Abstract
Background
Children with microcephaly due to vertical exposure to Zika virus are an interesting population for investigation. Highlighted among their unique aspects are those related to nutrition due to its impact on child growth and development. Knowledge about the nutrition of microcephalic infants can help mothers and caregivers provide better care. Thus, this study aimed to describe the nutritional status and feeding practices of infants with microcephaly due to Zika virus exposure at birth and 12–23 months of age.
Methods
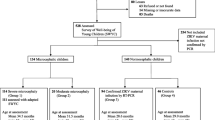
This is a descriptive study developed from a cohort of patients attending a public institution of reference. A total of 65 infants attended outpatient nutrition clinics. The food practices were described using the 24-h food recall and food consumption indicators. Anthropometric measurements and consultations were made using the Child Health Handbook to obtain information on the nutritional status (weight, height and head circumference) at the time of consultation and birth.
Results
There was a significant decrease in z-scores for weight, height and head circumference (HC) from birth to the time of the consultation. However, most infants did not show weight-for-height deficits. Additionally, HC was correlated with the anthropometric indices weight-for-age, height-for-age, body mass index-for-age and weight-for-height.
Conclusion
Infants exhibited a worsening of their nutritional status between birth and the time of their consultation, notably when we evaluated the indices of height and head circumference for age. The main inadequacies regarding dietary practices were low food diversity, use of ultra-processed products and low lipid intake.
Similar content being viewed by others
Introduction
Microcephaly is a condition in which the head circumference measurement is smaller than the typical range of values for children of the same age and gender [1, 2]. In 2015, there was an increased prevalence of microcephalic neonates in Brazil [3] related to the outbreak of the Zika virus infection during the gestational period, which established a new population [4] with a congenital infection to be investigated. Among the characteristics to be investigated in relation to these children, we highlight those related to nutrition due to its impact on children’s neurocognitive growth and development [5, 6], especially on children with neurological impairment [7].
Nutritional assessment is one of the components of child health care that can be encompassed by their primary care [8], and information related to the nutrition of infants with microcephaly can help mothers and health care providers.
Thus, this study aimed to describe the nutritional status and dietary practices of microcephalic infants vertically exposed to the Zika virus at birth and 12–23 months of age.
Methods
This is a descriptive study developed with data from the cohort “Vertical exposure to Zika virus and its consequences on the neurodevelopment of the child”, carried out at the Fernandes Figueira National Institute of Women, Children and Adolescent Health – Oswaldo Cruz Foundation (IFF/FIOCRUZ), a reference institution for this population. The Research Ethics Committee of IFF/FIOCRUZ approved the project under Opinion CAAE 52675616.0.0000.5269.
During outpatient nutrition visits, infants aged 12–23 months with microcephaly due to vertical exposure to the Zika virus were included according to head circumference (HC) z-scores for age of less than two (− 2) standard deviations and/or based on medical findings in imaging studies of the central nervous system (transfontanelle ultrasonography and tomography). The maternal infection with Zika virus was considered in the presence of symptoms (skin rash, fever associated with arthralgia, myalgia, non-purulent conjunctivitis or headache) and positive result of the transcription polymerase chain reaction (RT- PCR) during pregnancy.
Based on interviews with the mothers/guardians, the following information was obtained:
-
1-
Eating habits: a history of dietary practices; breastfeeding (BF) and exclusive breastfeeding (EBF) time; age of introduction of fruits, salty baby food (consistency - mashed/scraped or crushed/sieved), and foods such as sugar, soft drinks, processed juice and non-maternal milk.
-
2-
Food consumption at the time of the consultation (12–23 months) from the food recall of 24 h. The foods/preparations reported by the mothers/guardians were entered into the DietPro® software for the calculation of total energy and macronutrient (protein, carbohydrate and lipid) values. The nutritional information contained in the labels of the processed foods was included when they were not present in the program. We also collected data on the frequency of meals, the consumption of farinaceous and dairy products, the route of administration of food (oral or gastrostomy) and the presence of choking and reflux evidenced by infants (reported by the mother/guardians).
