Abstract
Background
Malaria remains a serious global public health problem, and continues to have a devastating impact on people’s health worldwide. Continuous monitoring and evaluation of current malaria transmission status in different seasons is a mainstay for the success of ongoing intervention strategies for malaria. The purpose of this study was to assess the dry-season transmission and determinants of malaria in Jawi district, northwest Ethiopia.
Methods
A community-based cross-sectional study was conducted from January 13 to February 11, 2020; among selected Kebeles in the Jawi district. A multistage sampling technique was used in this study. Random and systematic sampling techniques were carried out to select Kebeles and each household, respectively. Light microscopy and CareStart™ Malaria HRP2/pLDH (Pf/Pv) Combo RDT were implemented to determine the prevalence of malaria. Moreover, associated risk factors in the prevalence of malaria were assessed by using a bivariate and multivariate logistic regression model.
Results
A total of 219 study participants were enrolled in this study. Of the total enrolled individuals, malaria cases were found among 36 individuals with a positivity rate of 16.4% (95% CI 11.4–21.5). Plasmodium falciparum was the predominant species with an estimated prevalence of 87.0% in the study areas. Interrupted utilization of ITN (AOR = 4.411, 95% CI 1.401–13.880), using over 3 years older ITNs (AOR = 9.622, 95% CI 1.881–49.214), travel history (AOR = 12.703, 95% CI 2.441–66.114), living in a house with holes on the wall (AOR = 3.811, 95% CI 1.010–14.384), and living in a house with an eave (AOR = 4.23, 95% CI 1.065–16.801) significantly increased the probability of malaria positivity rate.
Conclusion
Malaria is still an important public health burden among individuals in the Jawi district. Interrupted utilization of ITNs, using over 3 years older ITNs, living in a house with holes on the wall, living in a house with an eave, and travel history were identified as the risk factors of malaria. Therefore, the District health office and Health extension workers should promote daily utilization of good ITNs and improve housing conditions to reduce malaria prevalence.
Similar content being viewed by others
Background
Malaria remains a serious global public health problem, and continues to have a devastating impact on people’s health worldwide [1, 2]. Available data showed that despite the mortality rates of malaria were declined by 7.2% from 2015 to 2017, the incidence has been reciprocally increased by 2.2% (from 214.2 million to 219 million cases) during the same time frame. In 2019, there were an estimated 229 million malaria cases and 409,000 malaria-associated deaths occurred worldwide, and it indicates still there is an increment of malaria incidence from 2015 to 2019. The World Health Organization (WHO) Africa region is home to the bulk of the global burden of malaria with an estimated 93% malaria cases and 94% malaria-associated deaths. Of whom, children aged under 5 years accounted for 67% of malaria cases and deaths. Plasmodium falciparum is the predominant species with an estimated burden of 99.7% of malaria cases in the regions [3, 4].
This disproportional share of the global malaria burden in Africa is most likely due to the existence of highly efficient mosquito vector species such as Anopheles gambiae, Anopheles coluzzii, Anopheles funestus, and Anopheles arabiensis, which are highly specialized vectors in seeking out and feeding on human blood [5,6,7]. These vectors are capable to survive and are widely distributed in the region during the long dry season, where surface water crucial for their reproduction is absent for up to 7 months [8]. Vectors’ dry season diapauses and quiescence adaptive mechanisms are presumably the reason for their extended and persistent survival [9]. In addition, recent findings revealed that Anopheles stephensi (Asian mosquito species) has been identified in the eastern parts of Africa, and is capable of transmitting both P. falciparum and P. vivax [7, 10].
Moreover, malaria parasites capable to exist persistently in the human host for long periods allow it to bridge the dry period and it resulting the disease burden remaining high regardless of the vector abundance [11, 12]. In addition to the impact of seasonal variation on the transmission of malaria, the role of climatic changes is also crucial for vector-borne disease [13]. In this regard, recent studies confirmed that the epidemiology of malaria becoming quite complex and highly sensitive to temperature, rainfall, and topographical variation, which are the main climatic variables that directly influence vector-borne diseases’ ecosystems, including the host behavior, development, and pathogen amplification [14,15,16,17,18,19,20].
