Abstract
Background
Cardiovascular disease (CVD) is the leading cause of mortality, and vascular calcification has been highly correlated with CVD events. Abdominal aortic calcification (AAC) has been shown to predict subclinical CVD and incident CVD events. However, the relationship between vitamin C and abdominal aortic calcification remains unclear.
Objective
To investigate the relationship of dietary vitamin C with AAC among the adult population in the US.
Methods
The National Health and Nutrition Examination Survey (NHANES) 2013–2014 provided the data for the cross-sectional study. 2297 subjects (1089 males) were included in the study. Two scoring systems, AAC 24-point scale (Kauppila) and AAC 8-point scale (Schousboe), were used for the measurement of AAC score. Dietary vitamin C intake was calculated as the average of two rounds of 24-h interview recall data and classified in tertiles for analysis. We applied weighted multiple regression analyses to assess the relationship of dietary vitamin C with AAC score and the risk of having AAC. To ensure the robustness of the findings, subgroup and sensitivity analyses were performed. Additionally, smooth curve fittings, using generalized additive models (GAM) were employed to visualize potential nonlinear relationships. Furthermore, an exploratory analysis on the relationship of vitamin C supplements with AAC was also conducted.
Results
The results showed that higher dietary vitamin C intake was related to a reduction in AAC score (AAC-24: β = -0.338, 95% confidence interval [CI] -0.565, -0.111, P = 0.004; AAC-8: β = -0.132, 95%CI -0.217, -0.047, P = 0.002), and lower risk of AAC (odds ratio [OR] = 0.807, 95%CI 0.659, 0.989, P = 0.038). However, the relationship of vitamin C supplements with AAC was not identified.
Conclusions
The study revealed that higher intake of dietary vitamin C rather than vitamin C supplements was related to reduced AAC score and lower risk of AAC, indicating that diets rich in vitamin C are recommended due to its potential benefits for protecting against vascular calcification and CVD among the adult population in the US.
Similar content being viewed by others
Introduction
Vascular calcification is typified by the pathological deposition of calcium phosphate crystals in the intima of arteries [1], which has been determined to be a risk factor for cardiovascular diseases (CVDs) and is highly linked to the increased risk of atherosclerotic plaque rupture, adverse cardiovascular events and all-cause mortalit [2]. To date, the understanding of the calcification mechanism remains incomplete, and the available treatments for calcification are restrictive. While, animal studies have shown the promising potential of statin [3] and calcium channel blockers (CCBs) in treating vascular calcification, their efficacy in human subjects remains controversial [4,5,6]. Although sodium thiosulfate has been validated as an effective treatment for vascular calcification in rats [7], further large randomized controlled trials are necessary to confirm its clinical efficacy and safety for human use. Given the association of vascular calcification with CVDs and mortality, and the therapeutic dilemma, the prevention and improvement method of vascular calcification deserve further exploration.
Abdominal aortic calcification (AAC), as a potential indicator related to vascular morbidity and mortality, occurs earlier than coronary artery calcification and has been shown to predict subclinical CVDs and incident CVD events, independently of traditional risk factors [8,9,10]. Moreover, AAC was found outperforming the Framingham risk score as a sensitive predictive factor of cardiovascular events in a retrospective study [11]. The Multi-Ethnic Study of Atherosclerosis (MESA) study examined the difference in cardiovascular outcomes between AAC and coronary artery calcification (CAC). Both AAC and CAC were identified as independent predictors for coronary heart disease and CVD. Nevertheless, it was AAC, not CAC, that demonstrated a significant association with cardiovascular disease mortality, and AAC was a more robust predictor for all-cause mortality than CAC [12]. Early prevention and treatment of AAC or slowing its progression is essential, even with lesser calcification. A 10-year longitudinal study demonstrated that both a rapid nonlinear increase and a late slow-increasing trajectory of abdominal aortic calcification were associated with an increased risk of death compared with a stable trajectory[adjusted hazard ratios (HR) 1.91; 95% CI 1.02–3.58 and adjusted HR 2.79; 95% CI: 1.44–5.11, respectively] [13]. A 5-year randomized controlled trial of calcium supplementation versus placebo enrolling 1471 healthy women indicated that compared with patients without AAC, the unadjusted HR for the occurrence of CVD events and myocardial infarction in patients with any AAC were 1.7 and 2.3, respectively. The relative risk of myocardial infarction remained significant after 5-year cardiovascular disease risk adjustment based on clinical risk factors. In addition, progression of AAC was significantly associated with incident CVD and incident myocardial infarction, with HR of 1.6 and 2.1, respectively [14]. Therefore, methods of reversing abdominal aortic calcification at any level or methods of slowing the progression of calcification are worth exploring and may be benefit to the patients. The AAC measurement is commonly carried out using the scoring systems of of Kauppila [15] and Schousboe [16].
