTT1 [11]. Homozygous deletion polymorphisms of GSTM1 and GSTT1 (GSTM1-null and GSTT1-null) can result in loss of function and increase the risk of faulty fetal development [12]. GSTM1/GSTT1 polymorphisms can significantly modify birth outcomes following maternal exposure to tobacco smoke [13,14,15,16], heavy metals [17], perfluorinated compounds [18], and particulate matter [19]. These polymorphisms also influence early neurodevelopment of infants born following maternal exposure to environmental tobacco smoke [6].
Breastfeeding is thought to be associated with better neurodevelopment. It is well known that breastfeeding provides nutritional and immunological benefits to infants and promotes cognitive development between the mother and the infant [20,21,22]. Improved cognitive development of infants who are breastfed longer has been described elsewhere [23]. Benefits of breastfeeding even extend to mothers who are current smokers [24].
Although the Korean government has established several public policies since 1995, 39.7% of non-smokers are still exposed to SHS. This percentage is higher than that in other countries. In addition, 18.5% of pregnant women responded affirmatively to SHS exposure in Korea [25]. Although concern about the effect of SHS on health has been increasing, very few researches have been conducted using Korean population.
Therefore, the aim of this study was to investigate the association between prenatal SHS exposure and neurodevelopment of infants 24 months after birth using Korean birth cohort study. We further identified whether the association could be modified by maternal genetic polymorphisms and breastfeeding behavior.
Methods
Study population
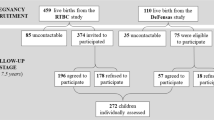
Data were from the Mothers and Children’s Environmental Health (MOCEH) study, a multicenter prospective cohort study initiated since 2006 in Korea. This study was designed to collect information related to environmental exposures during pregnancy and childhood to examine how exposure to environmental pollutants might affect growth, development, and disease in South Korea. Detailed information of the MOCEH study has been described previously [26]. Pregnant women in their first trimester were recruited from three university hospitals located in Seoul (metropolitan area), Cheonan (urban area, midwest), and Ulsan (metropolitan and industrial area, southeast) between 2006 and 2010. Participants were > 18 years of age. A total of 1751 pregnant women were enrolled in the beginning and 1516 mother-child pairs were followed up after birth (Fig. 1). Our study subjects were restricted to 801 children whose maternal urinary cotinine levels were determined between 12th and 20th gestational weeks. They also completed a neurodevelopmental follow-up assessment using Bayley tests at 24 months of age. There were no significant differences between children with Bayley scores (n = 801) and those without Bayley scores (n = 630) at 24 months with respect to maternal age, mother’s education level, genetic polymorphisms, sex of children, or breastfeeding behavior except for residential area and the primary caregiver up to 24 months after birth (Additional file 1: Table S1). Children (n = 53) were excluded if they were born with low birth weight (birth weight < 2500 g), preterm (gestational age < 37 weeks), or diagnosed with intrauterine growth restriction at birth. Written informed consent was obtained at the initial visit from all enrolled mothers on behalf of themselves and their children. Study protocols were approved by Institutional Review Boards of Ewha Womans University (Seoul), Dankook University Hospital (Cheonan), and Ulsan University Hospital (Ulsan).
Measurement of urine cotinine levels
Cotinine is considered a good biomarker of tobacco-smoke exposure. Urine can be obtained easily and non-invasively from pregnant women. Thus SHS exposure during pregnancy was quantified using urinary cotinine level in this study. Maternal urine was sampled once from 12th to 20th gestational weeks. Cotinine Direct ELISA Kits (Calibiotech, Spring Valley, CA, USA) were used for determinations. Urine was diluted 1:100 and 10 μl of the diluted sample was applied in duplicates to 96-well microtiter plates. Diluted urine sample was incubated with 100 μl of enzyme conjugate at room temperature for 60 min. Wells were washed six times with 300 μl distilled water prior to addition of 100 μl of substrate reagent to each well. Plates were then incubated at room temperature for 30 min. Absorbance was measured at 450 nm on an ELISA reader within 15 min after adding 100 μl of Stop Solution. Limit of detection (LOD) for urinary cotinine was 1.0 ng/ml. Values below this limit were converted to non-negative values by half of the detection limit (LOD/2) in the analysis. Urinary creatinine levels were adjusted in our analyses.
