Abstract
Background
Chronic low back pain (LBP) is the most common musculoskeletal pain that affects a person’s daily activities. This present study aimed at evaluating the relationship between major dietary pattern and Chronic LBP.
Methods
This cross-sectional analysis was examined 7686 Kurdish adults. The RaNCD cohort study physician diagnosed chronic LBP. Dietary patterns were derived using principal component analysis. The three identified dietary patterns derived were named: 1) the vegetarian diet included vegetables, whole grain, legumes, nuts, olive, vegetable oil, fruits, and fruit juice; 2) high protein diet related to higher adherence to red and white meat, legumes, nuts, and egg; and 3) energy-dense diet characterized with higher intake of salt, sweet, dessert, hydrogenated fat, soft drink, refined grain, tea, and coffee. Dietary pattern scores were divided into tertiles. Binary logistic regression in crude, adjusted odds ratios (OR) and 95% confidence intervals (CI) were used to determine this association.
Results
Twenty-two per cent of participants had chronic LBP. Higher adherence to high protein dietary pattern was inversely associated with chronic LBP in crude (OR: 0.79, 95% CI: 0.69–0.9) and adjusted model (for age, sex, smoking, drinking, diabetes, physical activity, body mass index, and waist circumference) (OR: 0.84, 95% CI: 0.72–0.97). In addition, after controlling for the mentioned potential confounders, participants in the highest category of energy dense diet were positively associated with chronic LBP compared with those in the lowest category (OR: 1.13, 95% CI: 1.01–1.32).
Conclusions
Higher adherence to the high protein diet was inversely related to chronic LBP prevalence. In addition, we found that following energy dense diet was positively associated with chronic LBP.
Similar content being viewed by others
Introduction
Low back pain (LBP) is the main cause of disability in the United States, with more than 1 in 5 adults experiencing chronic pain [1, 2]. In global disease burden studies, LBP usually ranks first when disease burden is measured by disability and is also in the top ten if both death and disability are considered [3]. LBP is caused by problems related to the intervertebral discs, nerves, muscles, etc., in the lumbar and sacral vertebrae [4]. Most LBP patients (up to 90%) have non-specific pain without apparent cause [5]. LBP is classified into three categories based on the duration of symptoms. Acute LBP is often the result of actual or near tissue injury or sprain, which has been present for 6 weeks or less, and it tends to settle on its own with personal care. Sub-acute LBP has a six- to 12-week duration, and chronic LBP lasts longer than 12 weeks. According to this category, chronic LBP often persists even though the initial injury has healed. These cases are more likely to be referred for treatment than the more acute cases that linger untreated [6].
People with chronic LBP have difficulty in social and occupational activities. Even the resulting pain affects a person’s mood and puts a heavy burden on the treatment system; overall, chronic LBP is the most common cause of disability in a person’s daily life activities [7]. It should be noted that many people may not see a doctor and consider a self-medication approach, so its prevalence is higher in communities [8]. Evidence suggests that stress, anxiety, sedentary lifestyle, hard work, obesity, and diet are involved in the etiology of chronic LBP [9].
Increased levels of pro-inflammatory mediators in the body can be involved in the pathogenesis of chronic LBP [10, 11]. Adherence to an unhealthy diet pattern by producing pro-inflammatory mediators upsets the balance of these mediators in the body [12]. Higher adhere to the Western diet, which is characterized by higher intake of refined grains, red meat, processed meat, high saturated fat, trans-fatty acids, sweet sugary foods, and caffeine, as an unhealthy diet is associated with the production of high levels of cytokines, interleukins, C- reactive protein (CRP) and tumor necrosis factor α (TNF-α) [4, 13]. A review study has recently indicated that adherence to the Mediterranean and plant-based diet, associated with consuming vegetable oils, especially olive oil, effectively reduces musculoskeletal pain [14]. A healthy dietary pattern seems related to an adequate and balanced intake of all food groups that can moderate the inflammatory conditions of the body [15, 16].