Dietary approaches have gained significant attention for their role in the primary prevention of CVDs, and other chronic disease [17]. Dietary Vitamin C, widely found in foods including fruits, vegetables, legumes, nuts and whole grains, is an essential dietary requirement for humans, and has been related to cardiovascular health [18, 19]. Vitamin C has antioxidant properties that reduce the production of reactive oxygen species, have anti-inflammatory effects, and the ability to reduce lipid peroxidation [20]. It has been suggested as a possible preventive therapy for CVDs and corresponding mortality [21,22,23], and may play a part in vascular calcification. A meta-analysis that included three interventional studies and 15 prospective cohort studies demonstrated that higher vitamin C intake was correlated with a lower risk of CVD mortality (RR = 0.79, 95% CI 0.68, 0.89) [24]. Previous observations indicating a lower risk of stroke and coronary heart disease with higher vitamin C intake may be attributed to the protective effect of vitamin C against calcification [25, 26]. Furthermore, vitamin C was discovered to interfere with the process of vascular calcification in a vitro experiment. The study demonstrated that vitamin C significantly reduced calcium accumulation produced and deposited by vascular smooth muscle cells [27]. However, the clinical evidence is lacking.
To our knowledge, there were no clinical studies that reported the relationship of dietary vitamin C with AAC. Thus, we applied cross-sectional research to validate the hypothesis that higher dietary vitamin C intake is in association with reduced AAC score and lower risk of AAC.
Materials and methods
Study population
Data were derived from the National Health and Nutrition Examination Survey (NHANES), a cross-sectional investigation aimed at obtaining preliminary information and assessing the health status of the US population. NHANES is conducted by National Center for Health Statistic (NCHS), using a non-institutionalized design that involves a multistage probability sample with stratification. NCHS Ethics Review Board had reviewed the ethics of the NHANES and had approved its conduction. All the subjects signed the informed consent before participating in the survey [28].
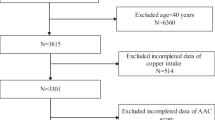
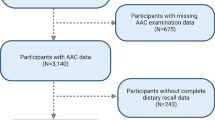
The selection of data from NHANES 2013–2014 cycle was based on its inclusion of AAC information, as no other cycle contained information of vascular calcification. Subjects were limited to those aged ≥ 40 years old, were not pregnant, and did not provide any self-reported radiation exposure over the previous seven-day period, due to the application of dual-energy X-ray absorptiometry (DXA) to measure AAC. We enrolled the subjects with data on both AAC and dietary vitamin C (n = 2640). Finally, the study included 2297 subjects after removing those with missing data on covariates. To further explore the relationship of vitamin C supplements with AAC, we also extracted the information of participants with data on vitamin C supplements (Fig. 1).
Exposure and outcome definitions
Dietary vitamin C as a continuous variable was the primary exposure in this study. While it was also classified into tertiles as a categorical variable for analysis, serving as the secondary exposure. Dietary data of NHANES, including information on food components, nutrients, and energy, was charged by Food Surveys Research Group (FSRG) regarding agriculture in the US. Dietary vitamin C was calculated as the average of two rounds of 24-h interview recall data. The initially collected recall was obtained in-person at the Mobile Examination Center (MEC), while the second interview was carried out over the phone after a period of 3 to 10 days.
AAC was the dependent variable in the study. The examination section of NHANES provided the data of AAC. The AAC was presented by images of lateral spine obtained through DXA, which could reduce radiant exposure. The lateral spine scans provide image resolution comparable to that observed in standard X-rays.The technologists were certified and annually trained. To determine the AAC score, Kauppila's approach (AAC-24 score) was employed, which divided the aorta's anterior and posterior walls into four sections adjacent to the lumbar vertebrae L1-L4. Each segment of the aorta was given a score ranging from 0 to 6 points based on the extent of calcification observed, resulting in a total score range of 0 to 24. AAC was deemed present if the Kauppila score was greater than 0, as evidenced by prior study [29]. All patients with AAC score were included in the analysis, not only for patients with severe calcification, to better exploring whether the dietary vitamin C could prevent or reverse the presence of calcification in the general population. In addition, we applied the Schousboe score (AAC-8 score) ranged from 0 to 8, as the outcome variable for sensitivity analysis. AAC-8 scoring method is similar to AAC-24 scoring, having the advantage of being less susceptible to the influence of small calcification distributed across 8 segments. However, AAC-8 measurement requires more expertise and skill [30].