In Ethiopia, 68% of the country’s landmass is favourable for malaria transmission and 60% of the population lives in malarious areas. In 2017, existing evidence revealed malaria remains one of the leading causes of morbidity and mortality in the nation [21]. The transmission pattern of malaria is unstable and mainly occurs in two major (September–December) and minor (April–June) transmissions seasons [22,23,24]. The mortality and morbidity of malaria has been reduced in Ethiopia following the nationwide distribution of rapid diagnostic tests (RDT), artemisinin-based combination therapy (ACT), long-lasting insecticidal nets (LLINs), and indoor residual sprays (IRS) in Ethiopia since 2005. However, recent findings published in 2018 revealed that the incidence of malaria has been increasing [21, 23]. It may be due to the implementation of control measures regardless of the evidence of shifting in malaria transmission pattern and it is constrained only to previously known stable malaria transmission areas [25]. It indicates the presence of challenges ahead of the national malaria elimination strategic plan.
Moreover, the current vector control approaches mainly aim to reduce the proliferation of the vectors, reduce transmission and, therefore, reduce the number of new malaria cases through the administration of insecticides at the beginning of a rainy season [2, 26]. However, these control strategies may not be expected to achieve the successful elimination programme due to it lacking the modified diapauses and quiescence vectors’ suppression strategies to maintain their survival during the dry season period [9, 11].
Ethiopia is implementing uninterrupted and integrated public health intervention measures to successfully eradicate malaria by 2030. Undergoing an in-depth examination of malaria parasite carriage, identification, and control of both asymptomatic and symptomatic infections at a community level is crucial for the success of this long-term plan. Besides, understanding dry season malaria transmission status could be a crucial issue to inform malaria and vector control strategies to strengthen the current designed malaria elimination programmes. Since no previous published data of malaria cases in the Jawi district at a community level, this study was conducted to provide preliminary information for the scientific community. Therefore, this study intended to assess the dry season’s malaria transmission status, the major Plasmodium species, and identifies potential associated risk factors for its transmission in the Jawi district from a deep sample at the community level.
Methods
Study area and period
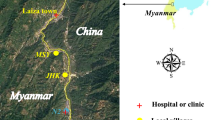
The study was conducted in two selected Kebeles (Wombelasi and Argabo) of Jawi district, Northwest Ethiopia, from January to February 2020. Jawi is one of the districts in the Amhara region specifically located in the Awi zone, northwest Ethiopia. According to the 2007 central statistical agency of Ethiopia report, Jawi has a total population of 79,090, of whom 9.76% are urban dwellers. Jawi district, the climate alternates with long summer rainfall (June–September) and a winter dry season (October–May) with a mean annual rainfall of 1569.4 mm. The mean temperature varies between 13.68 and 34.6 °C and the altitude ranges from 648 to 1300 m above sea level. Jawi is one of the renowned districts with high incidence and transmission of malaria as indicated by the unpublished district and zonal malaria reports.
Study design and population
A community-based cross-sectional study was conducted to determine the dry season transmission and associated risk factors of malaria infection among dwellers in the Jawi district. All settled community members of the residents or dwellers within the selected districts were the source population of the study. In the selected districts, family members of the selected households and those who were present during the study period were included in the study. The community members who had confirmed malaria parasites from a health facility, who were taking antimalarial drugs during the data collection period, who had been treated with antimalarial drugs 1 month before participant enrollment and under 6-months-old infants were excluded.
Sample size and sampling technique
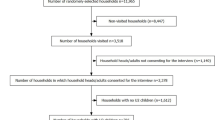
The intended sample size was calculated using a single proportion formula. It was calculated using the nearby area’s prevalence of 16.6% [27] with a margin of error of 5% and a confidence level of 95%. Then, the calculated sample size was 213. However, 5% of the estimated sample size was added to consider the possibility of a non-response rate, and the final calculated sample size was 224. A multistage sampling technique was used to select the required sample size. Before participant enrollment, two kebeles were randomly selected using the lottery technique in the district. Then households were selected using a systematic sampling method and the study participants were chosen using a simple random sampling method.
Data collection and laboratory methods
Prior to participant enrollment, a structured questionnaire that specifically designed to address the socio-demographic characteristics of participants and malaria risk factors was developed. The questionnaire was primarily developed in English and then translated to the local language Agewugna to check for consistency. Moreover, a pre-test was also undertaken on 5% of the total sample size among the community dwellers outside of the selected kebeles for this study, and some amendments were made. To assure and keep the consistency of data during collection, training was given for 2 consecutive days to the data collector and kebele administrators regard to the objective of the study, the data collection instrument, questionnaire administrating techniques, participant recruitment techniques among the household member, and other ethical issues by the principal investigators of this study.
