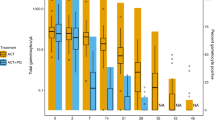
Abstract
Background
A goal of malaria epidemiological interventions is the detection and treatment of parasite reservoirs in endemic areas—an activity that is expected to reduce local transmission. Since the gametocyte is the only transmissible stage from human host to mosquito vector, this study evaluated the pre and post presence of gametocytes during a mass screening and treatment (MST) intervention conducted during 2013 in East Nusa Tenggara, Indonesia.
Methods
RT-qPCR targeting pfs25 and pvs25 transcripts—gametocyte molecular markers for Plasmodium falciparum and Plasmodium vivax, respectively, was performed to detect and quantify gametocytes in blood samples of P. falciparum and P. vivax-infected subjects over the course of the MST study. The presence of both asexual and sexual parasites in microscopic and submicroscopic infections was compared from the start and end of the MST, using proportion tests as well as parametric and non-parametric tests.
Results
Parasite prevalence remained unchanged for P. falciparum (6% = 52/811 versus 7% = 50/740, p = 0.838), and decreased slightly for P. vivax (24% = 192/811 versus 19% = 142/740, p = 0.035) between the MST baseline and endpoint. No significant difference was observed in gametocyte prevalence for either P. falciparum (2% = 19/803 versus 3% = 23/729, p = 0.353, OR = 1.34, 95%CI = 0.69–2.63), or P. vivax (7% = 49/744 versus 5% = 39/704, p = 0.442, OR = 0.83, 95%CI = 0.52–1.31). Even though there was an insignificant difference between the two time points, the majority of parasite positive subjects at the endpoint had been negative at baseline (P. falciparum: 66% = 29/44, P. vivax: 60% = 80/134). This was similarly demonstrated for the transmissible stage—where the majority of gametocyte positive subjects at the endpoint were negative at baseline (P. falciparum: 95% = 20/21, P. vivax: 94% = 30/32). These results were independent of treatment provided during MST activities. No difference was demonstrated in parasite and gametocyte density between both time points either in P. falciparum or P. vivax.
Conclusion
In this study area, similar prevalence rates of P. falciparum and P. vivax parasites and gametocytes before and after MST, although in different individuals, points to a negligible impact on the parasite reservoir. Treatment administration based on parasite positivity as implemented in the MST should be reevaluated for the elimination strategy in the community.
Trial registration Clinical trials registration NCT01878357. Registered 14 June 2013, https://www.clinicaltrials.gov/ct2/show/NCT01878357.
Similar content being viewed by others
Background
The sexual stage of malaria-causing parasites—gametocytes, originate from the asexual cycle in human red blood cells. However, among Plasmodium infecting reptiles and birds, gametocytes may also be produced from secondary exo-erythrocytic schizogony [1]. In the blood, only a small proportion of the asexual parasites differentiate into gametocytes [2]. In addition, gametocytes do not replicate in the blood, so their quantity depend on the number of progenitor asexual stages in the bloodstream [3]. There are characteristic differences in gametocyte development and longevity between the dominant malaria species [2]. In Plasmodium falciparum, the development of gametocytes typically takes 8–10 days where they sequester largely in the bone marrow until maturation [2]. Mature gametocytes re-enter the circulatory system where they remain for 3–4 weeks and are spontaneously cleared if not ingested by a mosquito vector [4]. Conversely, Plasmodium vivax gametocyte development takes 48 h to maturity with all stages of gametocytes present in the peripheral blood at the same time and are cleared within three days post maturation [2, 5]. It is not known if that clearance represents sequestration to another compartment, e.g. the microvasculature of skin accessible to anopheline mosquitoes.
Human-to-mosquito transmission requires the uptake of male and female gametocytes [2]. The infectivity of this stage to mosquitoes is positively associated with gametocyte density [6,7,8] with variations seen between species and study areas [9,10,11]. Non-linear relationships between gametocyte density and mosquito infection rates have been reported in P. falciparum [2], whereas in P. vivax, the relationship was demonstrated to be sigmoidal [8]. Furthermore, the gametocyte sex ratio, multiplicity of infection (MOI) [2], species, specific clones [12], and mixed infections [13] were also reported to impact transmission events. In addition, host factors also may affect gametocyte infectiousness [14], e.g. gametocytes from children were reported to be more infectious than those from adults, partly due to the higher gametocyte density in the younger age group [15]. Host immunity has also been documented to inhibit oocyst development in the mosquito [2, 16]. After about 10–12 days, oocysts develop into sporoblasts, which contains sporozoites [17]. Sporozoite will egress the sporoblast membrane and migrate to the salivary gland, ready to be transmitted [17].