Covariates
According to the clinical significance, demographics, economic status, metabolic indicators, comorbidities, and lifestyles were considered as confounding factors. The following covariates were included in our study: age, gender, body mass index (BMI), race/ethnicity, poverty ratio, cholesterol, creatinine, serum calcium, serum phosphorus, atherogenic index of plasma (AIP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), diabetes, hypertension, angina/angina pectoris history, coronary heart disease history, alcohol consumption, smoking status, and daily energy intake. The presentation of covariates was shown in the Supplementary Table 1. The measurements for the covariates mentioned above are accessible on the site: www.cdc.gov/nchs/nhanes/.
Sensitivity, subgroup and exploratory analyses
To ensure the robustness of the results, AAC-8 scoring method for the vascular calcification was used for sensitivity analysis. Subgroup analyses stratified by gender, BMI (BMI ≥ 30 kg/m2; BMI < 30 kg/m2) [31], age (age ≥ 65 years; age < 65 years) [32], diabetes, hypertension, angina/angina pectoris, coronary heart disease, alcohol consumption and smoking status were conducted to access the stability of the results among different population stratification. Moreover, vitamin C supplement was used as an exploratory exposure in the study. We applied exploratory analysis on the relationship between vitamin C supplements and AAC to investigate the effect of different sources of the vitamin C on vascular calcification. Vitamin C supplements were determined by averaging the data from two rounds of 24-h recall interviews. Besides, data on vitamin C supplements within the past 30 days provided directly by NHANES in the dietary data module were also included in the analysis.
Statistical analyses
Due to the complexity of the NHANES design, which includes multiple stages of cluster, weighted analysis was conducted according to the recommendation of the NCHS. Weighted means with standard error (SE) were used to describe continuous variables, whereas, frequency (percentage) was used to describe categorical variables. Dietary vitamins C was divided into tertiles for analysis. The difference in variables among different groups was determined using a weighted chi-squared test for categorical variables and a weighted linear regression model for continuous variables. We performed weighted multiple regression analyses to investigate the independent relationship of dietary vitamin C and vitamin C supplements with AAC after adjusting for covariates. Weighted logistic regression model was used for analyzing the association of vitamin C with AAC risk. Using stratified multivariate regression, we performed subgroup analysis, and all covariates except the stratification variable itself were adjusted. P for interaction was based on the log likelihood ratio test to assess the heterogeneity of relationship between the subgroups. Furthermore, we employed GAM-based smooth curve fittings to examine whether the relationship of dietary vitamin C with AAC was nonlinear. If the value of two-sided P was less than 0.05, it was considered statistically significant. We performed statistical analysis using R version 3.4.3 (http://www.R-project.org, The R Foundation) and Empower software (www.empowerstats.com; X&Y solutions, Inc., Boston MA).
Results
Baseline
The weighted characteristics of subjects are presented in Table 1. 2297 subjects with mean age of 57.81 ± 11.41 years were included in this study, of whom 51.34% were female and 71.72% were non-Hispanic White. The average dietary vitamin C intake was (82.46 ± 70.09) mg/day, and the mean intake of dietary vitamin C for tertiles was (21.57 ± 10.78) mg/day, (64.44 ± 15.47) mg/day, and (160.90 ± 64.85) mg/day, respectively. Subjects who had higher dietary intake of vitamin C tended to be older and have higher daily energy intake, and were less likely to have diabetes, angina/angina pectoris history and belong to a lower socioeconomic status. Furthermore, they were more likely to have lower BMI, cholesterol levels, serum phosphorus levels and AIP.
Inverse association of dietary vitamin C intake with AAC score and the risk of AAC
We conducted three weighted multiple regression models to evaluate the association of dietary vitamin C intake with AAC (Table 2). Age, race, gender, and BMI were adjusted in Model 1. Metabolic factors (cholesterol, creatinine, serum phosphorus, serum calcium, ALT, AST, and AIP) were added to adjust in Model 2. Model 3 was a fully adjusted model with the addition of economic status, comorbidities, and lifestyle factors (poverty ratio, hypertension, diabetes, angina/angina pectoris history, coronary heart disease history, smoking status, alcohol consumption, and daily energy intake). The inverse relationship of dietary vitamin C with AAC-24 score was evident in all models (Model 1: β = -0.376, 95%CI -0.550, -0.203, P < 0.001; Model 2: β = -0.368, 95%CI -0.541, -0.195, P < 0.001; Model 3: β = -0.338, 95%CI -0.565, -0.111, P = 0.004). It could be understood that each 100 mg increment in dietary vitamin C intake might be related to 0.338 decreases in AAC-24 score in the fully adjusted model. Furthermore, high-tertile group was related to decreased AAC-24 score (Model 3, T2: β = -0.484, 95%CI -0.870, -0.098, P = 0.014, T3: β = -0.723, 95%CI -1.128, -0.319, P < 0.001, P for trend < 0.001). When AAC score was treated as a categorical variable, inverse relationship was observed between dietary vitamin C intake and the risk of AAC (Model 3: OR = 0.807, 95%CI 0.659, 0.989, P = 0.038), which indicated that each 100 mg increment in dietary vitamin C intake might be related to 19.3% reduced risk of AAC.