Socio-demographic characteristics and malaria risk factor assessment
A standardized, pre-tested, and structured questionnaire was administered to gather information on the marker of socio-demographic characteristics, health-related factors, environmental-related factors, IRS coverage, housing structure, and ITN availability and utilization of the community. Every head of the household (HH) in the community either female or male present at home during visiting was interviewed using the local language (Agewugna).
Blood sample collection and laboratory procedures
Following the accomplishment of crucial data collection using the designed questionnaires, nearly 250 µL capillary blood samples from figure prick were collected aseptically from each study participant to prepare both thick and thin blood film using pre-labeled microscope slides for detection and identification of Plasmodium species. The prepared blood films were stained using 10% Giemsa solution for 10 min and it allows to be air-dried by putting horizontally in a slide tray at room temperature. After the stained slides were air-dried, malaria parasites were ruled out if no sexual or asexual forms of parasites are seen after reading 200 HPFs or 2500 WBCs [28].
CareStart™ Malaria HRP2/pLDH (Pf/Pv) Combo (RDT) was performed according to the manufacturer’s instructions. The kit was labeled with the respective sample code and 5 μL of blood specimen was added into the sample well of the test device. Two drops of lysis buffer were added into the buffer well to lyse the cells, release the antigen and facilitate the antigen–antibody reaction. Then, the result was read after 15–20 min and interpreted according to the manufacturer’s instruction [29].
Data quality control
To assure the quality of data, data collectors were trained, continuously supervised and a pre-test was done to keep the validity of the questionnaire. All test procedures and interpretation of results were done based on standard operating procedures (SOPs). Expiry dates, lot №s, and handling conditions of all reagents, materials, and Malaria HRP2/pLDH (Pf/Pv) RDTs were checked daily before and during the data collection period. The quality of Giemsa staining was tested with prefixed positive and negative control blood-smeared slides from Injibara General Hospital from the beginning. Then, all the blood films were examined carefully by the trained laboratory technician and principal investigators first and re-examined blindly by senior Medical Laboratory Technologists, who are working at malaria microscopy quality assurance centre in Injibara General Hospital with updated malaria training.
Data management and analysis
The data was coded and entered into Epi-data software to check its completeness and clearance, and then transferred to SPSS version-23 for further statistical analysis. Descriptive statistics were figured out to give a clear picture of dependent and independent variables. The association between each dependent and independent variable was explored by using binary logistic regression. Variables with a P-value < 0.25 in the bivariate analysis were subjected to further multivariate logistic regression analysis model to identify predictor variables and to control cofounders. Individuals diagnosed with confirmed Plasmodium infection using either a blood smear microscope or malaria HRP2/pLDH (Pf/Pv) RDT were considered to estimate the overall prevalence of malaria in this study (microscopy plus RDT results). Sensitivity, specificity, and predictive values for the malaria HRP2/pLDH (Pf/Pv) RDT were determined by using light microscopy as a reference. Agreement between malaria HRP2/pLDH (Pf/Pv) RDT and light microscopy in detecting both symptomatic and asymptomatic malaria were determined by Kappa value [30]. For any statistical analysis P-value < 0.05 was considered statistically significant.
Results
Socio-demographic characteristics
A total of 219 individuals were enrolled in this study. Of whom, 114 (52.1%) participants were female. The mean age of enrolled individuals was 22.3 ± 14.9 and 75% of the participants were farmers. Moreover, the remaining socio-demographic characteristics of study participants were indicated below (see Table 1).
Confirmed cases of Plasmodium infection
The overall prevalence of malaria infection was 16.4% ((36/219); 95% CI 11.4–21.5). Of this (32/219) 14.6% of malaria cases were identified using RDT, while 10.5% (23/219) using microscopy. Regarding Plasmodium species distribution, P. falciparum was the predominant species among confirmed cases, 28 (87.5%) by CareStartTM Pf/Pv RDT and 20 (87.0%) by light microscopy. Whereas, P. vivax accounted 12.5% (4 individual) by CareStartTM Pf/Pv RDT and 2 (8.7%) by microscopy, and only 1 (4.3%) participant was confirmed with P. falciparum and P. vivax mixed infection by microscopy. Statistically, significant correlation (P < 0.01) was observed between the prevalence of Plasmodium infections and the age categories. Age group 5–14 has accounted for the highest prevalence of the case with 22.7% (10/44) followed by the age group 15–24 with 21.8% (12/55) cases (see Table 2).