The human host, when infected with Plasmodium, may become symptomatic or asymptomatic [18]. Symptomatic infections can usually be detected microscopically, while asymptomatic infections are frequently below the detection threshold of microscopic examination and require more sensitive molecular detection methodologies [18]. These submicroscopic infections are reportedly less infectious than those detectable by microscopy, however, they may contribute more to transmission due to a much higher frequency in the population [6, 7]. Symptomatic individuals usually have a higher treatment-seeking rates and, on diagnosis, are treated with both a gametocytocidal and a blood schizonticide agent, such as primaquine and artemisinin-based combination therapy (ACT). In addition, the administration of ACT has been demonstrated to have a temporary prophylactic effect against new infections of blood [19,20,21].
Surveillance in the context of elimination strategies seeks to identify and treat asymptomatic carriers of malaria [22]. A consequent reduction in the human parasite reservoir is hence expected to lower the transmission rate [22]. Mass drug administration (MDA) and mass screen and treat (MST) strategies have been developed and studied as intervention strategies [23,24,25,26]. Most MST studies have reported the prevalence and/or incidence of parasitaemia as the outcomes, however data on the infective gametocytes has not been described despite it having an important role in transmission [27,28,29]. This study analysed the gametocyte carrier status of population receiving MST intervention [26] by examining gametocyte specific transcripts, pfs25 and pvs25.
Methods
Study area
This study was conducted in Wewiku subdistrict, Belu district, East Nusa Tenggara province, in eastern Indonesia (June–September 2013). The study area was reported to have the highest malaria endemicity in the province. The annual parasite incidence (API) per 1000 population was 72 and 124 in 2011 and 2012, respectively (Belu district health office, personal communication), whereas in the entire East Nusa Tenggara province it was 25.8 and 21.1 during the same period [30]. Malaria incidence peaked during the months of August and September which coincided with the end of this study [26]. Anopheles barbirostris was reported as the major malaria vector [26]. Other less dominant vectors included Anopheles subpictus and Anopheles vagus [26]. A more detailed description on the study site has previously been reported [26].
Sample collection
The original cluster-randomized trial evaluated the impact of MST conducted (a) twice, and (b) three times, both within 3 months [26]. Samples were collected in June (round-1), July (round-2) and August (round-3) [26]. Positivity for malaria parasites by microscopy was 8% in both groups at baseline, and 9% each at the last round of MST [26]. MST exerted no impact on measured incidence of diagnosed infections in both groups (RR = 0.89, 95%CI 0.43–1.83) [26]. Therefore, in this study, round-1 of MST from both treatment groups were pooled and considered the ‘baseline’, while the last time-point (round-3) from both groups was also pooled and considered the ‘endpoint’.
Finger-pricked blood samples from each of the study subjects were collected for malaria diagnosis using microscopy and 250 µL were also collected in EDTA tubes. Blood slides were air-dried overnight prior to Giemsa staining. Upon staining, microscopic readings were performed on the same day. P. falciparum and P. vivax-positive blood samples were screened for glucose-6-phosphate dehydrogenase (G6PD) deficiency using a fluorescence spot test (Trinity Biotech, Ireland), and subjects with deficient or intermediate G6PD activities were not given primaquine. The time required from the sample collection until treatment administration were 2–3 days. All microscopy-positive subjects were treated with dihydroartemisinin–piperaquine (DHP) and primaquine. Treatment consisted of DHP (each tab contains 40 mg dihydroartemisinin and 320 mg piperaquine) for 3-day with daily doses as follows: ≥ 60 kg = 4, 41–59 kg = 3, 31–40 kg = 2, 18–30 kg = 1.5, 11–17 kg = 1, 6–10 kg = 0.5 and ≤ 5 kg = 0.25 tablet(s). Primaquine administration for P. falciparum were 0.75 mg/kgBW single dose: ≥ 60 kg = 3, 41–59 kg = 2, 31–40 kg = 2, 18–30 kg = 1.5, 11–17 kg = 0.75 tablet(s). For P. vivax, the daily dose for 14 days were 0.25 mg/kgBW: 1, 1, 0.75, 0.5, 0.25 tablet for the same weight group. A pill cutter was used to cut 15 mg of primaquine tablet to meet the required dose according to the body weight. A pill grinder was used to make powder with added saccharine for small children, e.g. < 6 year old, who were unable to swallow tablets. Quality control with cross-checking of microscopic reading was performed post-hoc with kappa value = 0.82 and 0.85 for P. falciparum and P. vivax, respectively. Discordant speciation between microscopy and PCR was not an issue as same drugs were given for both species.
For RNA extractions, 50 µL of blood was mixed with 250 µL of RNAProtect (Qiagen, Germany) within 4 h of collection and stored at − 80 °C until further processing. During five years of storage, temperature was checked daily.