To explore the potential non-linear association between dietary vitamin C intake and AAC, smooth curve fittings based on GAM were conducted. After the adjustment of all covariates, the results indicated that a linear relationship existed between dietary vitamin C intake and AAC (Fig. 2). The trends were in line with the regression analyses.
We also observed the inverse relationship of dietary vitamin C intake with AAC-8 score in all models, and subjects in the second and third tertiles had lower AAC-8 score than those in the first tertile, which indicated that the results were stable (Table 2).
Consistent results in different stratification of subgroups
We conducted the subgroup analysis, grouped by gender, age, BMI, hypertension, diabetes, angina/angina pectoris history, coronary heart disease history, alcohol consumption and smoking status, to explore whether the inverse relationship of dietary vitamin C intake and AAC is stable among the different population (Table 3). After adjusting for covariates, the trends of β-coefficients remained steady across all stratification, implying that the relationship of dietary vitamin C intake with AAC score was robust, with no statistically significant interaction found.
No inverse association of vitamin C supplements with AAC
To explore the effect of vitamin C supplements on vascular calcification, we conducted multiple regression analyses between vitamin C supplements and AAC (Supplementary Table 2). The results showed that neither two rounds of dietary interviews nor the past 30 days of vitamin C supplements intake were related to AAC score and the risk of having AAC.
Discussion
The research investigated the relationship of dietary vitamin C intake with AAC, by applying NHANES 2013–2014 data that is both extensive and representative. The findings suggested that increased dietary vitamin C intake was related to reduced AAC score and lower risk of AAC, and this association was observed across different population stratification with steady consistency. Moreover, there was no inverse relationship of vitamin C supplements with AAC, implying that not all sources of vitamin C are beneficial for vascular calcification and indicating the importance of dietary sources of vitamin C.
Vascular calcification with oxidative stress and inflammation
Vascular calcification happens in the intimal and middle layers of the arteries. It has been considered an active osteogenic process of vascular cells, mainly vascular smooth muscle cells (VSMCs), similar to the formation of osteoblasts [33]. The mechanisms contributing to VSMCs differentiation have not been fully elucidated, although the major drivers identified so far are oxidative stress, inflammation, aging, and uremia [34]. Exposure of VSMCs to hydrogen peroxide or oxidized low-density lipoprotein implying the accumulation of ROS leads to the increased expression of the osteogenic transcription factor Runx2 [35, 36], which might drive the osteocytic VSMC phenotype and trigger vascular calcification. An animal experiment demonstrated that the antioxidant Tempol reduced aortic and systemic oxidative stress levels, and inhibited osteogenic differentiation of VSMCs and arterial calcification in uremic rats [37].
Furthermore, inflammation was discovered to be linked to osteogenic activity and vascular calcification in the cardiovascular system by distinct near-infrared fluorescent nanoparticle probes [38]. Inflammatory cells, especially macrophages/monocyte, were considered to regulate the phenotypic differentiation of VSMCs [39, 40]. The secretion of pro-inflammatory factors by activated macrophages stimulates the differentiation of VSMCs into bone cells [41]. TNF-α was identified as a pro-inflammatory factor that enhances the expression of alkaline phosphatase (ALP) through the NF-κB pathway, which then facilitates calcification in VSMCs [42]. Additionally, TNF-α is found to take a significant part in the process of medial calcification in people with diabetes and CKD [43]. Interleukin-1β (IL-1β), produced mainly by Rac1 of macrophages, can also promote calcification and is used as a predictive factor of cardiovascular mortality in patients with high coronary calcium burden[40]. Besides, IL-6 and Oncostatin M (OSM) could also realize the differentiation of VSMCs to osteoblast [44, 45].
The antioxidant and anti-inflammatory effects of vitamin C
Vitamin C, named as ascorbic acid, is a water-soluble natural ingredient found in food and could also be used as a dietary supplement. Due to its antioxidant, collagen-stabilizing, immune modulatory actions, and inflammation-slowing effects [1, 46, 47], there is growing interest in exploring the relationship between vitamin C and chronic diseases involving the above mechanisms.