This information on infant feeding was also used to construct five indicators proposed by Oliveira et al. [9] and the Ministry of Health [10]:
-
Intake of at least one iron-rich food (egg, liver, lentils, beans, beef, chicken, pork, fish or other meat) [9];
-
Consumption of at least one vitamin A-rich food (mango, papaya, pitanga, liver, carrot, broccoli, pumpkin or cabbage) [9];
-
Consumption of some ultra-processed products (sugar, chocolate milk, curd cheese, margarine, coffee, canned foods, fried foods, soft drinks, natural guaraná, mate, candies, biscuits, processed juices, salty snacks, ice creams and popsicles, pies, jellies, cakes and other sweets) [9];
-
Minimum dietary diversity: large meals (lunch and dinner) including one food from each group (cereals or tubers, vegetables, meats or eggs) and small meals including fruits and milk (including maternal milk) [9]; and
-
Continuous breastfeeding (consumption of breast milk on the day before the interview) [10].
To calculate energy requirements, the formula proposed by Culley in 1969 was recommended for children with mental retardation and motor dysfunction [11]. Energy consumption corresponding to 90–110% of the recommended intake was adequate, and values below and above this range were deemed to be inadequate or excessive, respectively. The macronutrients were evaluated through the AMDR (acceptable macronutrient distribution range) [12], with the following percentages of the total energy value considered to be adequate: 5–20% of total dietary calories from proteins, 45–65% from carbohydrates and 30–40% from lipids. Intake values below and above these parameters were classified as deficient and excessive, respectively.
Anthropometry to evaluate nutritional status at 12–23 months (weight, height and head circumference) was measured according to the recommendations of the Food and Nutrition Surveillance System [13].
The anthropometric indicators weight-for-age (W/A), height-for-age (H/A), body mass index-for-age (BMI/A) and weight-for-height (W/H) were described according to the following stratification: z-scores < − 2 (lower than expected); z-scores ≥ − 2 and ≤ 2 (adequate for age); and z-scores > 2 (higher than expected). For the nutritional diagnosis at 12–23 months, W/H z-scores < − 2 were classified as a growth restriction. HC/A z-scores < − 3 were considered to indicate severe microcephaly [14] according to the WHO Growth Curves, 2006 [15]. The Anthro® version 3.2.2 program was used to calculate z-score values [16].
Birth information (date, gender, delivery type, gestational age, weight, height and HC) was collected by consulting the medical records and the Child Health Handbook, and the classification of the anthropometric indicators was performed as described in the previous paragraph, taking as reference the INTERGROWTH-21st curves [17].
Data were handled using descriptive statistics. Paired Student’s t-tests were used to verify the associations among the W/A, H/A and HC/A indices at birth and 12–23 months. We also tested the correlation between the current anthropometric index and Pearson’s correlation coefficient.
Data were typed and stored in a spreadsheet in Excel® version 2016 and analysed in SPSS 21, with a significance level of less than or equal to 0.05.
Results
Sixty-five infants were included in the study. The infants had a mean age of 15 months, 50.8% were female, 54.8% were normal births, and 87.7% had a gestational age ≥ 37 weeks at birth.
We observed that most participants had adequate H/A and W/A z-scores at birth. Concerning nutritional status at 12–23 months, the anthropometric indices of W/A, BMI/A and W/H were within the parameters of adequacy for most infants. However, 56.9% had H/A deficits (Table 1). W/A, H/A and HC/A z-scores at birth and 12–23 months showed significant associations. There was a significant decrease in all mean z-scores between the periods of assessment (Table 2).
A significant correlation was identified between the values of HC/A z-scores and all anthropometric indices: H/A (correlation coefficient 0.606, p < 0.000), W/A (correlation coefficient 0.587, p < 0.000), W/H (correlation coefficient 0.377, p = 0.02) and BMI/A (correlation coefficient 0.314, p = 0.012).
Regarding the history of feeding practices, approximately 80% of the infants were not exclusively breastfed until the sixth month. Difficulties with breastfeeding were reported by 53.6% of the mothers. Among them, the mammary gland (33%) and hypogalactia (12.5%) were the most frequently mentioned. We found that these first foods were predominantly offered in “mashed and scraped” consistencies, as well as the first salty baby foods. Most participants had already tried sugar (Table 3).
At 12–23 months of age, few participants met the minimum dietary diversity criteria or were continuously breastfeeding. Ultra-processed foods were used by 52.3% of the participants. We found that many of the infants consumed fruit in less than two meals a day, and dairy foods were consumed more frequently. Most infants consumed farinaceous food twice or more a day and had adequate energy consumption. Regarding the distribution of macronutrients, we found that although participants’ overall protein and carbohydrate intake met the dietary recommendations, the lipid intake was poor in almost half of the participants (Table 4).