Molecular analysis
Microscopic and PCR screening results have been reported in the parent study [26]. All P. falciparum and a set of randomly selected P. vivax positive samples underwent RNA extraction (Fig. 1) using the Quick RNA Mini-prep kit (Zymo Research, USA) according to manufacturer’s instruction. DNase treatment (included in the kit) was done during the process. P. falciparum and P. vivax gametocytes were quantified by two-step RT-qPCR of the gametocyte markers pfs25 and pvs25 [31]. Transcriptor First Strand cDNA kit (Roche) was used to generate cDNAs for each sample in triplicates. The presence of mRNA transcripts was verified using RT-qPCR targeting 18S rRNA [32], and only those positive for 18S were analysed for pfs25 and pvs25. The pfs25 and pvs25 qPCR was conducted using FastStart Essential DNA SYBR Green master (Roche) with previously published primers [29]. Details on the assays are provided in the Additional file 1. Melting temperature (TM) for P. falciparum was 74–75 °C, and 79–80 °C for P. vivax (Additional file 2). For quantification, series of plasmid harbouring target sequence with concentration of 105, 104, 103, 102, 10, 5, and 1 copy(ies) per reaction were run in triplicate and a standard curve was generated for each run. Negative (no template) controls were included in triplicates. Minus-reverse transcriptase (−RT) qPCR using pfs25 and pvs25 was performed on 23% (19/83) of randomly selected of P. falciparum and 17% (40/231) of P. vivax RNA samples to check for genomic DNA contamination. Sixteen of 19 P. falciparum demonstrated no contamination with DNA while three showed the presence of DNA—with 0.01–1.28% of pfs25 RT qPCR result. Thirty-six of 40 P. vivax demonstrated the absence of DNA, while four showed presence of 0.4%–2.5% DNA with pvs25 RT qPCR results. The limit of detection (LOD) was assessed by running a serial dilution of the plasmid in quintuples and determined as 10 copies/µL for both pfs25 and pvs25. Performance of the assay is described in Additional file 3. All triplicates of the cDNA were run and recorded as positive if a minimum of two of the three demonstrated a positive result. Quantities of the transcript were reported as the average transcript numbers of the replicates to the nearest CT values. Conversion of transcript numbers into gametocyte density was made by extrapolating transcript numbers to the standard curve generated from transcript numbers of the known gametocyte density in the microscopy-positive subjects. Regression coefficient (r) was 0.758 and 0.835 for P. falciparum and P. vivax, respectively. The trendline equation for P. falciparum was: 100.251 [−0.607–1.109] * (pfs25 transcript numbers/µL)0.621 [0.357–0.886], whereas for P. vivax was: 100.673 [0.198–1.149] * (pvs25 transcript numbers/µL)0.406 [0.264–0.548] (Additional file 4). Using this equation, one gametocyte corresponds roughly to 0.4 (0.1–50.2) pfs25 transcripts/µL for P. falciparum and 0.02 (0.01–0.18) pvs25 transcripts/µL for P. vivax. DNA from samples which were tested for gametocytes were quantified to assess the relationship between asexual and sexual stages. This was performed by species-specific 18S qPCR as described previously with some modification [33]. Details of the procedure and performance of the qPCR were shown in Additional files 1 and 3.
All laboratory analyses were performed blindly to subject identification (ID). Upon completion of all laboratory work, the results were linked to the subject ID, MST time point, and treatment status. All laboratory analyses were conducted at the Indonesian Medical Education and Research Institute (IMERI), Faculty of Medicine, University of Indonesia, Jakarta.
Data analysis
This study was unable to be powered to detect any change in gametocyte prevalence during MST as originally planned given the much lower prevalence than estimation in the protocol. Moreover, the design of the study was modified to pre-post instead of a between-group analysis. Categorical variables were analysed using chi square test. Numerical variables were analysed using Student’s t-test when the distribution was normal or Mann–Whitney when it was not normal. 18S rRNA gene copies and pfs25/pvs25 transcripts were log transformed for parametric statistical analyses. Linear regression was conducted to investigate the correlation between parasite density and pfs25/pvs25 numbers. A p-value < 0.05 was considered as statistically significant. All analyses were performed using SPSS version 23 (IBM, Armonk, New York).