Moreover, experiments in vitro showed that vitamin C not only decrease the generation of superoxide radicals, hydrogen peroxide, and other reactive oxygen species (ROS) by inhibiting the Jak2/Stat1/IRF1 signaling pathway in endothelial cells [48], and it could also inhibit NF-κB-mediated inflammatory reaction [49] and suppress the synthesis of pro-inflammatory cytokine IL-6 and TNF-α in monocytes [50]. Hence, dietary vitamin C is likely to have a therapeutic effect on vascular calcification through antioxidant and anti-inflammatory actions. In a cross-sectional study of 7607 cases from the NHANES 2003–2006 surveys, subjects with high dietary vitamin C intake were related to lower levels of inflammatory biomarkers [51]. A randomized controlled trial indicated that vitamin C was beneficial in improving oxidative stress, inflammation, and endothelial dysfunction induced by acute hypoglycemia in patients with type 1 diabetes [52].
The potential benefit of vitamin C for vascular calcification
Previous studies have investigated the association between dietary factors and vascular calcification, and it has garnered significant attention from researchers. Vitamin K and vitamin E were found to attenuate the development of vascular calcification in animal experiments [53, 54]. A cross-sectional survey of 2535 participants indicated that higher dietary zinc intake was related to decreased risk of severe AAC among US adults [55]. Vitamin C has possible beneficial effects on cardiovascular health. A meta-analysis including 69 prospective studies revealed that greater dietary vitamin C intake was correlated with decreased risk of cardiovascular disease, total cancer, and all-cause mortality [18]. However, its connection with vascular calcification remains unclear. A cellular experiment demonstrated that vitamin C reduced calcification accumulation produced by VSMCs in the optimum extracellular matrix. This process was accompanied by reduced alkaline phosphatase activity and the expression of osteoblast marker protein, implying that the osteogenic transformation of VSMCs was blocked [27]. Dietary vitamin C may inhibit the differentiation of VSMCs and prevent vascular calcification through anti-inflammatory and antioxidant effects. Further research is still required to clarify the association and mechanisms.
Our results indicated that vitamin C supplements were not beneficial for vascular calcification, which were consistent with the results of previous randomized controlled studies and mendelian randomization studies [18, 56,57,58] on vitamin C supplements and CVDs, implying that fruits and vegetables rich in high amounts of natural vitamin C were recommended instead of dietary supplements. The possible reason for this result is that when vitamin C is extracted from a natural product, the intermediate product of this extraction or the process of artificial synthesis may lead to reduced bioavailability of the vitamin in plasma and decreased effects [59]. It was shown that vitamin C from fruit (kiwifruit) resulted in higher levels of ascorbate and superior bioavailability compared to synthetic vitamin C in mice with vitamin C deficienc [60].
Strengths and limitations
Of note, some strengths could be found in the study. It might be the first research to investigate the relationship of dietary vitamin C with AAC and may provide some support for future studies. Concerning the sensitivity and subgroup analyses, the findings consistently supported the relationship of dietary vitamin C intake with AAC, demonstrating the robustness of the conclusion. Moreover, an exploratory analysis of the relationship of vitamin C supplements with AAC was performed to investigate the effect of different sources of vitamin C on vascular calcification, suggesting that dietary sources of vitamin C are beneficial for vascular calcification rather than supplements. Finally, as a result of utilizing a sample that is representative of the population, the findings can be generally applied to the adult population in the United States.
The study also had certain limitations. First, as the research was conducted in a cross-sectional manner, it is not probable to infer a causal relationship of dietary vitamin C with AAC. Therefore, further longitudinal studies are necessary to elucidate the findings. Second, dietary data were collected via 24-h interview recall, which had the potential for recall bias. Third, NHANES 2013–2014 lacks data on serum vitamin C. Therefore, the relationship of serum vitamins C with AAC cannot be further explored. Additionally, it’s unavoidable that uncollected or unknown confounding factors may influence the study results. For instance, the use of medication may have an effect on the absorption of vitamin C or on vascular calcification. Finally, NHANES collects data on the American population. Due to the constraints of AAC measurement, the study only included individuals who were aged 40 years or above. Our study was centered towards the individuals aged 40 years or older within the general adult population, as this demographic is at a higher risk of developing vascular calcification. Focusing on this group is important in the prevention of calcification of blood vessels. Nevertheless, whether the findings are applied to the populations of different countries and other age groups needs further exploration.
Conclusion
The study conducted in a cross-sectional design indicated that there is an inverse relationship of dietary intake of vitamin C with the risk of AAC and AAC score. The findings suggest that increased vitamin C intake through diet could have a positive effect on reducing vascular calcification in adults, providing a new perspective on potential dietary approaches to prevent vascular calcification and CVDs. Although more prospective and mechanistic studies are needed to provide additional evidence and support this conclusion in the future.