The high prevalence of chronic LBP worldwide and the importance of proper diet in reducing inflammatory conditions necessitates studying the relationship between major dietary patterns and chronic LBP among the Kurdish population.
Material and methods
Study design and participants
This cross-sectional study was conducted on data from the recruitment phase of the Ravansar non-communicable diseases (RaNCD) cohort study. This population-based study was conducted amongst the Kurdish population (4770 men and 5289 women) aged 35–65 years residing in Ravansar, Kermanshah province, Western Iran. This study was developed by the PERSIAN (Prospective Epidemiological Research Studies in Iran) mega cohort study and was approved by the Ethics Committees in the Ministry of Health and Medical Education, the Digestive Diseases Research Institute, Tehran University of Medical Sciences, Iran. The details of this study have been published elsewhere [17, 18]. This cohort study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (No: KUMS.REC.1394.318).
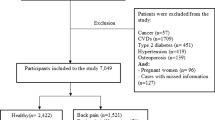
The inclusion criteria for this study were participants who provided complete information for the RaNCD cohort study. We also did not include participants with cardiovascular diseases (n = 1118), thyroid (n = 738), and cancer (n = 93) diseases due to possible dietary changes. Likewise, pregnant women (n = 134) were not included in this study. After excluding these participants, the participants whose calories intake was not in the range of 800–4200 Kcal/day (n = 437), were not included in the study. Furthermore, 41 participants with missing data were excluded. (Fig. 1).
Data sources/ measurements
The necessary data were obtained from the RaNCD cohort study, including demographics (age and gender), physical activity, dietary intake, anthropometric indices, and medical history of diseases including CVDs, diabetes, thyroid diseases, cancer, and chronic LBP. The RaNCD physician assessed the medical history. History of smoking and drinking was also evaluated based on the participants’ history of smoking, being a passive smoker, and alcohol consumption. All the data were recorded in the RaNCD cohort study [17].
Anthropometry.
Participants’ weight was measured with InBody 770 device (Inbody Co, Seoul, Korea) with as little as clothing possible and without shoes in the study site in Ravansar. The automatic stadiometer BSM 370 (Biospace Co., Seoul, Korea) was applied to measure their height in a standing position without shoes with a precision of 0.1 cm. Body mass index (BMI) was calculated by dividing weight in kg into height square in meters. The non-stretched and flexible tape was used to measure waist circumference (WC) in a- standing position at the level of the iliac crest three times, and the average was recorded.
Derivation of empirical dietary patterns
Participants’ diets were assessed using a valid, semi-quantitative 118-item food frequency questionnaire (FFQ) questionnaire developed for the RaNCD cohort study. The details of this questionnaire have been described in previous studies [17, 19]. 118 food items (in grams) were categorized into 31 food groups based on the nutrient content similarity to determine dietary patterns (Table 1). Principal component analysis was used to identify the major dietary patterns. The varimax rotation was applied to create a distinct and straightforward matrix in the factor analysis. The scree-plot was drawn to determine the number of matrix components (the major dietary patterns). We selected the first three major dietary patterns with eigenvalues greater than 1. Overall, the factor score for each dietary pattern was calculated by summing the food intakes of that group in terms of their factor loading, and each participant received a score for each pattern in terms of factor scores. Dietary pattern scores, i.e. factor scores, were divided into tertiles.
Physical activity
The physical activity level of the RaNCD participants was assessed using the standard questionnaire designed for PERSIAN Cohort. The questionnaire included 22 questions about the daily activity status. The responses were reported based on the metabolic equivalent of task per hour per day (MET/h/day). The detail of this questionnaire was described in the previous study [17].