Results
Characteristic of the study subjects
A total 866 subjects participated in this study: 811 samples were collected at baseline and 740 at the endpoint. These subjects represented 78% of the total parent study (reference) samples. Age and gender of study subjects were comparable between baseline and endpoint (33% were ≤ 15 year of age and 47% were male). Furthermore, 685 (84%) subjects were sampled at both time points.
pfs25/pvs25 RT qPCR assay
Of the 102 P. falciparum positive samples by microscopy and/or PCR from the two time points, RNA extractions were not conducted in three samples due to insufficient blood volume, and 13 samples due to human error. Of the remaining 86 RNA samples, 3 were negative for 18S transcripts, with 83 remaining for pfs25 analysis (Fig. 1). Of the 334 P. vivax positive samples by microscopy and/or PCR from the two time points, 246 were randomly selected for RNA extraction. Of these 246 RNA samples, 15 were negative for 18S transcripts—thus 231 samples were available for pvs25 analysis (Fig. 1).
In P. falciparum, gametocyte transcript numbers were not correlated with parasitaemia density by microscopy (r = 0.198, p = 0.202, Fig. 2a), whereas in P. vivax, positive correlation was demonstrated (r = 0.514, p < 0.001, Fig. 2b). However, gametocyte transcript numbers were positively correlated with 18S gene copy numbers for both species, with a stronger correlation observed for P. vivax than P. falciparum (P. falciparum: r = 0.372, p = 0.003; P. vivax: r = 0.770, p < 0.001, Figs. 3a, b).
Correlation between gametocyte transcript numbers and parasitaemia density by light microscopy (LM) in P. falciparum (a) and P. vivax (b). In P. falciparum, no correlation was found between gametocyte transcript numbers and parasitaemia density by microscopy (r = 0.198, p = 0.202), whereas in P. vivax, positive correlation was demonstrated (r = 0.514, p < 0.001)
Correlation between gametocyte transcript numbers and 18S copies by qPCR in P. falciparum (a) and P. vivax (b). A positive correlation was demonstrated between 18S gene copy numbers/µL and gametocyte transcript numbers/µL for a P. falciparum (pfs25) as well as for b P. vivax/pvs25. Linear regression analyses both demonstrated a significance correlation (p < 0.001). Microscopic infections (●) were showing higher transcripts than submicroscopic infections (○)
Prevalence and density of the parasite and gametocyte between baseline and endpoint
Plasmodium falciparum
Between baseline and endpoint, no significant difference in parasite prevalence was seen by microscopy, PCR, or either test (microscopy: 3% = 22/811 versus 5% = 34/740, p = 0.056, PCR: 6% = 49/811 versus 6% = 42/740, p = 0.829, either test: 6% = 52/811 versus 7% = 50/740, p = 0.838) (Table 1). No significant difference was also shown in parasite density by both microscopy and qPCR (median parasitaemia: 440 versus 860, p = 0.351; median 18S gene copies: 59.4 versus 713.5, p = 0.095) (Table 1). Furthermore, no significant difference was detected for gametocyte prevalence by microscopy, pfs25, or either test (microscopy: 1.4% = 11/811 versus 2% = 15/740, p = 0.328; pfs25: 2% = 19/803 versus 3% = 23/729, p = 0.353; either test: 2% = 19/803 versus 3% = 23/729, p = 0.353) (Table 1). Moreover, median gametocyte density by microscopy and pfs25 transcripts showed no significant difference either (microscopy: 80 versus 460, p = 0.085; pfs25: 132.5 versus 210.9, p = 0.625) (Table 1).
Prevalence of P. falciparum gametocytes (pfs25) was significantly higher in microscopic than in the submicroscopic group (63% = 12/19 versus 28% = 7/25, p = 0.032, OR = 4.41, 95% CI 1.04–19.22). Moreover, the microscopic group demonstrated higher transcripts than submicroscopic, albeit statistically insignificant (geometric mean: 182.8, 95%CI 29.1–1150.5 versus 39.6, 95%CI 3.3–472.1, t-test, p = 0.39) (Fig. 3a).
Plasmodium vivax
Although microscopic examination showed no significant difference in the parasite prevalence between baseline and endpoint (5% = 43/811 versus 5% = 35/740, p = 0.643), PCR and either test demonstrated a significantly lower prevalence at the endpoint (PCR: 23% = 183/811 versus 18% = 135/740, p = 0.038; either test: 24% = 192/811 versus 19% = 142/740, p = 0.035) (Table 2). However, parasite density by both microscopy and qPCR did not show any significant difference between the two time points (median parasitaemia by microscopy: 112 versus 80, p = 0.832; median 18S gene copies: 192.2 versus 177.7, p = 0.719) (Table 2). No significant difference was seen in gametocyte prevalence by microscopy, pvs25, or either test (microscopy: 1.1% = 9/811 versus 1.9% = 14/740, p = 0.215; pvs25: 7% = 49/744 versus 5% = 39/704, p = 0.442, either test: 7% = 49/744 versus 5% = 39/704, p = 0.442) (Table 2). Moreover, gametocyte density by microscopy and pvs25 transcripts did not demonstrate any significant difference (microscopy: 160 versus 64, p = 0.516, pvs25 transcripts: 56.4 versus 65.6, p = 0.810) (Table 2).