Symptomatic and asymptomatic Plasmodium infections
In the study area, the overall prevalence of symptomatic and asymptomatic malaria was 35.2% (25/71) and 7.4% (11/148), respectively using both RDT and microscopy. Of the total confirmed P. vivax individuals using RDT, 75% (3/4) were asymptomatic. But, only 37.9% (11/29) of P. falciparum positive study participants had no symptoms of Plasmodium infections. Among microscopically confirmed participants with malaria, asymptomatic cases were 50% (1/2) for P. vivax and 10% (2/20) for P. falciparum (see Table 3).
CareStart™ Pf/Pv RDT diagnostic performance using Giemsa-stained thick smear microscopy as a gold standard
Of the total 32 malaria parasite confirmed by RDT, only 19 were positive with microscopy. The remaining 13 RDT positive samples were negative by microscopy. In addition, of the total 23 microscopically detected cases, 4 malaria positives slides were negative by RDT. This made the positive predictive value of RDT low 59.4% (19/19 + 13). The negative predictive value was very high 97.9% (183/4 + 183). The overall sensitivity, specificity, positive predictive value, and negative predictive value of CareStart™ Pf/Pv RDT was 82.6%, 93.4%, 59.4%, and 97.9%, respectively. The level of agreement (Kappa value) of RDT and standard microscopy was 0.65 which is classified as a substantial agreement (kappa = 0.61–0.80).
Factors associated with malaria transmission in the community
After an adjustment was made for covariates, individuals who were not daily slept under LLIN (ITN) bed nets increased the risk of malaria infection by 4.41 times more than those who were sleeping daily under the net (AOR = 4.411, 95% CI 1.401–13.880). Participants who used one LLIN bed net for more than 3 years had a 9.62 times higher chance of contacting malaria than those who used it for less than a year (AOR = 9.622, 95% CI 1.881–49.214). While individuals who used a single LLIN (ITN) for 1 to 3 years had a 6.69 times increase in malaria positivity rate (AOR = 6.691, 95% CI 1.413–31.698) when compared to those who used it for less than a year. Individuals living in a house with eave were 4.23 times more likely to be infected with malaria (AOR = 4.23, 95% CI 1.065–16.801), while holes on the wall of the house increased malaria infection by 3.811 (AOR = 3.811, 95% CI 1.010–14.384) than those individuals living in houses with no hole. Similarly, individuals who had a travel history were also significantly associated with malaria prevalence (P = 0.003) (see Table 4).
Discussion
Despite sustained control efforts have been made nearly in the past two decades to fight malaria, it remains the major cause of morbidity, mortality, and socio-economic problems in Ethiopia [31, 32]. This study revealed that malaria continues to be a major public health concern among populations who reside in the Jawi district of Awi Zone. In the present study, a considerable prevalence of malaria was found with an estimated prevalence of 16.4% % despite there is a high coverage of LLITN in the community. This result confirms that the ongoing transmission of malaria persists with considerable level during dry/low transmission season. Studies conducted in different parts of Ethiopia such as in Dangila health center in Awi zone 16.6% [27], Pawe district 14.7% [33] and in Dilla, the southern parts of Ethiopia 16% [34] showed comparable findings to the current study. On the contrary, this is inconsistent with conducted in Jiga, Jabi Tehnan district 2.8% [35], Armachiho district with 32.6% [36], and Adi Arkay 36.1% [37]. The variations could be attributed to a difference in study design, seasonality of malaria transmission, epidemiology, and climatic conditions. For example, the present study was conducted during the dry season when there is low availability of rainfall and surface water which is essential for the breeding site of mosquito vectors unlike other studies mentioned above.
The overall Plasmodium species proportion was found to be 87.0% P. falciparum, 8.7% P. vivax, and 4.3% mixed infections. The distribution of Plasmodium species in this study supported with malaria parasite distribution report in Ethiopia [38, 39], the WHO malaria reports in the Africa region [40, 41], and with other many more studies such coincide with reports in Dembia [42], Metema [43], and Ataye district, North Shoa [44]. In this study, the overall symptomatic positives were 23.4% and the asymptomatic positives were 3.4%. The finding coincides with the result investigated in North Gondar 22.3% [45] or in Hadiya Zone; Southern Ethiopia 25.8% [46], and in Jiga 2.8% [35], respectively. In this study, the prevalence of symptomatic and asymptomatic malaria infections was 35.2% and 7.4%, respectively. The prevalence of symptomatic malaria in this study was comparable with the findings in Armachiho 32% [36], in central, north, and west Gondar zones 36% [47], in low transmission areas in Amhara Region 39.4% [48]. However, this is lower than a study conducted in another country in Tanzania [49]. Besides, the asymptomatic malaria prevalence (7.4%) in this study is also comparable to the study in Tanzania [42]. On the other hand, it is found to be lower than the prevalence reported in Armachiho 68.1% [36], in Jimma Zone [50], and other countries such as Kenya and South-Eastern Bangladesh [51, 52]. In general, the variations of this study from the other studies might be due to the included study populations immune status and timing of data collection.