Outcome measurement
All participants completed self-reports about chronic LBP. The pain area was surveyed based on the RaNCD cohort study physician’s opinions and participants’ responses to her questions 1) Do you experience LBP that lasted more than a few months and interfered with their daily activities? In addition, has it lasted so far? (Yes/ No); 2) Do you have a history of back stiffness for more than an hour in the morning? (Yes/ No); 3) Do you have a history of arthralgia? (Yes/ No); 4) Do you have a history of joint stiffness for more than an hour in the morning? (Yes/ No). These questions were administered by the PERSIAN mega cohort study to evaluate chronic diseases in all Iranian adults ages≥35 years. Based on self-report and their medical history after physical examination by the physician, chronic LBP has been diagnosed the presence of LBP for a few months, which led to limited daily activities and had been sought for its treatment, such as medication, medical consultation, or physiotherapy. Furthermore, the physician did not consider pain associated with malignancies in the spinal cord area, infections, and fractures as chronic LBP [20].
Statistical analysis
SPSS 20 (IBM Corp, Chicago, IL, USA) and Stata, version 14 (Stata Corp, College Station, TX) were applied for all statistical analysis. We reported quantitative variables by mean ± standard deviation (SD) and qualitative variables using frequency (%). Firstly, Dietary pattern scores, i.e. factor scores, were divided into tertiles. The comparison of participants’ baseline characteristics was evaluated using Chi-square and ANOVA tests based on the tertiles of all three dietary patterns. Binary logistic regression in crude and adjusted odds ratios (OR) and 95% confidence intervals (CI) was used to determine the association between chronic LBP and categories of three dietary patterns. In adjusted model 1, age (continuous), sex (categorical), smoking (categorical), and drinking (categorical) were adjusted. In adjusted model 2, we controlled the variables in model 1, diabetes (categorical), physical activity (continuous), body mass index (continuous), WC (continuous), energy intake (continuous), and treatment for chronic LBP (categorical). In all analyses, the first tertile of dietary patterns was considered as the reference category. Further, we considered a fractional polynomial plot for high protein, energy-dense diets concerning chronic LBP to illustrate this association better. P-values were considered significant at the level of < 0.05.
Results
Seven thousand six hundred eighty-six of the RaNCD participants met the study inclusion criteria in the current study.51.3% of them were male. We found that 22.5% of the participants had chronic LBP. The factor analysis results introduced three dietary patterns with a factor loading of food groups of more than 0.2 (Table 2). The major dietary patterns were identified are as follows: 1) the vegetarian diet included vegetables, whole grains, legumes, nuts, olive, vegetable oil, fruits, and fruit juice; 2) the high protein diet related to higher adherence to red and white meat, legumes, nuts, and egg; and 3) the energy-dense diet characterized by a higher intake of salt, sweets, dessert, hydrogenated fat, soft drink, refined grains, tea, and coffee. Table 2 shows the rotated component matrix of each food groups and the correlation coefficient between each food group and dietary patterns.
The highest tertiles of high protein and energy-dense diets were related to higher BMI and WC compared to the lowest tertile. (P < 0.001), while higher adherence to the vegetarian dietary patterns was significantly related to higher BMI and WC (P < 0.001) (Table 3).
Our results showed that the mean of PA in all participants was 41.27 ± 8.38, in which the third tertiles of the high protein and energy-dense diet, the mean of PA was significantly higher than their first tertiles (P < 0.001) (Table 3). According to Table 3, diabetes was prevalent in 6.5% of the studied participants. In this study, the prevalence of chronic LBP decreased significantly with higher adherence to high protein dietary pattern (P < 0.001). However, this prevalence was not significantly different with higher following the two other major dietary patterns (vegetarian and energy-dense diet). Other characteristics of the studied participants are presented in Table 3.
Multivariable-adjusted odds ratios and 95% confidence intervals for chronic LBP across categories of three dietary patterns are indicated in Table 4. The highest tertile of the high protein dietary pattern was associated with lower odds of chronic LBP as compared to the lowest tertile (OR: 0.79, 95% CI: 0.69–0.9); such that after controlling for age, sex, smoking, drinking, diabetes, physical activity, body mass index, WC, energy intake and treatment this association remained (OR: 0.84, 95% CI: 0.72–0.97).