Gametocyte prevalence (pvs25) was significantly higher in microscopic than in submicroscopic group (92% = 23/25 versus 26% = 26/100, p < 0.001, OR = 32.73, 95% CI 7.07–296.20). Moreover, the microscopic group demonstrated higher transcripts than the submicroscopic group (geometric mean: 356.6, 95%CI 97.2–1309.8 versus 9.6, 95%CI 5.1–18.2, t-test, p < 0.001) (Fig. 3b).
Dynamics of parasite and gametocyte at baseline and endpoint
Every parasitemic and gametocytemic subject was identified individually at each timepoint, and their infection status was followed or traced backwards. The treatment status was also linked for each identified individual.
Plasmodium falciparum
Figure 4 demonstrates changes of parasite and gametocyte status of subjects between baseline and endpoint. Most subjects who were parasite or gametocyte positive at baseline were negative at the endpoint regardless of treatment. Conversely, most positive subjects detected during the endpoint were previously negative at baseline. Furthermore, some subjects showed different infecting species between the two time points, e.g. P. vivax followed by P. falciparum or vice versa. This demonstrates the dynamic nature of Plasmodium infection reservoirs in the area.
Dynamics of parasite and gametocyte in P. falciparum at baseline and endpoint. At baseline, P. falciparum prevalence was 6% (52/811) by microscopy and/or PCR. Half of these 52 subjects were positive by microscopy thus treated (54% = 28/52, ■), and 46% (24/52) were submicroscopic and untreated (□). At the endpoint, only two of each microscopic and submicroscopic group were positive. However, 46 subjects other than the mentioned groups were detected positive by microscopy (n = 32) or PCR (n = 14) ( ). Forty of these 46 subjects had data at baseline: 73% (n = 29) were parasite negative and 27% (n = 11) were infected with other species (P. vivax). Furthermore, similar pattern was observed in gametocyte. At baseline, gametocyte prevalence was 2% (19/803). Seventy-nine percent (15/19) had microscopic parasite and treated (
). Forty of these 46 subjects had data at baseline: 73% (n = 29) were parasite negative and 27% (n = 11) were infected with other species (P. vivax). Furthermore, similar pattern was observed in gametocyte. At baseline, gametocyte prevalence was 2% (19/803). Seventy-nine percent (15/19) had microscopic parasite and treated ( ), and 21% (4/19) had submicroscopic thus untreated (
), and 21% (4/19) had submicroscopic thus untreated ( ). At the endpoint, only one submicroscopic parasite remained positive. However, 22 subjects other than the mentioned groups were positive for gametocyte (
). At the endpoint, only one submicroscopic parasite remained positive. However, 22 subjects other than the mentioned groups were positive for gametocyte ( ). Twenty of these 22 subjects were negative at baseline while two had no data
). Twenty of these 22 subjects were negative at baseline while two had no data
Plasmodium vivax
Changes of parasite and gametocyte status between baseline and endpoint among the study subjects were also observed in P. vivax (Fig. 5). A large proportion of subjects who were parasite/gametocyte positive at baseline were negative during the endpoint regardless of treatment. Similarly, the majority of positive subjects identified at the endpoint were negative at baseline. Moreover, a change in infection species between the two time points was also demonstrated in some subjects, e.g. P. vivax followed by P. falciparum or vice versa.
Dynamics of parasite and gametocyte in P. vivax at baseline and endpoint. At baseline, P. vivax prevalence was 24% (192/811) by microscopy and/or PCR. A third of these 192 subjects were microscopic positive thus treated (28% = 54/192, ■) and 72% (138/192) were submicroscopic and untreated (□). At the endpoint, six of the microscopic and 43 submicroscopic subjects were positive. In addition, 93 positive subjects (23 by microscopy and 70 by PCR) other than those groups ( ) appeared. Eighty-five of these 93 subjects had data at baseline: 80 were parasite negative and 5 were infected with other species (P. falciparum). A similar pattern was also observed in gametocyte. At baseline, gametocyte prevalence was 7% (49/744). Fifty-three percent (26/49) had microscopic parasite and treated (
) appeared. Eighty-five of these 93 subjects had data at baseline: 80 were parasite negative and 5 were infected with other species (P. falciparum). A similar pattern was also observed in gametocyte. At baseline, gametocyte prevalence was 7% (49/744). Fifty-three percent (26/49) had microscopic parasite and treated ( ), while 47% (23/49) had submicroscopic and untreated (
), while 47% (23/49) had submicroscopic and untreated ( ). At the endpoint, only two (one microscopic and one submicroscopic) were positive. However, 37 subjects other than the mentioned groups were positive for gametocyte (
). At the endpoint, only two (one microscopic and one submicroscopic) were positive. However, 37 subjects other than the mentioned groups were positive for gametocyte ( ). Thirty of these 37 subjects were negative at baseline, while seven had no data
). Thirty of these 37 subjects were negative at baseline, while seven had no data
Discussion
This study investigated pre and post prevalence of parasite and gametocyte reservoirs of P. falciparum and P. vivax during 3-months of MST activities in East Nusa Tenggara, Indonesia. Dynamic temporal changes in species-specific infection among subjects occurred, but overall community-wide prevalence of parasites and gametocytes did not change. This indicates that the MST intervention with high compliance, though successful in treating diagnosed cases during its duration, was unsuccessful in reducing the parasite reservoir in the community to an extent that impacted overall transmission in this setting.