Availability of data and materials
The data used in the study were obtained from NHANES: https://www.cdc.gov/nchs/nhanes/.
References
Bardeesi ASA, et al. A novel role of cellular interactions in vascular calcification. J Transl Med. 2017;15:95. https://doi.org/10.1186/s12967-017-1190-z.
Kendrick J, Chonchol M. The role of phosphorus in the development and progression of vascular calcification. Am J Kidney Dis. 2011;58:826–34. https://doi.org/10.1053/j.ajkd.2011.07.020.
Lin CP, et al. Correction: Simvastatin Attenuates Oxidative Stress, NF-κB Activation, and Artery Calcification in LDLR-/- Mice Fed with High Fat Diet via Down-regulation of Tumor Necrosis Factor-α and TNF Receptor 1. PLoS ONE. 2016;11:e0148590. https://doi.org/10.1371/journal.pone.0148590.
Shimizu T, Tanaka T, Iso T, Kawai-Kowase K, Kurabayashi M. Azelnidipine inhibits Msx2-dependent osteogenic differentiation and matrix mineralization of vascular smooth muscle cells. Int Heart J. 2012;53:331–5. https://doi.org/10.1536/ihj.53.331.
Motro M, Kirwan BA, de Brouwer S, Poole-Wilson PA, Shemesh J. Tracking coronary calcification and atherosclerotic lesions in patients with stable angina pectoris undergoing nifedipine therapy. Cardiology. 2007;107:165–71. https://doi.org/10.1159/000095308.
Saremi A, Bahn G, Reaven PD. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care. 2012;35:2390–2. https://doi.org/10.2337/dc12-0464.
Pasch A, et al. Sodium thiosulfate prevents vascular calcifications in uremic rats. Kidney Int. 2008;74:1444–53. https://doi.org/10.1038/ki.2008.455.
Bastos Gonçalves, F. et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart 98, 988–994, https://doi.org/10.1136/heartjnl-2011-301464 (2012).
Wilson PW, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–34. https://doi.org/10.1161/01.cir.103.11.1529.
Schousboe JT, et al. Abdominal aortic calcification (AAC) and ankle-brachial index (ABI) predict health care costs and utilization in older men, independent of prevalent clinical cardiovascular disease and each other. Atherosclerosis. 2020;295:31–7. https://doi.org/10.1016/j.atherosclerosis.2020.01.012.
O’Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does Nonenhanced CT-based Quantification of Abdominal Aortic Calcification Outperform the Framingham Risk Score in Predicting Cardiovascular Events in Asymptomatic Adults? Radiology. 2019;290:108–15. https://doi.org/10.1148/radiol.2018180562.
Criqui MH, et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:1574–9. https://doi.org/10.1161/atvbaha.114.303268.
Inoue H, et al. Impact of trajectories of abdominal aortic calcification over 2 years on subsequent mortality: a 10-year longitudinal study. Nephrol Dial Transplant. 2018;33:676–83. https://doi.org/10.1093/ndt/gfx253.
Bolland MJ, et al. Abdominal aortic calcification on vertebral morphometry images predicts incident myocardial infarction. J Bone Miner Res. 2010;25:505–12. https://doi.org/10.1359/jbmr.091005.
Kauppila LI, et al. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–50. https://doi.org/10.1016/s0021-9150(97)00106-8.
Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281–9. https://doi.org/10.1007/s00198-005-2010-5.
Hartley, L., May, M. D., Loveman, E., Colquitt, J. L. & Rees, K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 2016, Cd011472, https://doi.org/10.1002/14651858.CD011472.pub2 (2016).
Aune D, et al. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of prospective studies. Am J Clin Nutr. 2018;108:1069–91. https://doi.org/10.1093/ajcn/nqy097.
Cimmino L, Neel BG, Aifantis I. Vitamin C in Stem Cell Reprogramming and Cancer. Trends Cell Biol. 2018;28:698–708. https://doi.org/10.1016/j.tcb.2018.04.001.
Righi NC, et al. Effects of vitamin C on oxidative stress, inflammation, muscle soreness, and strength following acute exercise: meta-analyses of randomized clinical trials. Eur J Nutr. 2020;59:2827–39. https://doi.org/10.1007/s00394-020-02215-2.
Moser, M. A. & Chun, O. K. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int J Mol Sci 17, https://doi.org/10.3390/ijms17081328 (2016).
