Abstract
Background
While sub-microscopic malarial infections are frequent and potentially deleterious during pregnancy, routine molecular detection is still not feasible. This study aimed to assess the performance of a Histidine Rich Protein 2 (HRP2)-based ultrasensitive rapid diagnostic test (uRDT, Alere Malaria Ag Pf) for the detection of infections of low parasite density in pregnant women.
Methods
This was a retrospective study based on samples collected in Benin from 2014 to 2017. A total of 942 whole blood samples collected in 327 women in the 1st and 3rd trimesters and at delivery were tested by uRDT, conventional RDT (cRDT, SD BIOLINE Malaria Ag Pf), microscopy, quantitative polymerase chain-reaction (qPCR) and Luminex-based suspension array technology targeting P. falciparum HRP2. The performance of each RDT was evaluated using qPCR as reference standard. The association between infections detected by uRDT, but not by cRDT, with poor maternal and birth outcomes was assessed using multivariate regression models.
Results
The overall positivity rate detected by cRDT, uRDT, and qPCR was 11.6% (109/942), 16.2% (153/942) and 18.3% (172/942), respectively. Out of 172 qPCR-positive samples, 68 were uRDT-negative. uRDT had a significantly better sensitivity (60.5% [52.7–67.8]) than cRDT (44.2% [36.6–51.9]) and a marginally decreased specificity (93.6% [91.7–95.3] versus 95.7% [94.0–97.0]). The gain in sensitivity was particularly high (33%) and statistically significant in the 1st trimester. Only 28 (41%) out of the 68 samples which were qPCR-positive, but uRDT-negative had detectable but very low levels of HRP2 (191 ng/mL). Infections that were detected by uRDT but not by cRDT were associated with a 3.4-times (95%CI 1.29–9.19) increased risk of anaemia during pregnancy.
Conclusions
This study demonstrates the higher performance of uRDT, as compared to cRDTs, to detect low parasite density P. falciparum infections during pregnancy, particularly in the 1st trimester. uRDT allowed the detection of infections associated with maternal anaemia.
Similar content being viewed by others
Background
In sub-Saharan Africa (SSA), around 39 million pregnant women are exposed to malaria every year [1]. Malaria in pregnancy (MiP) due to Plasmodium falciparum is one of the leading causes of maternal anaemia, low birthweight and fetal growth restriction, which are high risk factors for neonatal and infant morbidity and mortality [1, 2].
Several studies have evidenced high proportions of sub-microscopic infections—that are not detectable by microscopy because of low parasite densities—among pregnant women [3]. In Benin, this proportion was as high as 25% at the first antenatal care visit in early first-trimester [4]. Besides, these infections have been associated with a reduction in birth weight, as well as an increase in low birth weight and maternal anaemia [3, 5, 6], especially those occurring early in pregnancy or in primigravidae [3]. Although intermittent preventive treatment (IPTp) with sulfadoxine-pyrimethamine (SP) could theoretically clear and reduce the prevalence of such infections during pregnancy [3], resistance of parasites to SP and the low IPTp coverage in most SSA countries [1] are factors that limit its effectiveness. Besides, the administration of IPTp-SP is only recommended from the second trimester onwards. The accurate identification and treatment of women with sub-microscopic infections in the first trimester of pregnancy may be of high clinical relevance considering the high prevalence and significant deleterious effects of these early infections [7,8,9].
Sub-microscopic infections can be detected by nucleic acid amplification techniques (such as Polymerase Chain Reaction (PCR) or Loop-mediated isothermal Amplification), however these require highly trained staff, relatively sophisticated laboratory infrastructure and cannot be used in a point-of-care (POC) manner for malaria. Malaria rapid diagnostic test (RDT) are useful POC tool to screen pregnant women for malaria. Recently, a Histidine Rich Protein 2 (HRP2)-based ultrasensitive rapid diagnostic test (uRDT) has been made available (Alere Malaria Ag Pf ultra-sensitive RDT), with an analytical sensitivity (i.e. a detection threshold) ten times higher than conventional RDTs [10]. This test showed good performances compared to PCR in pregnant women in low transmission malaria areas [11, 12].
This study aimed to assess the performance of this uRDT, compared to conventional RDT (cRDT) and qPCR, for the detection of P. falciparum malaria in peripheral and placenta blood from pregnant women in Benin, a high malaria-endemic area. Also, the association of uRDT-positive/cRDT-negative infections with poor maternal and birth outcomes was assessed.
Methods
Study site and population
This retrospective study was performed using blood samples collected during the RECIPAL study conducted in Southern Benin (2014–2017) [13]. Briefly, RECIPAL aimed to assess the prevalence and consequences of malaria in the first trimester of pregnancy on maternal and child health. It was based on a cohort of 411 pregnant women who were recruited before conception and then followed monthly from early pregnancy to delivery. In April 2018, 378 out of the 411 pregnant women (92%) consented to have the samples collected during the RECIPAL study used for the present study. Among the remaining women, 20 had migrated to Nigeria, 9 refused to participate, 2 were lost to follow-up and 2 were deceased.