Complex infection dynamics surely influenced these findings. Studies have demonstrated that the numbers of circulating blood-stage parasites and gametocytes may fluctuate [6, 7] presenting the possibility that a proportion of infected subjects may have escaped a positive diagnosis with transient parasite levels below the detection limit at the point of blood collection (baseline/endpoint). Moreover, negativity in peripheral blood may conceal positivity in other compartments of the host (sequestration) that may later be released to peripheral blood [34] with vasculature of skin representing one possible such compartment [35, 36]. In the specific instance of P. vivax, there may be a considerable proportion of the population that are negative for parasitaemia but have dormant infections in their livers, i.e. latency [37]. New infections from the local carriers or introduced by travel from areas outside of the MST intervention area may also partly explain the findings. Natural clearing of parasites may have occurred among some of those who were positive at MST baseline (by PCR but not microscopy, i.e. not treated in the intervention) but negative at endpoint. The sum of these factors, of unknown proportional individual impacts, likely account for the apparently poor overall impacts on asexual and sexual parasitaemia prevalence rates resulting from the MST intervention.
In this context, a safe and efficacious MDA interventions of good coverage may be more effective than MST. Previous MDA studies have demonstrated that this approach may reduce incidence in low endemic malaria areas [23, 38]. Furthermore, the combination of MDA with vector control was shown to have a bigger impact in lowering incidence rates [23]. In addition, the prophylactic effect of the administered blood-schizonticide may prevent new infections for a certain period [19,20,21].
The primary limitation of this study is the limited sample size that reduces the power of the study. Furthermore, for a longitudinal study, the presence of more than two sampling time points would be preferable and better able to capture the temporal dynamics of the parasite reservoir in the study area.
This study demonstrates that there is a need to reconsider MST as a primary surveillance strategy, while alternative strategies such as MDA and complementary vector interventions should be considered and evaluated for efficacy in the drive towards malaria elimination.
Conclusion
This study demonstrates the dynamic persistence of P. falciparum and P. vivax infectious reservoirs over the course of the MST intervention in West Timor, Indonesia. Overall, ongoing and continuing transmission is unaffected by the intervention. MDA with or without vector control may be considered as an alternative epidemiological intervention towards a more efficacious transmission reduction strategy.
Availability of data and materials
The dataset generated by this study is available from the corresponding author upon request.
References
Venugopal K, Hentzschel F, Valkiūnas G, Marti M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat Rev Microbiol. 2020;18:177–89.
Meibalan E, Marti M. Biology of malaria transmission. Cold Spring Harb Perspect Med. 2017;7:a025452.
Koepfli C, Yan G. Plasmodium gametocytes in field studies: do we measure commitment to transmission or detectability? Trends Parasitol. 2018;34:378–87.
Gebru T, Lalremruata A, Kremsner PG, Mordmüller B, Held J. Life-span of in vitro differentiated Plasmodium falciparum gametocytes. Malar J. 2017;16:330.
Karl S, Laman M, Moore BR, Benjamin JM, Salib M, Lorry L, et al. Risk factors for Plasmodium falciparum and Plasmodium vivax gametocyte carriage in Papua New Guinean children with uncomplicated malaria. Acta Trop. 2016;160:1–8.
Slater HC, Ross A, Felger I, Hofmann NE, Robinson L, Cook J, et al. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat Commun. 2019;10:1433.
Tadesse FG, Slater HC, Chali W, Teelen K, Lanke K, Belachew M, et al. The relative contribution of symptomatic and asymptomatic Plasmodium vivax and Plasmodium falciparum infections to the infectious reservoir in a low-endemic setting in Ethiopia. Clin Infect Dis. 2018;66:1883–91.
Kiattibutr K, Roobsoong W, Sriwichai P, Saeseu T, Rachaphaew N, Suansomjit C, et al. Infectivity of symptomatic and asymptomatic Plasmodium vivax infections to a Southeast Asian vector Anopheles dirus. Int J Parasitol. 2017;47:163–70.