In this study, there was a slight increase in RDT positivity rate (7.5% or 33/438) compared with another study investigated with a much higher sample size (1.9% or 153/7878) [53]. In addition, by considering Giemsa light microscopy as a gold standard test method for malaria detection, the overall sensitivity, specificity, positive predictive value, and negative predictive value of CareStart™ Pf/Pv RDT was 83.3%, 96.9%, 60.6%, and 99.0% respectively. The sensitivity and specificity of this study are similar to the study in the Amhara region of a higher malarious area [54]. These findings were also comparable with the results of the study conducted in Cameroon [55]. In this study, the positive predictive value was lower, and this revealed that CareStart™ Pf/Pv RDT test was less specific relative to light microscopy. This might be due to the probability of false positivity results from RDT that could be caused by previously treated individuals [56] with the high eagerness of being diagnosed by lying for the exclusion criteria. A substantial agreement was found between diagnoses of the RDT and light microscopy (Kappa = 0.68) which could be consistent with the result of a study conducted in Colombia (Kappa = 0.61–0.80) [57].
In the current study, utilization of LLIN, the oldness of LLIN, house with eave, house with holes on the wall, and travel history of individuals were found to be significantly associated with malaria prevalence (P < 0.05). This finding is supported by different studies conducted in Ethiopia [42, 46, 58, 59]. In this study, individuals who were not daily slept under bed nets (ITNs) increased the risk of malaria infection by 4.411 times more than those who were sleeping daily under the net. This is supported by studies conducted in the Hadiya zone [46], and in Dembia district [42]. Moreover, in this study, the IRS operation coverage was 82.6% in the lowland which is again lower than the country-wide coverage [60], and it was absent or extremely lower (1.4%) in the highland areas.
In this study, the odds of malaria infection among individuals who had a history of travel were higher than those who did not. This finding is consistent with that of a study conducted in Dembia, in Northwest Ethiopia [42] and Jimma town, Southwest Ethiopia [61]. A challenge to address malaria control tools to mobile migrant workers from highland homes to organic mechanized farms in Metema–Humera lowland during the agricultural rainy season (travel history) was reported as the main reason for persistent high malaria prevalence in Central, west and North Gondar zones [62].
Above all, assessing any association for single malaria infection with a variety of risk factors in the highland area showed insignificant relation. The possible reasons for the differences of this study from the other studies could be due to differences in quality of houses, nature of the study population, the implemented malaria control and prevention strategies (LLIN and IRS coverage), and culture of ITN utilization in the study area. Even though this research was conducted at the community level considered as a strength of the study, enrolled small sample-sized study participants and unable to consider the design effect to minimize sampling errors were considered as a limitation of the study.
Conclusion and recommendations
Malaria, especially the predominant falciparum malaria, is an important public health concern among dwellers in the study area. Individuals in the age group of 5–14 were the most vulnerable segments of the population for malaria. Despite the protective role of LLIN/ITN is confirmed against malaria, its application for a long period was found to be a risk factor. Besides, the nature of housing (presence of eave in the house and a hole in the wall of the house) and travel history were identified as risk factors for malaria. Therefore, during the implementation of malaria control and prevention activities, awareness creation should be given regarding risk factors like housing structure and utilization of single ITN for a long period alongside the distribution of ITN. Moreover, attention should be given to travellers to use the most appropriate prevention approaches. The local health office should facilitate and motivate the health extension workers to increase the utilization of ITN and create awareness about the way how and how often they should use ITNs.
Availability of data and materials
The data generated or analysed during this study is included in this manuscript. Other data will be available from the corresponding author upon request.
Abbreviations
- ACT:
-
Artemisinin-based combination therapy
- HRP2:
-
Histidine rich protein 2
- IRS:
-
Indoor-Residual Spraying
- ITN:
-
Insecticide treated net
- LLIN:
-
Long-lasting insecticidal net
- RDT:
-
Rapid diagnostic test
- SOP:
-
Standard operating procedure
- SPSS:
-
Statistical Package for Social Sciences
- WHO:
-
World Health Organization
- AOR:
-
Adjusted odds ratio
- COR:
-
Crude odds ratio
References
WHO. The potential impact of health service disruptions on the burden of malaria: a modeling analysis for countries in sub-Saharan Africa. Geneva: World Health Organization; 2020.