Martín-Calvo, N. & Martínez-González, M. Vitamin C Intake is Inversely Associated with Cardiovascular Mortality in a Cohort of Spanish Graduates: the SUN Project. Nutrients 9, https://doi.org/10.3390/nu9090954 (2017).
Kobylecki, C. J., Afzal, S., Davey Smith, G. & Nordestgaard, B. G. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am J Clin Nutr 101, 1135–1143, https://doi.org/10.3945/ajcn.114.104497 (2015).
Jayedi A, Rashidy-Pour A, Parohan M, Zargar MS, Shab-Bidar S. Dietary and circulating vitamin C, vitamin E, β-carotene and risk of total cardiovascular mortality: a systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2019;22:1872–87. https://doi.org/10.1017/s1368980018003725.
Yang, C. et al. Association between Dietary Total Antioxidant Capacity of Antioxidant Vitamins and the Risk of Stroke among US Adults. Antioxidants (Basel) 11, https://doi.org/10.3390/antiox11112252 (2022).
Morelli, M. B., Gambardella, J., Castellanos, V., Trimarco, V. & Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants (Basel) 9, https://doi.org/10.3390/antiox9121227 (2020).
Ivanov V, Ivanova S, Niedzwiecki A, Rath M. Vitamin C inhibits the calcification process in human vascular smooth muscle cells. Am J Cardiovasc Dis. 2020;10:108–16.
Zipf G, et al. National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat. 2013;1:1–37.
Lv L, Wu S, Yang Y, Yue X. Modified effect of active or passive smoking on the association between age and abdominal aortic calcification: a nationally representative cross-sectional study. BMJ Open. 2021;11: e047645. https://doi.org/10.1136/bmjopen-2020-047645.
Schousboe JT, Wilson KE, Hangartner TN. Detection of aortic calcification during vertebral fracture assessment (VFA) compared to digital radiography. PLoS ONE. 2007;2: e715. https://doi.org/10.1371/journal.pone.0000715.
Ornelas-Loredo A, et al. Association Between Obesity-Mediated Atrial Fibrillation and Therapy With Sodium Channel Blocker Antiarrhythmic Drugs. JAMA Cardiol. 2020;5:57–64. https://doi.org/10.1001/jamacardio.2019.4513.
Kirshner JJ, et al. Prevention of pegfilgrastim-induced bone pain: a phase III double-blind placebo-controlled randomized clinical trial of the university of rochester cancer center clinical community oncology program research base. J Clin Oncol. 2012;30:1974–9. https://doi.org/10.1200/jco.2011.37.8364.
Lian JB, Stein GS. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118–40.
Durham AL, Speer MY, Scatena M, Giachelli CM, Shanahan CM. Role of smooth muscle cells in vascular calcification: implications in atherosclerosis and arterial stiffness. Cardiovasc Res. 2018;114:590–600. https://doi.org/10.1093/cvr/cvy010.
Byon CH, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–27. https://doi.org/10.1074/jbc.M800021200.
Farrokhi E, Samani KG, Chaleshtori MH. Oxidized low-density lipoprotein increases bone sialoprotein expression in vascular smooth muscle cells via runt-related transcription factor 2. Am J Med Sci. 2015;349:240–3. https://doi.org/10.1097/maj.0000000000000381.
Yamada S, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res. 2012;27:474–85. https://doi.org/10.1002/jbmr.539.
Aikawa E, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–50. https://doi.org/10.1161/circulationaha.107.732867.
Callegari A, Coons ML, Ricks JL, Rosenfeld ME, Scatena M. Increased calcification in osteoprotegerin-deficient smooth muscle cells: Dependence on receptor activator of NF-κB ligand and interleukin 6. J Vasc Res. 2014;51:118–31. https://doi.org/10.1159/000358920.
Ceneri N, et al. Rac2 Modulates Atherosclerotic Calcification by Regulating Macrophage Interleukin-1β Production. Arterioscler Thromb Vasc Biol. 2017;37:328–40. https://doi.org/10.1161/atvbaha.116.308507.
Li Y, et al. Role of Macrophages in the Progression and Regression of Vascular Calcification. Front Pharmacol. 2020;11:661. https://doi.org/10.3389/fphar.2020.00661.
Lee HL, Woo KM, Ryoo HM, Baek JH. Tumor necrosis factor-alpha increases alkaline phosphatase expression in vascular smooth muscle cells via MSX2 induction. Biochem Biophys Res Commun. 2010;391:1087–92. https://doi.org/10.1016/j.bbrc.2009.12.027.
Al-Aly Z. Medial vascular calcification in diabetes mellitus and chronic kidney disease: the role of inflammation. Cardiovasc Hematol Disord Drug Targets. 2007;7:1–6. https://doi.org/10.2174/187152907780059047.