Discussion
Our results indicate a deteriorating nutritional status of infants from birth to the time of the consultation (12–23 months). The most negatively affected anthropometric indices (p < 0.000) were H/A and HC/A, which showed significant declines, eventually changing the growth z-score channel. There was a correlation between the HC/A z-score and all other current anthropometric indices, mainly for H/A and W/A, showing that the weight and size of the skull and brain have a greater impact on these indicators. Del Campo et al. [18] also found similar correlations among HC/A, W/A and H/A scores at the birth of children with (probable or confirmed) congenital Zika virus syndrome, with and without microcephaly. In describing the growth of children with the same (presumed) syndrome from birth to the 8th month, Silva et al. [19] found monthly decreases in the z-scores for mean weight, height and especially HC, which continued to decline with time among cases both with and without microcephaly. However, in our findings, unlike the compromised anthropometric indicators above, at the moment of the consultation, W/H was proportional in more than 60% of infants; that is, most of these children did not evidence a deficit of this indicator. Thus, this population is characterized by a unique anthropometric profile such that their height and weight were affected. However, this population also showed harmony between these two parameters at the time of the consultation. Thus, the W/H index is an important parameter for the nutritional assessment of these infants.
Regarding dietary practices, approximately 78% of infants remained under EBF for less than six months. Before the first year of life, more than 90% of them were already consuming non-maternal milk. At the time of the nutritional consultation, less than 20% were continuously breastfeeding, whereas in children without microcephaly, this frequency at 12 months was approximately 35% [20]. The early weaning observed in our results can be explained, in part, by the difficulties with breastfeeding reported by mothers, especially vis-à-vis the mammary gland. This is to be expected, since children with microcephaly due to Zika virus exposure may show dysphagia from the third month [21] when changes in oral-motor coordination, swallowing and sucking make breastfeeding a challenging task [15].
In the introduction of complementary feeding, most infants consume the first salty baby food and fruits in “mashed and scraped” consistencies, adjusting to the consistencies recommended for children in this age group [22], despite the neurological impairment that implies adaptations in their dietary practice. An intake of fruits in less than two meals a day and a higher frequency of dairy consumption characterized a poorly diversified diet. A small percentage (27.7%) met the criteria of minimum dietary diversity [10]. A survey with data from more than 100 countries showed that only 29% of children between 6 and 23 months of age have minimal food variety [23]. This raises the challenge of how to change this situation, regardless of the health status of the child, paying particular attention to their dietary practices, considering the impact of adequate nutrition during the first thousand days of life on the child’s current and future health and life conditions [24].
In addition, we identified the introduction of the consumption of ultra-processed foods as inadequate from the point of view of nutritional quality, especially in children under two years of age, given their high levels of sodium, sugar and fat, high energy density and low nutritive content [25], which are associated with chronic noncommunicable diseases in adulthood [26]. The values found for the intake of these foods (52%) were close to those in the study by Marinho et al. [27] (58%) in children under two years of age without neurological impairment.
Most infants (68%) had an adequate or excessive energy intake. However, since the balance between the macronutrient percentage distribution, which is as important as the energy intake, is desired, we found that 50% of the babies had poor lipid intake. This is a factor of concern, given the importance of lipids and, more specifically, essential fatty acids, in child growth, as well as in visual and central nervous system development [28]. Considering that these systems may be affected by the congenital Zika virus syndrome [29, 30], the adequate consumption of lipids for these children exposed to the virus becomes even more crucial. The inadequate dietary intake of lipids may be explained by the main challenge to eating in this study (choking), when mothers thicken food through the use of carbohydrate-rich farinaceous food. These foods were used at least two times a day for more than 70% of the children, possibly displacing other lipid-rich foods, thus contributing to meeting the energy requirements.
A possible limitation of this study is the use of a single 24-h food recall, which does not consider dietary variability. However, this limitation may have been minimized by the food monotony of the age group and population studied. We hope that these results can provide insight into this stage of research, since there are still few studies in the literature that address the food consumption of these babies. We also hope that these findings help to provide initial subsidies for the elaboration of future care protocols, which can be used in the clinical practice of professionals involved in the healthcare of these children.
Conclusion
In conclusion, the infants studied exhibited a deterioration in their nutritional status between the two assessment points, notably when we evaluated the H/A and HC/A indices. The main inadequacies related to dietary practices were low food diversity, ultra-processed consumption and low lipid intake. We then considered the efforts that must be invested to ensure that care for these children is provided by a team of interdisciplinary professionals. The nutritionist should have an essential role on this team, especially concerning the guidelines for an age-appropriate diet, which consider the limitations of this population and aim to minimize nutritional deficits and promote a better quality of life.