Assessment of infants’ MDI and PDI at 24 months
Neurodevelopment of infant at 24 months of age was measured using the Korean version of Bayley Scale of Infant Development II (K-BSID-II) [27], a standardized tool for assessing infant neurodevelopment [28]. This produces developmental indices and composite scores to compare developmental performance of a child with norms taken from typically developing Korean children of the same age. K-BSID-II also assesses habituation, problem solving, memory, classification, vocalization, and language skills. The resulting score represents mental development index (MDI). Psychomotor development index (PDI) is scored for the degree of body control, muscle coordination, postural control, and finer manipulatory skills. K-BSID-II has shown excellent test-retest stability and inter-rater agreement [27]. Test score was standardized, having a mean of 100 and a standard deviation of 15 [29]. To increase the inter-rater consistency, annual rater training sessions with video monitoring were held (inter-rater consistency: kappa value > 0.8) [30].
Genotyping of GSTM1 and GSTT1
Genomic DNA was extracted from maternal whole blood using a QIAamp DNA blood kit (Qiagen, Valencia, CA, USA). Polymerase chain reaction (PCR) was used to genotype GSTM1 and GSTT1 polymorphisms. As a positive control, a 268-bp fragment of β-globin gene was amplified at the same time. PCR mixture (20 μl) for GSTM1 and GSTT1 genotyping contained 10 mM Tris-HCl (pH 9.0), 40 mM KCl, 1.5 mM MgCl2, 0.25 mM of each dNTP, 1 unit Taq polymerase (Bioneer, Seoul, Korea), 20 pmol of forward and reverse primers each, and 50–100 ng of the genomic DNA as a template. The following primer sets of GSTM1 and GSTT1 genes were used for PCR reaction: 5′-GAACTCCCTGAAAAGCTAAAGC-3′ (forward) and 5′-GTTGGGCTCAAATATACGGTGG-3′ (reverse) for GSTM1, and 5′-TCACCGGATCATGGCCAGCA-3′ (forward) and 5′-TTCCTTACTGGTCCTCACATCTC-3′ (reverse) for GSTT1. Amplifications were performed using an initial denaturation at 94 °C for 5 min; 35 cycles of denaturation at 94 °C for 1 min, annealing at 65 °C for 1 min, and extension at 72 °C for 1 min; and a final extension at 72 °C for 7 min. PCR amplification of the reaction mixture was carried with a PTC-200 thermal cycler (MJ Research, Watertown, MA, USA).
To evaluate PCR-amplified fragments, electrophoresis was performed using 3% 3:1 NuSieve/agarose gel (Cambrex Bio Science, Rockland, ME, USA). Genotyping of GSTM1 and GSTT1 genes was performed based on the presence of a 215-bp product and a 459-bp product, respectively. Null genotype was defined as a homozygous deletion of the gene. To confirm results of analyses, 10% of samples were randomly selected and genotyped again, with identical results.
Covariates
Potential confounders were selected through literature review. Sociodemographic data including maternal age, maternal education level, and region were reported in the baseline questionnaire at the first visit. Each variable was considered as a categorical variable: ≤ 30 years old or > 30 years old for maternal age; ≤ high school or ≥ university for maternal education; and Seoul, Cheonan, and Ulsan for region. Information of infant sex as a categorical variable and gestational age as a continuous variable was obtained at delivery. At 6-month follow-up, mothers were asked about how they were feeding their babies. We categorized children into two groups: breastfeeding only for up to 6 months as exclusive breastfeeding or others. We also defined the primary caregiver during the first 24 months after birth based on the questionnaire at each follow-up: mother during the whole period or others.
Statistical analyses
First, we conducted receiver operating characteristic (ROC) curve analysis [31] to obtain the optimal cut-off point of urinary cotinine levels for distinguishing active smokers from non-active smokers during pregnancy. Based on our database, active smoker was defined as a mother who responded positively to a question, ‘Do you smoke now?’ in early or mid-pregnancy. Everyone else was considered a non-active smoker. ROC analysis is a graphical and quantitative technique that can determine the optimal cut-off point for a classified decision for a given continuous criterion variable. Here, the criterion variable is urinary cotinine. The classified condition is self-reported smoking status during pregnancy. Sensitivity is the percentage of smokers exceeding the cut-off point and specificity represents the percentage of nonsmokers below the cut-off point.