In addition, after controlling for the mentioned potential confounders, The highest tertile of the energy-dense diet was associated with higher odds of chronic LBP than the lowest tertile (OR: 1.13, 95% CI: 1.01–1.32). Figure 2 shows odds ratios and 95% confidence intervals for chronic LBP across high protein and energy-dense diet categories.
However, no significant association was found between adherence to vegetarian dietary pattern and chronic LBP either before or after adjusting the confounders (Table 4).
Discussion
In the current study, we found that higher adherence to the high protein dietary pattern was inversely associated with chronic LBP, while odds of chronic LBP were increased with higher adherence to the energy-dense diet. LBP is a common pain experienced during adulthood, and it is believed that nutrition can affect the formation and severity of chronic LBP [21, 22]. Accordingly, the current study evaluated the relationship between major dietary patterns and chronic LBP.
In the current study, the prevalence of chronic LBP was significantly decreased with higher adherence to high protein dietary pattern. There was a significant association between high protein dietary pattern and chronic LBP. After controlling for potential confounders, participants in the third tertile of high protein dietary pattern were 12% lower odds of chronic LBP compared to participants in the lowest group. A randomized clinical trial by Kirk et al. [23] showed that dietary protein supplementation significantly improved skeletal muscle function. Another clinical trial by Shell et al. [24] showed that administration of amino acids precursors could improve chronic LBP and decrease the level of IL-6 and CRP. Nutritional mechanisms in the development of chronic LBP include affecting brain-gut axis neurotransmitters and changes in gut-derived neurotransmitters such as glutamate, which also affect the brain system and induce chronic pain [25]. Essential and semi-essential amino acids deficiency interfere with the production of neurotransmitter precursors that can affect pain sensation [24]. Other factors worsening chronic LBP include decreased muscle mass and some degree of sarcopenia [26, 27]. Skeletal muscle strength begins to decline in middle age in both men and women [28]. Adequate protein intake is one of the main factors in maintaining this muscle strength [29]. The type and amount of protein determine muscle mass’s effect [30, 31]. In this study, the high protein diet related to higher adherence to red and white meat, legumes, nuts, and egg involving protein with high biological value and essential micronutrients (e.g., calcium, iron, zinc, choline, vitamin B12) that are important for growth and development, developing of neurotransmitters, improving skeletal muscle mass and strength [32, 33].
Our study also found that adherence to the unhealthy diet was positively associated with chronic LBP. After adjusting the potential confounders, participants in the third tertile energy-dense diet were 15% higher odds of chronic LBP compared to participants in the lowest.
The unhealthy diet components in our study are most similar to the Western diet involving a higher intake of refined grains, red meat, processed meat, high saturated fat, trans-fatty acids, sweet sugary foods, and caffeine [34]. Following this dietary pattern was associated with an increased level of inflammatory markers such as IL-6 and CRP, leading to a decrease in pain threshold in chronic LBP [35,36,37]. Song et al. [38] reported that a high-fat diet was related to increased chronic LBP in the animal model. Another study was shown that higher adherence to sugary foods was decreased muscle strength (OR: 1.06 CI 95%: 1.01–1.12) [39]. Other studies also found that low-protein, high-sugar, high-fat diets were associated with more chronic LBP and higher CRP levels [9, 40,41,42]. Therefore, the unhealthy diet in this study was characterized by intake of salt, sweets, desserts, hydrogenated fat, soft drink, refined grain, tea, and coffee, which these dietary components can increase inflammation and consequence chronic LBP.
No significant relationship was found between the vegetarian diet and chronic LBP in this study. Although we controlled potential confounders, we did not observe any association between the vegetarian diet and chronic LBP. In fact, this dietary pattern contains high levels of essential antioxidants such as vitamin C, vitamin E, vitamin A, and all carotenoids [43, 44]. These antioxidants have anti-inflammatory effects and can reduce the pain threshold in these patients [45]. On the other hand, vegetable-based diets produce short-chain fatty acids that stabilize the beneficial intestinal microbiome, and substances derived from this microbiome environment can affect the brain-gut system and reduce systemic and central inflammation [46]. Furthermore, this vegetable diet can relieve musculoskeletal pain [47]. In the present study, the vegetarian dietary pattern was related to the intake of vegetables, whole grains, legumes, nuts, olive, vegetable oil, fruits, and fruit juice. The intake of these food groups in participants with and without chronic LBP seems to be the same and we could not find any association.