References
World Health Organization. Assessment of infants with microcephaly in the context of Zika virus - interim guidance. Genebra; 2016. http://www.chinacdc.cn/jkzt/crb/ablcxr_8561/zstd_8600/201602/W020160227443710998127.pdf.
World Health Organization. Screening, assessment and management of neonates and infants with complications associated with Zika virus exposure in utero. Genebra; 2016. http://apps.who.int/iris/bitstream/handle/10665/204475/WHO_ZIKV_MOC_16.3_eng.pdf;sequence=1.
Marinho F, Araújo VEM, Porto DL, Ferreira LH, Coelho MRS, Lecca RCR, et al. Microcefalia no Brasil: prevalência e caracterização dos casos a partir do Sistema de Informações sobre Nascidos Vivos (Sinasc), 2000-2015. Epidemiol Serv Saude. 2016;25:1–12.
Brasil. Ministério da Saúde. Ministério da Saúde confirma relação entre vírus Zika e microcefalia, 28 de novembro de 2015. Nota à imprensa [Internet] 2015. Available at: http://www.blog.saude.gov.br/index.php/combate-ao-aedes/50399-ministerio-da-saude-confirma-relacao-entre-virus-zika-e-microcefalia. Accessed 10 June 2016.
Mozetic RM, Silva SDC, Ganen AP. A importância da nutrição nos primeiros mil dias. REAS. 2016;8(2):876–84.
Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72(4):267–84.
Penagini F, Mameli C, Fabiano V, Brunetti D, Dilillo D, Zuccotti GV. Dietary intakes and nutritional issues in neurologically impaired children. Nutrients. 2015;7:9400–15.
Leandro CG. Nutritional status and gross motor function in children with cerebral palsy, and implications for Zika virus infection. Dev Med Child Neurol. 2016;58(9):893–4.
Oliveira JM, Castro IRR, Silva GB, Venancio SI, Saldiva SRDM. Avaliação da alimentação complementar nos dois primeiros anos de vida: proposta de indicadores e de instrumento. Cad Saúde Pública. 2015;31(2):377–94.
Brasil. Ministério da Saúde. Orientações para avaliação de marcadores de consumo alimentar na atenção básica [online]. Secretaria de Atenção à Saúde, Departamento de Atenção Básica – Brasília, 2015.
Culley WJ, TO M. Caloric requirements of mentally retarded children with and without motor dysfunction. J Pediatr. 1969;75(3):380–4.
Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber fat, protein and amino acids (macronutrients). Washington: National Academy Press; 2002.
Brasil. Ministério da Saúde. Orientações para a coleta e análise de dados antropométricos em serviços de saúde: Norma Técnica do Sistema de Vigilância Alimentar e Nutricional – SISVAN. Secretaria de Atenção à Saúde, Departamento de Atenção Básica. – Brasília, 2011.
Brasil. Ministério da Saúde. Orientações integradas de vigilância e atenção à saúde no âmbito da Emergência de Saúde Pública de Importância Nacional: procedimentos para o monitoramento das alterações no crescimento e desenvolvimento a partir da gestação até a primeira infância, relacionadas à infecção pelo vírus Zika e outras etiologias infeciosas dentro da capacidade operacional do SUS. In: Secretaria de Vigilância em Saúde – Brasília; 2017.
Brasil. Ministério da Saúde. Sistema de Vigilância Alimentar e Nutricional. Curvas de Crescimento da Organização Mundial da Saúde - OMS 2006/2007. Access on January 17, 2017. Available at: http://dab.saude.gov.br/portaldab/ape_vigilancia_alimentar.php?conteudo=curvas_de_crescimento
World Health Organization – WHO. WHO Anthro version 3.2.2: Child growth standards [online]. 2011. Available at: http://www.who.int/childgrowth/software/en/
Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG. International fetal and newborn growth consortium for the 21st century (INTERGROWTH-21st). International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. 2014;384:857–68.
el Campo M, Feitosa IML, Ribeiro EM, Horovitz DDG, Pessoa ALS, França GVA, et al. The phenotypic spectrum of congenital Zika syndrome. Am J Med Genet. 2017;173(4):841–57.
Silva AAM, Ganz JSS, Sousa OS, Doriqui MJR, Ribeiro MRC, Branco MRFC, et al. Early growth and neurologic outcomes of infants with probable congenital Zika virus syndrome. Emerg Infect Dis. 2016;22(11):1953–6.