Magombedze G, Ferguson NM, Ghani AC. A trade-off between dry season survival longevity and wet season high net reproduction can explain the persistence of Anopheles mosquitoes. Parasite Vectors. 2018;11:576.
WHO. Malaria key facts. Geneva: World Health Organization; 2020. https://www.who.int/news-room/fact-sheets/detail/malaria.
WHO. Regional and global trends in burden of malaria cases and deaths. Geneva: World Health Organization; 2019. https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019.
Wiebe A, Longbottom J, Gleave K, Shearer FM, Sinka ME, Massey NC, et al. Geographical distributions of African malaria vector sibling species and evidence for insecticide resistance. Malar J. 2017;16:85.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2010;3:117.
Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA. 2020;117:24900–8.
Yaro AS, Traoré AI, Huestis DL, Adamou A, Timbiné S, Kassogué Y, et al. Dry season reproductive depression of Anopheles gambiae in the Sahel. J Insect Physiol. 2012;58:1050–9.
Diniz DFA, de Albuquerque CMR, Oliva LO, de Melo-Santos MAV, Ayres CFJ. Diapauses and quiescence: dormancy mechanisms that contribute to the geographical expansion of mosquitoes and their evolutionary success. Parasit Vectors. 2017;10:310.
Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89.
University of Exeter. Malaria parasites adapt to survive the dry season. Science Daily. 2020 Oct 30. https://www.sciencedaily.com/releases/2020/10/201030111811.htm.
Andrade CM, Fleckenstein H, Thomson-Luque R, Doumbo S, Lima NF, Anderson C, et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nat Med. 2020;26:1929–40.
Cella W, Baia-da-Silva DC, de Melo GC, Tadei WP, Sampaio VdS, Pimenta P, et al. Do climate changes alter the distribution and transmission of malaria? Evidence assessment and recommendations for future studies. Rev Soc Bras Med Trop. 2019;52:e20190308.
Chuang T-W, Soble A, Ntshalintshali N, Mkhonta N, Seyama E, Mthethwa S, et al. Assessment of climate-driven variations in malaria incidence in Swaziland: toward malaria elimination. Malar J. 2017;16:232.
Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis Poverty. 2019;8:51.
Tesfaye S, Belyhun Y, Teklu T, Mengesha T, Petros B. Malaria prevalence pattern observed in the highland fringe of Butajira, Southern Ethiopia: a longitudinal study from parasitological and entomological survey. Malar J. 2011;10:153.
Lowe R, Chirombo J, Tompkins AM. Relative importance of climatic, geographic and socio-economic determinants of malaria in Malawi. Malar J. 2013;12:416.
Abeku TA, van Oortmarssen GJ, Borsboom G, de Vlas SJ, Habbema JDF. Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop. 2003;87:331–40.
Segun OE, Shohaimi S, Nallapan M, Lamidi-Sarumoh AA, Salari N. Statistical modelling of the effects of weather factors on malaria occurrence in Abuja, Nigeria. Int J Environ Res Public Health. 2020;17:3474.
Endo N, Yamana T, Eltahir EAB. Impact of climate change on malaria in Africa: a combined modelling and observational study. Lancet. 2017;389 Special issue:S7.
Ethiopian Public Health Institute. Ethiopia National Malaria Indicator Survey 2015. Addis Ababa, 2016. https://www.ephi.gov.et/images/pictures/download2009/MIS-2015-Final-Report-December-_2016.pdf.
Federal Ministry of Health. National malaria guidelines. Addis Ababa: Federal Ministry of Health; 2012. p. 3–59.
President’s Malaria Initiative. Ethiopia—malaria operational plan FY 2018. https://reliefweb.int/report/ethiopia/president-s-malaria-initiative-ethiopia-malaria-operational-plan-fy-2018.
President’s Malaria Initiative. Ethiopia—Malaria Operational Plan FY 2017. https://reliefweb.int/report/ethiopia/president-s-malaria-initiative-ethiopia-malaria-operational-plan-fy-2017.
Vajda É, Webb C. Assessing the risk factors associated with malaria in the highlands of Ethiopia: what do we need to know? Trop Med Infect Dis. 2017;2:4.
Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, et al. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122.
Belew A. The prevalence of malaria in outpatients attending dangila health center, North Central Ethiopia. Masters thesis, Addis Ababa University. 2017. http://thesisbank.jhia.ac.ke/id/eprint/5229.
WHO. Research Malaria Microscopy Standards Working Group. Microscopy for the detection, identification and quantification of malaria parasites on stained thick and thin films. Geneva: World Health Organization; 2015. https://apps.who.int/iris/bitstream/10665/163782/1/9789241549219_eng.pdf.
FIND. Methods manual for laboratory quality control testing of malaria rapid diagnostic tests. Geneva: Foundation for Innovative Diagnostics, 2014. http://www.finddiagnostics.org/export/sites/default/programs/malaria-afs/docs/Methods_manual_for_laboratory_qc_testing_malaria_RDTs_vers7.pdf.
McHugh ML. Interrater reliability: the kappa statistic. Biochem Med. 2012;22:276–82.
Shiferaw M, Alemu M, Tedla K, Tadesse D, Bayissa S, Bugssa G. The prevalence of malaria in Tselemti Wereda, North Ethiopia: a retrospective study. Ethiop J Health Sci. 2018;28:539–46.
Girum T, Shumbej T, Shewangizaw M. Burden of malaria in Ethiopia, 2000–2016: findings from the Global Health Estimates 2016. Trop Dis Travel Med Vaccines. 2019;5:11.
Bedimo BH. Knowledge, attitude and practice on malaria and associated factors among residents in Pawe District, North West Ethiopia: a cross-sectional study. Sci J Public Health. 2015;3:303.
Basha Ayele EM. Prevalence of malaria and associated factors in Dilla town and the surrounding rural areas, Gedeo Zone, Southern Ethiopia. J Bacteriol Parasitol. 2015;6:5.
Ayalew S, Mamo H, Animut A, Erko B. Assessment of current malaria status in light of the ongoing control interventions, socio-demographic and environmental variables in Jiga Area, Northwest Ethiopia. PLoS ONE. 2016;11:e0146214.
Aschale Y, Mengist A, Bitew A, Kassie B, Talie A. Prevalence of malaria and associated risk factors among asymptomatic migrant laborers in West Armachiho District, Northwest Ethiopia. Res Rep Trop Med. 2018;9:95–101.
Tesfa H, Bayih AG, Zeleke AJ. A 17-year trend analysis of malaria at Adi Arkay, north Gondar zone, northwest Ethiopia. Malar J. 2018;17:155.
WHO. The global health observatory. Geneva: World Health Organization; 2018. https://www.who.int/data/gho/data/themes/malaria.
Ethiopian Public Health Institute. Ethiopia national malaria indicator Survey 2015. Addis Ababa, 2015. https://www.ephi.gov.et/images/pictures/download2009/MIS-2015-Final-Report-December-_2016.pdf.
WHO. World malaria report 2018. Geneva: World Health Organization. 2018. https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf.
WHO. World malaria report 2019. Geneva: World Health Organization; 2019.
Fekadu M, Yenit MK, Lakew AM. The prevalence of asymptomatic malaria parasitemia and associated factors among adults in Dembia district, northwest Ethiopia, 2017. Arch Public Health. 2018;76:74.
Ferede G, Worku A, Getaneh A, Ahmed A, Haile T, Abdu Y, et al. Prevalence of malaria from blood smears examination: a seven-year retrospective study from Metema Hospital, Northwest Ethiopia. Malar Res Treat. 2013;2013:704730.
Feleke DG, Gebretsadik D, Gebreweld A. Analysis of the trend of malaria prevalence in Ataye, North Shoa, Ethiopia between 2013 and 2017. Malar J. 2018;17:323.
Getnet G, Getie S, Srivastava M, Birhan W, Fola AA, Noedl H. Diagnostic performance of rapid diagnostic tests for the diagnosis of malaria at public health facilities in north-west Ethiopia. Trop Med Int Health. 2015;20:1564–8.
Delil RK, Dileba TK, Habtu YA, Gone TF, Leta TJ. Magnitude of malaria and factors among febrile cases in low transmission areas of Hadiya Zone, Ethiopia: a facility based cross sectional study. PLoS ONE. 2016;11:e0154277.
Lankir D, Solomon S, Gize A. A five-year trend analysis of malaria surveillance data in selected zones of Amhara region, Northwest Ethiopia. BMC Public Health. 2020;20:1175.