Characteristics of the study population are presented as mean ± standard deviation (SD) for continuous variables or numbers and percentages for categorical variables. Difference was compared using Chi-squared test or t-test. Maternal urinary cotinine level was natural log (ln)-transformed in all analyses because of its skewed distribution. Summary statistics for urinary cotinine level (including arithmetic and geometric means, and percentiles) were calculated.
Generalized additive model (GAM) [32] was used to examine the non-linear association between maternal urinary cotinine level at early pregnancy and offspring’s K-BSID-II test score at 24 months. Based on results from GAM analysis, children were divided into two groups according to their mothers’ urinary cotinine levels: above or below the median (1.90 ng/ml). For each group, associations between maternal urinary cotinine levels and offspring’s K-BSID-II test scores were separately assessed using a multiple linear regression model after adjusting for ln-transformed urinary creatinine level, sex of a child, maternal age, maternal education, gestational age, region, breastfeeding up to 6 months, and primary caregiver during the first 24 months after birth. Data were further analyzed after data stratification by maternal genetic polymorphisms or breastfeeding to investigate their modification effect on the association. Maternal GSTM1/GSTT1 genotype was classified as any present or both null. We then tested the significance of interaction term between maternal urinary cotinine level at early pregnancy and each stratum.
A supplementary analysis was conducted to examine the robustness of results. We repeated all analyses without adjusting for urinary creatinine because using urine cotinine values not corrected for urine creatinine values showed high correlation with smoking behavior [33]. All analyses were considered to be statistically significant if p-values were less than 0.05. All data preparation and analyses were performed using SAS statistical package, version 9.4 (SAS Institute Inc., Cary NC, USA).
Results
From ROC curve analysis, the optimal cut-off level for discriminating active smokers from non-smokers during pregnancy was 42.7 ng/ml, with a sensitivity of 81.8%, a specificity of 96.5%, and an area under the curve of 0.91 (Fig. 2). We then further excluded 28 children whose mothers were considered as active smokers because their urine cotinine levels exceeded 42.7 ng/ml. Finally, 720 mother-child pairs were included in the our analyses.
ROC curve analysis of urinary cotinine levels for differentiating current smokers from nonsmokers. The optimal urine cotinine cut-off level to distinguish active smokers from non-active smokers was 42.7 ng/ml, with sensitivity of 81.8% and specificity of 96.5%. The area under the curve (AUC) was 0.91
Demographic characteristics of study participants are presented in Table 1. Sixty percent of the women were ≤ 30 years old and 51.7% had at least a university education. MDI scores at 24 months were significantly higher in children with younger mothers. MDI and PDI scores at 24 months varied significantly according to mother’s education level and region. Girls displayed significantly higher scores of MDI and PDI at 24 months than boys. The median of maternal urinary cotinine level assessed at early pregnancy was 1.90 ng/ml.
Figure 3 presents a nonlinear relationship between ln-transformed maternal urinary cotinine level and residualized unadjusted MDI and PDI scores. Concerning MDI, no association was evident for lower level of urinary cotinine, although a negative association was observed for higher level of urinary cotinine (Fig. 3 (a)). Therefore, we divided our data into two cohorts and analyzed separately. One cohort included children whose maternal urinary cotinine levels at early pregnancy were at or below the median and the other cohort included children whose maternal urinary cotinine levels at early pregnancy were above the median. However, this pattern was not observed for PDI (Fig. 3 (b)).
Unadjusted associations of ln-transformed maternal urinary cotinine levels and Bayley scores in infants at 24 months of age. a Mental Development Index (MDI) and (b) Psychomotor Development index (PDI). The Generalized Additive Model was used. Dashed vertical straight line at 0.64 in each figure represents the median level of urinary cotinine, exp. (0.64) = 1.90
MDI scores of infants at 24 months of age and maternal urinary cotinine levels during early pregnancy were negatively associated if maternal urinary cotinine level exceeded the median (β = − 2.73, 95% CI: -5.32 to − 0.15 from adjusted analysis) (Table 2). However, such negative association between infant’s PDI score at 24 months and maternal urinary cotinine level disappeared. No association was found when maternal urinary cotinine level in early pregnancy was lower than the median.