Saldan PC, Venancio SI, Saldiva SRDM, Pina JC, Mello DF. Práticas de aleitamento materno de crianças menores de dois anos de idade com base em indicadores da Organização Mundial da Saúde. Rev Nutr [online] 2015;28(4); 409–420 [acesso em 25 de jan. 2018]. Available at: http://www.scielo.br/pdf/rn/v28n4/1415-5273-rn-28-04-00409.pdf
Leal MC, van der Linden V, Bezerra TP, de Valois L, Borges ACG, Antunes MMC. Characteristics of dysphagia in infants with microcephaly caused by congenital Zika virus infection, Brazil, 2015. Emerg Infect Dis. 2017;23(8):1253–9.
Brasil. Ministério da Saúde. Dez passos para uma alimentação saudável: guia alimentar para crianças menores de dois anos: um guia para o profissional da saúde na atenção básica. Secretaria de Atenção à Saúde, Departamento de Atenção Básica. 2ª ed. 2015.
White JM, Bégin F, Kumapley R, Murray C, Krasevec J. Complementary feeding practices: current global and regional estimates. Maternal & Child Nutrition. 2017;13(2):12505.
Cunha AJLA, Leite AJM, Almeida IS. The pediatrician’s role in the first thousand days of the child: the pursuit of healthy nutrition and development. J Pediatr. 2015;91:544–51.
Monteiro CA. The big issue is ultra-processing. World Nutrition. 2010;1(6):237–69.
Claro RM, Sanos MAS, Oliveira TP, Pereira CA, Szwarcwald CL, Malta DC. Consumo de alimentos não saudáveis relacionados a doenças crônicas não transmissíveis no Brasil: Pesquisa Nacional de Saúde, 2013. Epidemiol Serv Saúde. 2015;24(2):257–65.
Marinho LMF, Capelli JCS, Rocha CMM, Bouskela A, Carmo CN, Freitas SEAP, et al. Situação da alimentação complementar de crianças entre 6 e 24 meses assistidas na Rede de Atenção Básica de Saúde de Macaé, RJ, Brasil. Ciência & Saúde Coletiva. 2016;21(3):977–86.
Cozzolino SMF, Cominetti C. Bases bioquímicas e fisiológicas da nutrição nas diferentes fases da vida, na saúde e na doença; 2013. p. 1257.
Eickmann SH, Carvalho MDCG, Ramos RGF, Rocha MAW, Linden VV, Silva PFS. Síndrome da infecção congênita pelo vírus Zika. Cad. Saúde Pública. 2016;32(7):1–3.
Zin AA, Tsui I, Rossetto J, Vasconcelos Z, Adachi K, Valderramos S, et al. Screening criteria for ophthalmic manifestations of congenital Zika virus infection. JAMA Pediatr. 2017;171(9):847–54.
Acknowledgements
The authors thank the participants for their contributions to the trial.
Funding
This study was supported by the Wellcome Trust (205377/Z/16/Z) and the United Kingdom’s Department for International Development; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (441098/2016–9); Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)(E_18/2015TXB) and European Union’s Horizon 2020 research and innovation programme under Zika-PLAN grant agreement no. 734584.
Availability of data and materials
Upon request, the dataset of the study is available from the corresponding author.
Prior presentation
Not applicable
Author information
Authors and Affiliations
Contributions
SFMS collected the data, interpreted the results and drafted the manuscript. FVMS collected the data, contributed to the interpretation of the results and the drafting and oversight of the manuscript. ADA collected the data, contributed to interpretation of the results and the drafting of the manuscript. ACCC conducted the statistical analysis. MELM contributed to the interpretation of the results. VMF interpreted the results, participated in the writing of the manuscript, and guided and supervised the preparation of the manuscript. All authors reviewed, read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was approved by the Research Ethics Committee of the IFF (Certificate of Presentation for Ethical Appreciation - CAAE 52675616.0.00005269) in May 2016. Families could participate in the research if mothers/guardians of the infants signed the Term of Free and Informed Consent. This study was carried out in accordance with the norms applicable to the research, contained in Resolution number 466 of December 12, 2012 - Brazil.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
dos Santos, S.F.M., Soares, F.V.M., de Abranches, A.D. et al. Infants with microcephaly due to ZIKA virus exposure: nutritional status and food practices. Nutr J 18, 4 (2019). https://doi.org/10.1186/s12937-019-0429-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-019-0429-3