Scott CA, Yeshiwondim AK, Serda B, Guinovart C, Tesfay BH, Agmas A, et al. Mass testing and treatment for malaria in low transmission areas in Amhara Region, Ethiopia. Malar J. 2016;15:305.
Sumari D, Mwingira F, Selemani M, Mugasa J, Mugittu K, Gwakisa P. Malaria prevalence in asymptomatic and symptomatic children in Kiwangwa, Bagamoyo district, Tanzania. Malar J. 2017;16:222.
Zemene E, Koepfli C, Tiruneh A, Yeshiwondim AK, Seyoum D, Lee M-C, et al. Detection of foci of residual malaria transmission through reactive case detection in Ethiopia. Malar J. 2018;17:390.
Starzengruber P, Fuehrer H-P, Ley B, Thriemer K, Swoboda P, Habler V, et al. High prevalence of asymptomatic malaria in south-eastern Bangladesh. Malar J. 2014;13:16.
Idris ZM, Chan CW, Kongere J, Gitaka J, Logedi J, Omar A, et al. High and heterogeneous prevalence of asymptomatic and sub-microscopic malaria infections on Islands in Lake Victoria, Kenya. Sci Rep. 2016;6:36958.
Yalew WG, Pal S, Bansil P, Dabbs R, Tetteh K, Guinovart C, et al. Current and cumulative malaria infections in a setting embarking on elimination: Amhara, Ethiopia. Malar J. 2017;16:242.
Beyene BB, Yalew WG, Demilew E, Abie G, Tewabe T, Abera B. Performance evaluation of rapid diagnostic test for malaria in high malarious districts of Amhara region, Ethiopia. J Vector Borne Dis. 2016;53:63–9.
Mfuh KO, Achonduh-Atijegbe OA, Bekindaka ON, Esemu LF, Mbakop CD, Gandhi K, et al. A comparison of thick-film microscopy, rapid diagnostic test, and polymerase chain reaction for accurate diagnosis of Plasmodium falciparum malaria. Malar J. 2019;18:73.
Okangba CC. Parasite-based confirmation of malaria with rapid diagnostic tests: challenges and advantages. Sci J Pure Appl Sci. 2019;8:840–57.
Rodríguez Vásquez C, Barrera Escobar S, Tobón-Castaño A. Low frequency of asymptomatic and submicroscopic plasmodial infections in Urabá Region in Colombia. J Trop Med. 2018;2018:1–8.
Ayele DG, Zewotir TT, Mwambi HG. Prevalence and risk factors of malaria in Ethiopia. Malar J. 2012;11:195.
Haji Y, Fogarty AW, Deressa W. Prevalence and associated factors of malaria among febrile children in Ethiopia: a cross-sectional health facility-based study. Acta Trop. 2016;155:63–70.
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee M, Kazura J, et al. Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty. 2018;7:103.
Alemu A, Tsegaye W, Golassa L, Abebe G. Urban malaria and associated risk factors in Jimma town, south-west Ethiopia. Malar J. 2011;10:173.
Lemma W. Impact of high malaria incidence in seasonal migrant and permanent adult male laborers in mechanized agricultural farms in Metema–Humera lowlands on malaria elimination program in Ethiopia. BMC Public Health. 2020;20:320.
Acknowledgements
We would like to thank the Amhara regional health office for financial support of this research. We are grateful to all study participants, parents/guardians, and west Gojjam health office, and data collectors.
Funding
This study was fully granted by Amhara regional health office ($387.35). This fund was provided only for covering and supporting us at the expense of the thesis work. The funding body has no other role regarding the study design, data collection, analysis, interpretation, and manuscript write-up.
Author information
Authors and Affiliations
Contributions
All authors made a significant contribution to the conception, design, and execution, acquisition of data, analysis, and interpretation; took part in drafting the initial manuscript, revising it critically for final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Ethical and Review Committee of the School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences University of Gondar, Ethiopia. Permission and support letters were also obtained from the Zonal Health Bureau and District Health Office. Parents/guardians had provided written informed consent on behalf of any participants under the age of 16. Thumbprint was also taken from each parent/guardian who was unable to read and write after having been read the full informed consent form by a data collector. Besides, verbal assent was sought from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amare, A., Eshetu, T. & Lemma, W. Dry-season transmission and determinants of Plasmodium infections in Jawi district, northwest Ethiopia. Malar J 21, 45 (2022). https://doi.org/10.1186/s12936-022-04068-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-022-04068-y