The effect of maternal urinary cotinine level at early pregnancy on infant’s MDI score at 24 months of age differed depending on genetic polymorphism and breastfeeding behavior (Additional file 1: Figure S1). The observed significant negative association from Table 2 became much stronger for infants whose mothers had GSTM1/GSTT1 double deletion (β = − 5.78, 95% CI: -10.69 to − 0.87) (Table 3). The significance of this negative association disappeared in infants whose mothers had high cotinine levels when infants received breastmilk exclusively up to 6 months of age (β = − 0.24, 95% CI: -4.69 to 4.20). However, we found that none of the interaction terms between urinary cotinine level and each stratum was statistically significant.
These results did not change substantially when we conducted analyses without adjusting for urinary creatinine level (Additional file 1: Tables S2 and S3).
Discussion
We explored the association between maternal SHS exposure during early pregnancy and infant neurodevelopment at 24 months of age. We also examined the effect of genetic polymorphism and exclusive breastfeeding on such association. A significant association was found in children whose mothers had higher (greater than median) levels of cotinine. Cognitive development of 24 months old infants decreased significantly with increasing maternal cotinine level. For mothers with both GSTM1 and GSTT1 null types, the negative association became stronger. An association between PDI of 24 months old infants and maternal SHS exposure early in the pregnancy was not evident.
Previous studies have investigated the impact of prenatal maternal SHS exposure on infants’ neurodevelopment. Secondhand smoke exposure during pregnancy could be measured by parental self-reports or biomarkers such as cotinine in cord blood and cotinine in maternal urine during pregnancy. Based on self-reported prenatal exposure to SHS, previous studies have shown a negative impact of such exposure on cognitive development of infants aged 6–36 months [9, 34, 35]. Such negative association has also been observed by using cord blood cotinine level [6] and cotinine level in saliva during pregnancy [7]. We showed a consistent result using urinary cotinine as a biomarker of SHS exposure. A recent study has shown that maternal exposure to SHS during pregnancy measured through urine cotinine is associated with a decrease in gross motor function among children 18 months old [8]. However, no association was found for the impact of SHS exposure on cognitive function [8].
The biological mechanism by which SHS influences neurodevelopment delay has not been established yet. Huizink et al. [36] have provided potential mechanisms to explain the association between prenatal exposure to maternal smoking and neurobehavioral and cognitive outcomes based on animal and human studies. Results using rodent exposure models have suggested that prenatal nicotine exposure during critical periods of development can disrupt corticothalamic circuitry, resulting in long-lasting dysregulation of sensory information procession in the cortex [37]. A recent review study has concluded that the effect of SHS is consistent with the effect of direct smoking during pregnancy [3]. During the embryonic and fetal period, the development of central nervous system is vulnerable to toxic chemicals. Exposure to such chemicals might not only have short-term deficit, but also have long-term implications. Cigarette smoke contains thousands of noxious compounds, including mutagenic, neurotoxic, and fetotoxic agents such as nicotine, PAHs, aromatic amines, and carbon monoxide. They could pass through the placenta into the fetus [38,39,40]. These agents could cause fetal hypoxia-ischemia by reducing utero-placental blood flow. They might also influence fetal brain development [9]. Although cognition and motor function are known to be related to each other as the prefrontal cortex and the cerebellum may play an important role in both [41], our study demonstrated that infant’s MDI score rather than PDI score at 24 months of ages was affected by maternal SHS exposure.
Phase I and phase II metabolic enzymes are important in biotransformation of toxicants. GSTs consist of a superfamily of dimeric phase II metabolic enzymes that catalyze the conjugation of reduced glutathione with various electrophilic compounds [42]. Since GST enzymes play a vital role in cellular defense against environmentally toxic compounds, polymorphisms of GST gene can increase susceptibility to diseases caused by chemicals such as those in cigarettes. Therefore, the effect of prenatal SHS exposure on pregnancy outcomes can differ by maternal metabolic gene polymorphisms. Several studies have reported significant gene modification effects of maternal smoking or SHS exposure on birth outcomes [14,15,16, 43, 44]. Hsieh et al. [6] have reported that metabolic genes, GSTT1, as well as cytochrome P450 1A1 (CYP1A1) Ile462Val can modify the effect of cord blood cotinine level on early child neurodevelopment, especially for language and fine motor development using a small number of study subjects. Our present study in Korea with more participants using maternal urinary cotinine level in early pregnancy also provided evidence that metabolic genetic polymorphisms had modifying effect on the association between maternal SHS exposure in early pregnancy and neurodevelopment scores at 24 months. The proportion of double deletion of GSTM1 and GSTT1 observed in the Korean population has been found to be higher than that observed in Caucasian population, South Indians, or Afro-Americans [42]. Therefore, our study suggests the importance of considering maternal genetic polymorphisms when counseling about mother and their offspring’s health.