Lin JT, Ubalee R, Lon C, Balasubramanian S, Kuntawunginn W, Rahman R, et al. Microscopic Plasmodium falciparum gametocytemia and infectivity to mosquitoes in Cambodia. J Infect Dis. 2016;213:1491–4.
Martins-Campos KM, Kuehn A, Almeida A, Duarte APM, Sampaio VS, Rodriguez ÍC, et al. Infection of Anopheles aquasalis from symptomatic and asymptomatic Plasmodium vivax infections in Manaus, western Brazilian Amazon. Parasit Vectors. 2018;11:288.
Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther. 2013;11:623–39.
Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410.
Beri D, Balan B, Tatu U. Commit, hide and escape: the story of Plasmodium gametocytes. Parasitology. 2018;145:1772–82.
Stone W, Gonçalves BP, Bousema T, Drakeley C. Assessing the infectious reservoir of falciparum malaria: past and future. Trends Parasitol. 2015;31:287–96.
Bousema T, Drakeley C. Determinants of malaria transmission at the population level. Cold Spring Harb Perspect Med. 2017;7:a025510.
de Jong RM, Tebeje SK, Meerstein-Kessel L, Tadesse FG, Jore MM, Stone W, et al. Immunity against sexual stage Plasmodium falciparum and Plasmodium vivax parasites. Immunol Rev. 2020;293:190–215.
Matuschewski K. Getting infectious: formation and maturation of Plasmodium sporozoites in the Anopheles vector. Cell Microbiol. 2006;8:1547–56.
Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–40.
Lwin KM, Phyo AP, Tarning J, Hanpithakpong W, Ashley EA, Lee SJ, et al. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin–piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother. 2012;56:1571–7.
Sikora SA, Poespoprodjo JR, Kenangalem E, Lampah DA, Sugiarto P, Laksono IS, et al. Intravenous artesunate plus oral dihydroartemisinin-piperaquine or intravenous quinine plus oral quinine for optimum treatment of severe malaria: lesson learnt from a field hospital in Timika, Papua. Indonesia Malar J. 2019;18:448.
Kakuru A, Jagannathan P, Muhindo MK, Natureeba P, Awori P, Nakalembe M, et al. Dihydroartemisinin–piperaquine for the prevention of malaria in pregnancy. N Engl J Med. 2016;374:928–39.
Cheaveau J, Mogollon DC, Mohon MAN, Golassa L, Yewhalaw D, Pillai DR. Asymptomatic malaria in the clinical and public health context. Expert Rev Anti Infect Ther. 2019;17:997–1010.
Hsiang MS, Ntuku H, Roberts KW, Dufour MK, Whittemore B, Tambo M, et al. Effectiveness of reactive focal mass drug administration and reactive focal vector control to reduce malaria transmission in the low malaria-endemic setting of Namibia: a cluster-randomised controlled, open-label, two-by-two factorial design trial. Lancet. 2020;395:1361–73.
Samuels AM, Odero NA, Odongo W, Otieno K, Were V, Shi YP, et al. Impact of community-based mass testing and treatment on malaria infection prevalence in a high transmission area of western Kenya: a cluster randomized controlled trial. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa471 (online ahead of print).
Mulebeke R, Wanzira H, Bukenya F, Eganyu T, Collborn K, Elliot R, et al. Implementing population-based mass drug administration for malaria: experience from a high transmission setting in North Eastern Uganda. Malar J. 2019;18:271.
Sutanto I, Kosasih A, Elyazar IRF, Simanjuntak DR, Larasati TA, Dahlan MS, et al. Negligible impact of mass screening and treatment on mesoendemic malaria transmission at West Timor in eastern Indonesia: a cluster-randomized trial. Clin Infect Dis. 2018;67:1364–72.
Larsen DA, Bennett A, Silumbe K, Hamainza B, Yukich JO, Keating J, et al. Population-wide malaria testing and treatment with rapid diagnostic tests and artemether-lumefantrine in southern Zambia: a community randomized step-wedge control trial design. Am J Trop Med Hyg. 2015;92:913–21.
Cook J, Xu W, Msellem M, Vonk M, Bergström B, Gosling R, et al. Mass screening and treatment on the basis of results of a Plasmodium falciparum-specific rapid diagnostic test did not reduce malaria incidence in Zanzibar. J Infect Dis. 2015;211:1476–83.
Tiono AB, Guelbeogo MW, Sagnon NF, Nébié I, Sirima SB, Mukhopadhyay A, et al. Dynamics of malaria transmission and susceptibility to clinical malaria episodes following treatment of Plasmodium falciparum asymptomatic carriers: results of a cluster-randomized study of community-wide screening and treatment, and a parallel entomology study. BMC Infect Dis. 2013;13:535.