Limitations
This is the first study to evaluate the relationship between major dietary patterns and chronic LBP among the Kurdish population; however, this study suffered from some limitations. Firstly, this is a cross-sectional study and the cause-and-effect relationship was unclear. Second, dietary intake was assessed by FFQ, and the error of recalling food intake should not be ignored. However, the questionnaire was presented by trained nutritionists. In addition, the degree and severity of chronic LBP in the RaNCD cohort study were not measured. Therefore, further studies are recommended without these limitations.
Conclusions
According to the findings of this study, higher adherence to high protein diet had protective effects on chronic LBP prevalence. In addition, we found that following energy dense diet was associated with higher odds of chronic LBP. Therefore, it is recommended that people prone to chronic LBP consider a high biological value protein in their daily diet, and reduce their intake of salt, sweets, dessert, hydrogenated fat, soft drink, refined grain, tea, and coffee.
Availability of data and materials
Data will be available upon request from the corresponding author.
Abbreviations
- LBP:
-
Low back pain
- CRP:
-
C- reaction protein
- TNF-α:
-
tumor necrosis factor alpha
- RaNCD:
-
Ravansar non- communicable diseases
- BMI:
-
Body mass index
- OR:
-
Odds ratios
- CI:
-
Confidence intervals
References
Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–e32.
Stevans JM, Delitto A, Khoja SS, Patterson CG, Smith CN, Schneider MJ, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4(2):e2037371.
Tamrakar M, Kharel P, Traeger A, Maher C, O'Keeffe M, Ferreira G. Completeness and quality of low back pain prevalence data in the global burden of disease study 2017. BMJ Glob Health. 2021;6(5):e005847.
Torlak MS, Bagcaci S, Akpinar E, Okutan O, Nazli MS, Kuccukturk S. The effect of intermittent diet and/or physical therapy in patients with chronic low back pain: a single-blinded randomized controlled trial. Explore. 2022;18(1):76-81.
Shamsi M, Sarrafzadeh J, Jamshidi A, Arjmand N, Ghezelbash F. Comparison of spinal stability following motor control and general exercises in nonspecific chronic low back pain patients. Clin Biomech. 2017;48:42–8.
Bratton RL. Assessment and management of acute low back pain. Am Fam Physician. 1999;60(8):2299.
Bento TPF, dos Santos Genebra CV, Maciel NM, Cornelio GP, Simeão SFAP, de Vitta A. Low back pain and some associated factors: is there any difference between genders? Braz J Phys Ther. 2020;24(1):79–87.
Shamsi M, Safari A, Samadzadeh S, Yoosefpour N. The prevalence of musculoskeletal pain among above 50-year-old population referred to the Kermanshah-Iran health bus in 2016. BMC Research Note. 2020;13(1):72.
Zick SM, Murphy SL, Colacino J. Association of chronic spinal pain with diet quality. Pain Report. 2020;5(5):e837.
Philpot U, Johnson MI. Diet therapy in the management of chronic pain: better diet less pain? Future Medicine. 2019;9(4):335–8.
Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific low Back pain: inflammatory profiles of patients with acute and chronic pain. Clin J Pain. 2019;35(10):818.
Barbaresko J, Rienks J, Oluwagbemigun K, Jacobs G, Lieb W, Laudes M, et al. Dietary patterns associated with inflammatory biomarkers in a northern German population. Eur J Nutr. 2020;59(4):1433–41.
Field R, Pourkazemi F, Turton J, Rooney K. Dietary interventions are beneficial for patients with chronic pain: a systematic review with meta-analysis. Pain Med. 2021;22(3):694–714.
Mendonça CR, Noll M, Castro MCR, Silveira EA. Effects of nutritional interventions in the control of musculoskeletal pain: an integrative review. Nutrients. 2020;12(10):3075.