Kurozumi A, et al. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone. 2019;124:53–61. https://doi.org/10.1016/j.bone.2019.04.006.
Zhang X, et al. Oncostatin M receptor β deficiency attenuates atherogenesis by inhibiting JAK2/STAT3 signaling in macrophages. J Lipid Res. 2017;58:895–906. https://doi.org/10.1194/jlr.M074112.
Peterkofsky B. Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr. 1991;54:1135s–40s. https://doi.org/10.1093/ajcn/54.6.1135s.
Carr, A. C. & Maggini, S. Vitamin C and Immune Function. Nutrients 9, https://doi.org/10.3390/nu9111211 (2017).
Chaghouri, P. et al. Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation. Nutrients 13, https://doi.org/10.3390/nu13030791 (2021).
Du YT, et al. Prooxidative inhibition against NF-κB-mediated inflammation by pharmacological vitamin C. Free Radic Biol Med. 2022;180:85–94. https://doi.org/10.1016/j.freeradbiomed.2022.01.007.
Härtel C, Strunk T, Bucsky P, Schultz C. Effects of vitamin C on intracytoplasmic cytokine production in human whole blood monocytes and lymphocytes. Cytokine. 2004;27:101–6. https://doi.org/10.1016/j.cyto.2004.02.004.
Crook, J. M., Horgas, A. L., Yoon, S. L., Grundmann, O. & Johnson-Mallard, V. Vitamin C Plasma Levels Associated with Inflammatory Biomarkers, CRP and RDW: Results from the NHANES 2003–2006 Surveys. Nutrients 14, doi:https://doi.org/10.3390/nu14061254 (2022).
Ceriello A, et al. Vitamin C further improves the protective effect of glucagon-like peptide-1 on acute hypoglycemia-induced oxidative stress, inflammation, and endothelial dysfunction in type 1 diabetes. Diabetes Care. 2013;36:4104–8. https://doi.org/10.2337/dc13-0750.
Schurgers LJ. Vitamin K: key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013;83:782–4. https://doi.org/10.1038/ki.2013.26.
Peralta-Ramírez A, et al. Vitamin E protection of obesity-enhanced vascular calcification in uremic rats. Am J Physiol Renal Physiol. 2014;306:F422-429. https://doi.org/10.1152/ajprenal.00355.2013.
Chen W, et al. Association between dietary zinc intake and abdominal aortic calcification in US adults. Nephrol Dial Transplant. 2020;35:1171–8. https://doi.org/10.1093/ndt/gfz134.
Al-Khudairy, L. et al. Vitamin C supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev 3, Cd011114, https://doi.org/10.1002/14651858.CD011114.pub2 (2017).
Myung SK, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346: f10. https://doi.org/10.1136/bmj.f10.
Yuan S, Zheng JS, Mason AM, Burgess S, Larsson SC. Genetically predicted circulating vitamin C in relation to cardiovascular disease. Eur J Prev Cardiol. 2022;28:1829–37. https://doi.org/10.1093/eurjpc/zwab081.
Pawlowska E, Szczepanska J, Blasiak J. Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations. Oxid Med Cell Longev. 2019;2019:7286737. https://doi.org/10.1155/2019/7286737.
Vissers MC, Bozonet SM, Pearson JF, Braithwaite LJ. Dietary ascorbate intake affects steady state tissue concentrations in vitamin C-deficient mice: tissue deficiency after suboptimal intake and superior bioavailability from a food source (kiwifruit). Am J Clin Nutr. 2011;93:292–301. https://doi.org/10.3945/ajcn.110.004853.
Acknowledgements
All authors thanked the NHANES administration and data collection team for the availability of the relevant data.
Funding
This study was supported by China Academy of Chinese Medical Sciences Innovation Fund (CACMS Innovation Fund) (No. CI2021A00917 and CI2021B004).
Author information
Authors and Affiliations
Contributions
HX, WW, and JJ designed the study. JJ and JZ drafted the manuscript and analyzed data. QH and MW collected data. Forms were made by QL and TW. Pictures were graphed by XC. HX and WW reviewed the paper. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The NCHS Ethic Review Board approved the implementation of NHANES, and all the participants signed the informed consent.
Consent for publication
All authors provided consent for the publication of this work, and are accountable for ensuring the accuracy and integrity of the content.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Description of covariates. Supplementary Table 2. Associations of vitamin C supplements with AAC score and the risk of AAC.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jia, J., Zhang, J., He, Q. et al. Association between dietary vitamin C and abdominal aortic calcification among the US adults. Nutr J 22, 58 (2023). https://doi.org/10.1186/s12937-023-00889-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-023-00889-y