Sample collection and handling
During the RECIPAL study, each month during pregnancy, EDTA preserved capillary blood samples were collected to detect malaria parasites using microscopy. At the same occasions, 50µL of whole blood was blotted on filter paper to make dried blood spot, which were dried, preserved at −20 °C and used for DNA extraction. The different types and volumes of blood that were used for microscopy, qPCR, RDTs and HRP2 level determination are presented in Additional file 1.
In addition, EDTA preserved venous blood samples were collected twice during pregnancy (at 1st and 3rd trimester, for haemoglobin determination), as well as twice at delivery (one sample from maternal peripheral blood and one sample from placental blood), from which 500µL of whole blood was stored in −20 °C freezers. Those frozen samples were used for RDT testing, consisting in one to four samples per woman depending on whether she completed the study follow-up until delivery.
Women with uncomplicated microscopic malaria were treated immediately with oral quinine in the 1st trimester and artemether-lumefantrine in the 2nd and 3rd trimesters. Those with severe malaria received intravenous artesunate until oral medication could be tolerated. Women received a long-lasting insecticide-treated net at their first ANC visit. Besides, the maternity staff was encouraged to administer at least three doses of IPTp as recommended by national guidelines [13].
RDT and uRDT testing
The cRDT SD BIOLINE Malaria Ag Pf (05FK50, batch 05CDD019AA, Abbott USA) and the HRP2 ultrasensitive RDT Alere Malaria Ag Pf (05FK140, batch 05LDD001AA, Abbott USA) were used according to the manufacturer’s instructions. Each RDT reaction was performed in duplicate using 5 µL of previously frozen EDTA-anticoagulated venous whole blood and read in a blind manner. For each RDT reaction, the result of each test line was quantitatively recorded by two independent readers on a scale from 0 (no line) to 4 (strong line) using a standardized scoring chart. A P. falciparum positive blood sample was used as positive control to have a positive example of scales before RDTs testing. Therefore, a total of 4 readings were performed with each product for a given specimen. A specimen was considered RDT positive if at least one reader identified one repeat as positive (i.e. test line intensity > 0) and negative if all readings were negative (i.e. test line intensity = 0).
Microscopy and qPCR testing
Microscopy and quantitative PCR (qPCR) testing was performed in the framework of RECIPAL study. Thick blood smears (TBS) were stained with Giemsa and parasitaemia was quantified by the Lambaréné method [14]. Blood smears were considered negative if no parasites were seen in all the 10µL TBS. The presence of P. falciparum was also tested in duplicate by a qPCR that targeted the 18S rDNA after 40 cycles of amplification [15, 16]. Starting from 50 µL of blood spotted on filter paper, the extraction procedure leads to a final volume of 150 µL extracted DNA. A test sampling of 5 µL extracted DNA thus corresponds to approximately 1.7 µL of blood. All parasite density estimates for this study were determined by qPCR. Parasitaemia was determined by extrapolation of cycle thresholds (Ct) from a standard curve generated with purified DNA from 3D7 P. falciparum infected erythrocyte culture. Samples without amplification (no cycle thresholds detected) were considered negative, and a density of 2 parasites/μL was assigned if amplification was observed out of the lower range of the standard curve (5 parasites/μL). Purified DNA 3D7 parasite strain was used as a positive control while negative control with no DNA template was run in all reactions. Plasmodium falciparum infections detected by qPCR, but not by microscopy, were classified as sub-microscopic. A quality check qPCR (using FTD Malaria, FastTrack Diagnostics) was performed in Hôpital Bichat (France) on same DNA extracts from a 10% randomly selected sub-sample. This reagent is for detection of Plasmodium spp. DNA. The limit of detection was estimated by the manufacturer of 0.1 target copy/µl of DNA extract. Considering that the PCR was carried out using 10 μL of DNA extract (equivalent to 3.4 μl of whole blood), the detection limit was equivalent to 0.3 parasites/μl of whole blood. If the sample displayed an exponential trace under cycle threshold 38, this sample was considered as positive.
Plasmodium falciparum HRP2 quantification
HRP2 was quantified using a highly sensitive laboratory based quantitative bead suspension array (qSA) based on Luminex technology [17]. After incubating 2’000 magnetic beads per analyte with blood sample at 1:5 dilution, beads were sequentially incubated with in-house biotinylated antibody α-HRP2 (MBS832975, MyBiSource, San Diego, CL) and streptavidin-PE (42250-1ML, Sigma Aldrich, St. Louis, MO). After a final wash, a minimum of 50 microspheres per analyte were acquired using the Luminex xMAP® 100/200 analyser (Luminex Corp., Austin, TX). After subtracting background (blank) values, median fluorescent intensities (MFI) were normalized to account for plate to plate variation and quantification was performed against a 5-parameter logistic (5-PL) regression curve consisting of recombinant protein HRP2 type A (890015, Microcoat GmbH, Germany).