Calle MC, Andersen CJ. Assessment of dietary patterns represents a potential, yet variable, measure of inflammatory status: a review and update. Dis Markers. 2019;2019:3102870.
Pasdar Y, Moradi S, Moludi J, Darbandi M, Niazi P, Nachvak SM, et al. Risk of metabolic syndrome in non-alcoholic fatty liver disease patients. Med J Nutrition Metab. 2019;12(4):353–63.
Pasdar Y, Najafi F, Moradinazar M, Shakiba E, Karim H, Hamzeh B, et al. Cohort profile: Ravansar non-communicable disease cohort study: the first cohort study in a Kurdish population. Inter J Epidemiol. 2019;48(3):682–3f.
Poustchi H, Eghtesad S, Kamangar F, Etemadi A, Keshtkar A-A, Hekmatdoost A, et al. Prospective epidemiological research studies in Iran (the PERSIAN cohort study): rationale, objectives, and design. Am J Epidemiol. 2018;187(4):647–55.
Moradi S, Pasdar Y, Hamzeh B, Najafi F, Nachvak SM, Mostafai R, et al. Comparison of 3 nutritional questionnaires to determine energy intake accuracy in Iranian adults. Clin Nutr Res. 2018;7(3):213–22.
Pasdar Y, Hamzeh B, Moradi S, Cheshmeh S, Najafi F, Moradinazar M, et al. Better muscle strength can decrease the risk of arthralgia and back &joint stiffness in Kurdish men; a cross-sectional study using data from RaNCD cohort study. BMC Musculoskeletal Disord. 2020;21(1):686.
Manchikanti L, Singh V, Datta S, Cohen SP, Hirsch JA. Comprehensive review of epidemiology, scope, and impact of spinal pain. Pain physician. 2009;12(4):E35–70.
Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the global burden of disease study 2010. Lancet (London, England). 2012;380(9859):2224–60.
Kirk B, Mooney K, Amirabdollahian F, Khaiyat O. Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: Liverpool Hope University–Sarcopenia Aging Trial (LHU-SAT). Front Physiol. 2019;10:445.
Shell WE, Pavlik S, Roth B, Silver M, Breitstein ML, May L, et al. Reduction in pain and inflammation associated with chronic low Back pain with the use of the medical food Theramine. Am J Ther. 2016;23(6):e1353–e62.
Kawase T, Nagasawa M, Ikeda H, Yasuo S, Koga Y, Furuse M. Gut microbiota of mice putatively modifies amino acid metabolism in the host brain. Br J Nutr. 2017;117(6):775–83.
Sakai Y, Matsui H, Ito S, Hida T, Ito K, Koshimizu H, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporos Sarcopenia. 2017;3(4):195–200.
Kim WJ, Kim KJ, Song DG, Lee JS, Park KY, Lee JW, et al. Sarcopenia and Back muscle degeneration as risk factors for Back pain: a comparative study. Asian Spine J. 2020;14(3):364–72.
Kuo Y-K, Lin Y-C, Lee C-Y, Chen C-Y, Tani J, Huang T-J, et al. Novel insights into the pathogenesis of spinal sarcopenia and related therapeutic approaches: a narrative review. Int J Mol Sci. 2020;21(8):3010.
Chen TJ, Blum K, Payte JT, Schoolfield J, Hopper D, Stanford M, et al. Narcotic antagonists in drug dependence: pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Med Hypotheses. 2004;63(3):538–48.
Børsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283(4):E648–E57.
Samadi M, Moradi S, Azadbakht L, Rezaei M, Hojati N. Adherence to healthy diet is related to better linear growth with open growth plate in adolescent girls. Nutr Res. 2020;76:29–36.
Headey D, Hirvonen K, Hoddinott J. Animal sourced foods and child stunting. Am J Agric Econ. 2018;100(5):1302–19.