Breastfeeding up to 6 months is recommended by WHO because it has several health benefits [45]. It can protect against many diseases and medical conditions. It is also beneficial for the child’s development and behavior [46]. A specific examination of the relationship between breastfeeding and childhood cognition has revealed that children who are exclusively breastfed for the first 6 months have higher scores in Peabody Picture Vocabulary Test and Kaufman Brief Intelligence Test at 3 and 7 years of age than those not exclusively breastfed [47]. Some studies have suggested that the effect of breastfeeding on child neurodevelopment has to be investigated after adjusting for important confounders [48]. After controlling for some primary confounders such as maternal education level and primary caregiver during the first 24 months after birth, our study showed that the negative impact of SHS exposure at early pregnancy on MDI score at 24 months of age was diluted when children received breast milk exclusively for 6 months after birth.
We restricted mothers whose urine cotinine levels were ≤ 42.7 ng/ml and regarded them as non-active smokers because we focused on the effect of SHS exposure during pregnancy. There has been no standardized cut-off level of urinary cotinine to distinguish active smokers from non-active smokers. Several studies have suggested cut-off levels ranging from 50 to 550 ng/ml [49, 50]. These cut-off levels vary by race [51]. When we repeated analyses in all mothers without excluding mothers with high urinary cotinine levels, results were consistent (data not shown).
Previous studies have defined prenatal SHS exposed infants as those with cord blood cotinine level ≥ 0.16 ng/ml [6] or with maternal cotinine level in saliva during pregnancy ≥1.5 ng/ml [7]. Negative association was found between SHS exposure and early neurodevelopment of infants up to 24 months of age in the exposed group [6]. A negative association was also found in our study among women whose urinary cotinine levels exceeded the median of 1.90 ng/ml. However, further research is necessary concerning the threshold cotinine level in urine, saliva, or cord blood that affects neurodevelopment of infants.
The main strength of this study was its prospective cohort design with data collected from early pregnancy. We were able to study prenatal exposure in relation to children’s neurodevelopment. This prospective design enabled us to control for various potential epidemiological biases. Second, SHS exposure was not based on self-reported surveys. Instead, it was assessed using urinary cotinine as a biomarker. The use of self-reported SHS exposure might result in biased estimates because of recall bias. Several studies have described the advantage of using biomarkers for the assessment of SHS exposure compared to the use of self-reporting [52,53,54].
This study also has several limitations. Exposure to SHS reflects urinary cotinine level for a short time. The level of urine cotinine is associated with the amount of exposure to SHS. However, we used a single measurement of urinary cotinine level for the whole pregnancy which might not provide an accurate estimate of the exposure. Serial measurements of urinary cotinine throughout pregnancy are needed in future studies. Second, we did not include possible confounding factors such as quality of the home environment and ventilation in our analyses. Instead, we adjusted for maternal education as a surrogate marker. Third, numerous chemical compounds in SHS were activated and detoxified by both phase I and phase II enzymes. However, we could not investigate the role of other genes except GSTs due to the lack of such information in our database. Fourth, although we considered certain substantial confounders in our analyses, the effect of other environmental neurotoxins was not adjusted in our analysis. Further study is needed to investigate co-exposure of SHS and other environmental factors. Fifth, human neurodevelopment continues after birth. However, we did not consider the whole effect of postnatal exposure that could affect children’s neurodevelopment after birth except for breastfeeding behavior up to 6 months and primary caregiver up to 2 years of age. We showed that prenatal exposure to SHS with high urinary cotinine level at early pregnancy was negatively associated with neurodevelopment delay at 24 months, proposing that early pregnancy might be a critical window of public health intervention to reduce the effect. Fetal development is highly likely to be susceptible to environmental factors during the prenatal period. Thus, further analysis is needed to understand the overall association between exposure to SHS (including postnatal exposure) and children’s neurodevelopment.