Purba IE, Hadi UK, Hakim L. Analisis Pengendalian Malaria Di Provinsi Nusa Tenggara Timur Dan Rencana Strategis Untuk Mencapai Eliminasi Malaria. 2017.
Wampfler R, Mwingira F, Javati S, Robinson L, Betuela I, Siba P, et al. Strategies for detection of Plasmodium species gametocytes. PLoS ONE. 2013;8:e76316.
Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB Jr, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–40.
Rosanas-Urgell A, Mueller D, Betuela I, Barnadas C, Iga J, Zimmerman PA, et al. Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malar J. 2010;9:361.
Koepfli C, Schoepflin S, Bretscher M, Lin E, Kiniboro B, Zimmerman PA, et al. How much remains undetected? Probability of molecular detection of human Plasmodia in the field. PLoS ONE. 2011;6:e19010.
Nixon CP. Plasmodium falciparum gametocyte transit through the cutaneous microvasculature: a new target for malaria transmission blocking vaccines? Hum Vaccin Immunother. 2016;12:3189–95.
Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–4.
Robinson LJ, Wampfler R, Betuela I, Karl S, White MT, Li Wai Suen CS, et al. Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med. 2015;12:e1001891.
Eisele TP, Bennett A, Silumbe K, Finn TP, Porter TR, Chalwe V, et al. Impact of four rounds of mass drug administration with dihydroartemisinin-piperaquine implemented in Southern Province Zambia. Am J Trop Med Hyg. 2020;103(Suppl 2):7–18.
Acknowledgements
We would like to thank the field team (Dedi R. Simanjuntak, Tri A. Larasati, Cathy Wabang and Emerensiana Meno) for their dedication and hard work. We also thank the IMERI head of infectious diseases and immunology research cluster Dr. Erni J. Nelwan, and members (Suwarti, Yunita Windi Anggraini and Mila Amalia) for their support and assistance. We also appreciate the outstanding work from laboratory staff at the Eijkman-Oxford Clinical Research Unit (Decy Subekti, Jeny, Lia Waslia, Damian A. Oyong, and Saraswati Soebianto). Our sincere gratitude to Ayu Nurdiantika for her assistance in the laboratory. Our deep gratitude to Dwi Ari Pujianto and Rintis Noviyanti for their valuable advice during the study and analysis. Our deep gratitude to the Dean of Faculty of Medicine, University of Indonesia, Prof. Dr. Ari Fahrial Syam, PhD, and director of IMERI, Prof. Dr. Badriul Hegar, PhD, for the opportunity to work at the laboratory facility in IMERI.
Funding
The work was supported by Bill & Melinda Gates Foundation through Malaria Transmission Consortium (45114), and the Ministry of Research Technology and Higher Education of Republic of Indonesia (120/SP2H/PTNBH/DRPM/2018).
Author information
Authors and Affiliations
Contributions
WAH, IM, NFL and IS designed the study. AK was involved in the collection of data, performed the laboratory work, analysed the data and drafted the manuscript. AK, CK, MSD, WAH, FHC, JKB, IM, NFL, IS contributed to the analysis and interpretation of the data. All the authors reviewed the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by Ethical committee of Medical Faculty, University of Indonesia no. 37/H2.F1/ETIK/2013. Informed consent was obtained from all study subjects prior to samples taking.
Consent for publication
Not applicable.
Competing interests
The author(s) declare(s) that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Primer sequences and reaction conditions for 18S qPCR. The amplification and quantification of Pf- or Pv-specific 18S rRNA gene copies were conducted using SYBR-Green-based qPCR.
Additional file 2.
Amplification and melting curve of gametocyte assay. The amplification and melting curve was generated by the 7500 Fast Real-Time PCR (Applied Biosystem) software.
Additional file 3.
Performance of 18S qPCR and pfs25/pvs25 RT-qPCR. The amplification and melting curve was generated by the 7500 Fast Real-Time PCR (Applied Biosystem) software. Mean CT, CT standard deviation, PCR efficiency, and r2 were calculated by running serial dilutions of plasmid in quintuples.
Additional file 4.
Correlation between pfs25/pvs25 transcript numbers and gametocyte density. A regression analysis was conducted to obtain the trendline for the relationship between transcript numbers and gametocyte density from samples with known gametocyte density.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kosasih, A., Koepfli, C., Dahlan, M.S. et al. Gametocyte carriage of Plasmodium falciparum (pfs25) and Plasmodium vivax (pvs25) during mass screening and treatment in West Timor, Indonesia: a longitudinal prospective study. Malar J 20, 177 (2021). https://doi.org/10.1186/s12936-021-03709-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-021-03709-y