Pasdar Y, Moradi S, Esfahani NH, Darbandi M, Niazi P. Intake of animal source foods in relation to risk of metabolic syndrome. Prev Nutr Food Sci. 2020;25(2):133.
Samadi M, Moradi S, Moradinazar M, Mostafai R, Pasdar Y. Dietary pattern in relation to the risk of Alzheimer’s disease: a systematic review. Neurol Sci. 2019;40(10):2031–43.
Sullivan PM. Influence of Western diet and APOE genotype on Alzheimer's disease risk. Neurobiol Dis. 2020;138:104790.
Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27.
Briggs MS, Givens DL, Schmitt LC, Taylor CA. Relations of C-reactive protein and obesity to the prevalence and the odds of reporting low back pain. Arch Phys Med Rehabil. 2013;94(4):745–52.
Song Z, Xie W, Chen S, Strong JA, Print MS, Wang JI, et al. High-fat diet increases pain behaviors in rats with or without obesity. Sci Rep. 2017;7(1):1–14.
Pasdar Y, Moradi S, Moradinazar M, Hamzeh B, Najafi F. Better muscle strength with healthy eating. Eat Weight Disord. 2020;1-8.
Lim YZ, Wang Y, Cicuttini FM, Hughes HJ, Chou L, Urquhart DM, et al. Association between inflammatory biomarkers and nonspecific low Back pain: a systematic review. Clin J Pain. 2020;36(5):379–89.
Bärebring L, Winkvist A, Gjertsson I, Lindqvist HM. Poor dietary quality is associated with increased inflammation in Swedish patients with rheumatoid arthritis. Nutrients. 2018;10(10):1535.
Elma Ö, Yilmaz ST, Deliens T, Coppieters I, Clarys P, Nijs J, et al. Do nutritional factors interact with chronic musculoskeletal pain? A systematic review. J Clin Med. 2020;9(3):702.
Steck S, Shivappa N, Tabung F, Harmon B, Wirth M, Hurley T, et al. The dietary inflammatory index: a new tool for assessing diet quality based on inflammatory potential. Digest. 2014;49(3):1–10.
Pasdar Y, Hamzeh B, Moludi J, Mehaki B, Darbandi M, Moradi S. Dietary intake and risk of depression among male and female with HIV/AIDS. Eat Weight Disord. 2019;25(4):1029–38.
Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–8.
Pimentel GD, Micheletti TO, Pace F, Rosa JC, Santos RV, Lira FS. Gut-central nervous system axis is a target for nutritional therapies. Nutr J. 2012;11:22.
Towery P, Guffey JS, Doerflein C, Stroup K, Saucedo S, Taylor J. Chronic musculoskeletal pain and function improve with a plant-based diet. Complement Ther Med. 2018;40:64–9.
Acknowledgments
RaNCD is part of PERSIAN national cohort and we would like to thank Professor Reza Malekzadeh, Deputy of Research and Technology at the Ministry of Health and Medical Education of Iran and Director of the PERSIAN cohort, and also Dr. Hossein Poustchi Executive Director of PERSIAN cohort for all their supports during design and running of RaNCD.
Informed consent
Written informed consent was obtained from each studied subject after explaining the purpose of the study. The right of subjects to withdraw from the study at any time and subject’s information is reserved and will not be published.
Funding
This study was supported by Ministry of Health and Medical Education of Iran and Kermanshah University of Medical Science (Grant No: 92472).
Author information
Authors and Affiliations
Contributions
SM and YP equally contributed to the conception and design of the research; FN, BH, and YP contributed to data collection; SM, YP and MM contributed to the acquisition and analysis of the data; SM, SK and AM contributed to the interpretation of the data; and SM, SK, AM and YP contributed to draft the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics approval number: KUMS.REC.1394.318).
Consent for publication
Not applicable.
Competing interests
All authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pasdar, Y., Hamzeh, B., Karimi, S. et al. Major dietary patterns in relation to chronic low back pain; a cross-sectional study from RaNCD cohort. Nutr J 21, 28 (2022). https://doi.org/10.1186/s12937-022-00780-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-022-00780-2