Conclusion
Exposure to maternal SHS during early pregnancy, especially in those who have high cotinine levels, may affect neurodevelopment of infants at 24 months of age. Genetic polymorphism and breastfeeding may modify the effect of SHS exposure on neurodevelopment.
Abbreviations
- CI:
-
Confidence Interval
- GAM:
-
Generalized Additive Model
- GSTM1:
-
Glutathione S-transferases mu 1
- GSTT1:
-
Glutathione S-transferases theta 1
- K-BSID-II:
-
Korean version of the Bayley Scales of Infant Development II
- LOD:
-
Limit of Detection
- MDI:
-
Mental Developmental Index
- MOCEH:
-
Mothers and Children Environmental Health
- PAHs:
-
Polycyclic Aromatic Hydrocarbons
- PDI:
-
Psychomotor Developmental Index
- ROC:
-
Receiver Operating Characteristic
- SHS:
-
Secondhand Smoke
- WHO:
-
World Health Organization
References
Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377(9760):139–46.
Osseiran NC, K. The cost of a polluted environment: 1.7 million child deaths a year, says WHO 2017 [Available from: http://www.who.int/mediacentre/news/releases/2017/pollution-child-death/en/.
Chen R, Clifford A, Lang L, Anstey KJ. Is exposure to secondhand smoke associated with cognitive parameters of children and adolescents?--a systematic literature review. Ann Epidemiol. 2013;23(10):652–61.
Windham GC, Von Behren J, Waller K, Fenster L. Exposure to environmental and mainstream tobacco smoke and risk of spontaneous abortion. Am J Epidemiol. 1999;149(3):243–7.
Miyake Y, Tanaka K, Arakawa M. Active and passive maternal smoking during pregnancy and birth outcomes: the Kyushu Okinawa maternal and child health study. BMC Pregnancy Childbirth. 2013;13:157.
Hsieh CJ, Liao HF, Wu KY, Hsieh WS, Su YN, Jeng SF, et al. CYP1A1 Ile462Val and GSTT1 modify the effect of cord blood cotinine on neurodevelopment at 2 years of age. Neurotoxicology. 2008;29(5):839–45.
Polanska K, Krol A, Merecz-Kot D, Ligocka D, Mikolajewska K, Mirabella F, et al. Environmental Tobacco Smoke Exposure during Pregnancy and Child Neurodevelopment. Int J Environ Res Public Health. 2017;14:7.
Evlampidou I, Bagkeris M, Vardavas C, Koutra K, Patelarou E, Koutis A, et al. Prenatal second-hand smoke exposure measured with urine cotinine may reduce gross motor development at 18 months of age. J Pediatr. 2015;167(2):246–52 e2.
Lee BE, Hong YC, Park H, Ha M, Kim JH, Chang N, et al. Secondhand smoke exposure during pregnancy and infantile neurodevelopment. Environ Res. 2011;111(4):539–44.
Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31(1):14–30.
Park EY, Hong YC, Lee KH, Im MW, Ha E, Kim YJ, et al. Maternal exposure to environmental tobacco smoke, GSTM1/T1 polymorphisms and oxidative stress. Reprod Toxicol. 2008;26(3–4):197–202.
Jain M, Kumar S, Rastogi N, Lal P, Ghoshal UC, Tiwari A, et al. GSTT1, GSTM1 and GSTP1 genetic polymorphisms and interaction with tobacco, alcohol and occupational exposure in esophageal cancer patients from North India. Cancer Lett. 2006;242(1):60–7.
Danileviciute A, Grazuleviciene R, Paulauskas A, Nadisauskiene R, Nieuwenhuijsen MJ. Low level maternal smoking and infant birthweight reduction: genetic contributions of GSTT1 and GSTM1 polymorphisms. BMC Pregnancy Childbirth. 2012;12:161.
Hong YC, Lee KH, Son BK, Ha EH, Moon HS, Ha M. Effects of the GSTM1 and GSTT1 polymorphisms on the relationship between maternal exposure to environmental tobacco smoke and neonatal birth weight. J Occup Environ Med. 2003;45(5):492–8.
Infante-Rivard C, Weinberg CR, Guiguet M. Xenobiotic-metabolizing genes and small-for-gestational-age births: interaction with maternal smoking. Epidemiology. 2006;17(1):38–46.
Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287(2):195–202.
Lee BE, Hong YC, Park H, Ha M, Koo BS, Chang N, et al. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ Health Persp. 2010;118(3):437–42.
Kwon EJ, Shin JS, Kim BM, Shah-Kulkarni S, Park H, Kho YL, et al. Prenatal exposure to Perfluorinated compounds affects birth weight through GSTM1 polymorphism. J Occup Environ Med. 2016;58(6):e198–205.
Kim E, Park H, Park EA, Hong YC, Ha M, Kim HC, et al. Particulate matter and early childhood body weight. Environ Int. 2016;94:591–9.
Bachrach VR, Schwarz E, Bachrach LR. Breastfeeding and the risk of hospitalization for respiratory disease in infancy: a meta-analysis. Arch Pediatr Adolesc Med. 2003;157(3):237–43.
Bartels M, van Beijsterveldt CE, Boomsma DI. Breastfeeding, maternal education and cognitive function: a prospective study in twins. Behav Genet. 2009;39(6):616–22.
Bhandari N, Bahl R, Mazumdar S, Martines J, Black RE, Bhan MK, et al. Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet. 2003;361(9367):1418–23.
Lee H, Park H, Ha E, Hong YC, Ha M, Park H, et al. Effect of breastfeeding duration on cognitive development in infants: 3-year follow-up study. J Korean Med Sci. 2016;31(4):579–84.
Dorea JG. Maternal smoking and infant feeding: breastfeeding is better and safer. Matern Child Health J. 2007;11(3):287–91.
Center for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey 2011.
Kim BM, Ha M, Park HS, Lee BE, Kim YJ, Hong YC, et al. The mothers and Children's environmental health (MOCEH) study. Eur J Epidemiol. 2009;24(9):573–83.
Park HC, Cho BH. Korean Bayley scales of infant development. Interpretation manual. 2nd ed. Kidspop publishing corporation. 2006:Seoul, Korea.
Bayley N. Bayley scales of infant development. 2nd ed. San Antonio, TX: Psychological Corporation; 1993.
Kim Y, Ha EH, Park H, Ha M, Kim Y, Hong YC, et al. Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: the mothers and Children's environmental health (MOCEH) study. Neurotoxicology. 2013;35:15–22.
Kim E, Park H, Hong YC, Ha M, Kim Y, Kim BN, et al. Prenatal exposure to PM10 and NO2 and children's neurodevelopment from birth to 24 months of age: mothers and Children's environmental health (MOCEH) study. Sci Total Environ. 2014;481:439–45.
Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–7.
Hastie T, Tibshirani R. Generalized additive models. London: Chapman and Hall/CRC; 1990.
Jatlow P, McKee S, O'Malley SS. Correction of urine cotinine concentrations for creatinine excretion: is it useful? Clin Chem. 2003;49(11):1932–4.
Jedrychowski W, Perera FP, Jankowski J, Maugeri U, Mrozek-Budzyn D, Mroz E, et al. Early wheezing phenotypes and cognitive development of 3-yr-olds. Community-recruited birth cohort study. Pediatr Allergy Immunol. 2010;21(3):550–6.
Rauh VA, Whyatt RM, Garfinkel R, Andrews H, Hoepner L, Reyes A, et al. Developmental effects of exposure to environmental tobacco smoke and material hardship among inner-city children. Neurotoxicol Teratol. 2004;26(3):373–85.
Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;30(1):24–41.
Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122(2):125–39.
Lackmann GM, Salzberger U, Töllner U, Chen M, Carmella SG, Hecht SS. Metabolites of a tobacco-specific carcinogen in urine from newborns. J Natl Cancer Inst. 1999;91(5):459–65.
Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Paediatr. 2008;97(10):1331–7.
Weitzman M, Byrd RS, Aligne CA, Moss M. The effects of tobacco exposure on children's behavioral and cognitive functioning: implications for clinical and public health policy and future research. Neurotoxicol Teratol. 2002;24(3):397–406.
Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71(1):44–56.
Cho HJ, Lee SY, Ki CS, Kim JW. GSTM1, GSTT1 and GSTP1 polymorphisms in the Korean population. J Korean Med Sci. 2005;20(6):1089–92.
Sasaki S, Kondo T, Sata F, Saijo Y, Katoh S, Nakajima S, et al. Maternal smoking during pregnancy and genetic polymorphisms in the ah receptor, CYP1A1 and GSTM1 affect infant birth size in Japanese subjects. Mol Hum Reprod. 2006;12(2):77–83.
Wu T, Hu Y, Chen C, Yang F, Li Z, Fang Z, et al. Passive smoking, metabolic gene polymorphisms, and infant birth weight in a prospective cohort study of Chinese women. Am J Epidemiol. 2007;166(3):313–22.
Kramer MS, Kakuma R. The optimal duration of exclusive breastfeeding: a systematic review. Adv Exp Med Biol. 2004;554:63–77.
Horta BL, Loret de Mola C, Victora CG. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):14–9.
Belfort MB, Rifas-Shiman SL, Kleinman KP, Guthrie LB, Bellinger DC, Taveras EM, et al. Infant feeding and childhood cognition at ages 3 and 7 years: effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013;167(9):836–44.
Walfisch A, Sermer C, Cressman A, Koren G. Breast milk and cognitive development--the role of confounders: a systematic review. BMJ Open. 2013;3(8):e003259.
Jung S, Lee IS, Kim SB, Moon CS, Jung JY, Kang YA, et al. Urine cotinine for assessing tobacco smoke exposure in Korean: analysis of the Korea National Health and nutrition examination survey (KNHANES). Tuberc Respir Dis (Seoul). 2012;73(4):210–8.
Zielinska-Danch W, Wardas W, Sobczak A, Szoltysek-Boldys I. Estimation of urinary cotinine cut-off points distinguishing non-smokers, passive and active smokers. Biomarkers. 2007;12(5):484–96.
Signorello LB, Cai Q, Tarone RE, McLaughlin JK, Blot WJ. Racial differences in serum cotinine levels of smokers. Dis Markers. 2009;27(5):187–92.
Aranda Regules JM, Mateos Vilchez P, Gonzalez Villalba A, Sanchez F. Luna del Castillo Jde D. Validity of smoking measurements during pregnancy: specificity, sensitivity and cut-off points. Rev Esp Salud Publica. 2008;82(5):535–45.
DeLorenze GN, Kharrazi M, Kaufman FL, Eskenazi B, Bernert JT. Exposure to environmental tobacco smoke in pregnant women: the association between self-report and serum cotinine. Environ Res. 2002;90(1):21–32.
Jhun HJ, Seo HG, Lee DH, Sung MW, Kang YD, Syn HC, et al. Self-reported smoking and urinary cotinine levels among pregnant women in Korea and factors associated with smoking during pregnancy. J Korean Med Sci. 2010;25(5):752–7.
Acknowledgements
This study was supported by MOCEH (Mothers and Children’s Environmental Health) project of the National Institute of Environmental Research, Republic of Korea.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A3A110335).
Availability of data and materials
Datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
ML conceptualized and designed the study, conducted the analysis and interpretation of study data, drafted the initial manuscript, and reviewed and revised the manuscript; EK and YK provided use of neurodevelopment assessments; MH, YH, HP, and YK conceptualized and designed the study, and critically reviewed and revised manuscript; and EH conceptualized and designed the study, and reviewed and revised the manuscript; All authors critically reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study protocols were approved by Institutional Review Boards of Ewha Womans University (Seoul), Dankook University Hospital (Cheonan), and Ulsan University Hospital (Ulsan).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Comparison of general characteristics between children with K-BSID-II scores and children without K-BSID-II scores at 24 months. Table S2. Association between maternal urine cotinine in early pregnancy and children’s neurodevelopment at 24 months without adjusting for creatinine level. Table S3. Association between maternal urine cotinine in early pregnancy and children’s neurodevelopment at 24 months stratified by genetic polymorphism and breastfeeding behavior without adjusting for creatinine level. Figure S1. Unadjusted associations of ln-transformed maternal urinary cotinine levels and Bayley scores in infants at 24 months of age stratified by genetic polymorphism and breastfeeding behavior. (DOCX 562 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lee, M., Ha, M., Hong, YC. et al. Exposure to prenatal secondhand smoke and early neurodevelopment: Mothers and Children’s Environmental Health (MOCEH) study. Environ Health 18, 22 (2019). https://doi.org/10.1186/s12940-019-0463-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12940-019-0463-9