Statistical analysis
For both RDTs, Kappa coefficients were estimated to assess agreement among the different readings. The positivity rate by diagnostic test (qPCR, uRDT, cRDT and microscopy) as well as the proportion of sub-microscopic infections were calculated. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR +) and negative likelihood ratio (LR-) of each RDT were assessed compared to qPCR considered as reference standard. Secondary analyses were performed to assess RDTs’ performance according to gravidity (primi- and secundigravidae vs. multigravidae), trimester of pregnancy (1st trimester, 3rd trimester, and delivery), type of blood sample (peripheral vs. placental blood at delivery), and symptoms (symptomatic vs. asymptomatic women). Symptomatic women were those who presented fever (axillary temperature ≥ 37.5 °C) or history of fever in the preceding 24 h, whether malaria infection was detected by qPCR, uRDT or cRDT. For each sub-group, sensitivity and specificity were compared between uRDT and cRDT using McNemar test for matched pairs. For all performance characteristics, exact binomial confidence intervals were computed. The geometric mean (95% Confidence Interval) of parasite density and HRP2 concentration were presented according to qPCR and RDT positivity.
The clinical impact of infections detected by the uRDT and not the cRDT was assessed. For that purpose, at each time-point of malaria screening using uRDT (i.e., at the 1st trimester, at the 3rd trimester and at delivery), women were categorized in four exclusive groups as detailed in Table 1. Groups 3 and 4 included both qPCR positive and qPCR negative specimens in order to assess the impact of uRDT and cRDT infections in a pragmatic way, regardless of qPCR result. The first analysis consisted in assessing the association between maternal haemoglogin (Hb) level and anaemia (defined as Hb level < 11 g/dL based on WHO recommendations for pregnant women) during pregnancy (in the 1st and 3rd trimester) with concomitant malarial infection according to women’s group. Mixed models were used in order to take into account the correlation between Hb concentrations measured in the same woman. The following variables were considered as potential confounding factors: maternal age (categorized according to the tertiles: < 23, 23–30, > 30 years old), gravidity (primi-secundigravidae vs. multigravidae), pre-pregnancy body mass index (BMI), anaemia before conception (defined as Hb level < 12 g/dL based on WHO recommendations for non-pregnant women), maternal education level (illiterate vs. literate), socioeconomic level, and ethnicity (Toffin vs. other). Pre-pregnancy BMI was classified into low (< 18.5 kg/m2), normal (18.5−24.9 kg/m2) and high (> 25 kg/m2) according to WHO classification. Socioeconomic level was approximated using a synthetic score combining occupation and ownership of assets, which was then categorized according to the tertiles. All variables with a P value below 0.2 in univariate analysis were included in the multivariate analysis. Then, the variables were eliminated step-by-step using the backward selection procedure, leaving only those variables with a P-value < 0.05. Gravidity and trimester of pregnancy were forced in the final multivariate models. The second analysis consisted in assessing the association between birthweight and low birthweight (LBW, defined as < 2500 grams in live-birth babies) with malaria at delivery according to women’s group. Women were classified according to the highest group to which they belonged in either peripheral or placental blood. Because of convergence issues, probably due to the small sample size, both models were only adjusted for gravidity. Stata version 13 for Windows (Stata Corp., College Station, TX) was used for all statistical analyses.
Results
Study participants characteristics
A total of 942 blood specimens were used for this study corresponding to 327 women who consented to participate and from whom sufficient archived whole blood samples were available. Primigravidae accounted for 7.7% of the study population (Table 2). Before pregnancy, 57.8% of women were anaemic (Hb level < 12 g/dL). During pregnancy, 66% of women received 2 or 3 doses of IPTp with SP. The proportion of women with at least one microscopic malaria infection during pregnancy was 40.0% (131/327), with 22.3% (71/319), 17.3% (52/300) and 14.3% (39/272) infected women in the 1st, 2nd and 3rd trimester of pregnancy, respectively. Thirty-eight percent and 58.5% of women were anaemic in the 1st and 3rd trimester of pregnancy, respectively (see Additional file 2). The prevalence of low birth weight was 8.0%. Women included in the present study had similar characteristics compared to the 411 women included in the RECIPAL study, except for malaria in pregnancy (defined as at least one microscopic infection), which was marginally higher in women included in the present study (40% vs. 34%, p = 0.10).
Malaria infection rate by diagnostic test
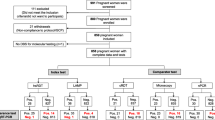
A total of 319, 246, 183 and 194 specimens were tested with uRDT, cRDT and qPCR in the 1st trimester, in the 3rd trimester, at delivery in peripheral blood and in placental blood, respectively (Fig. 1). Mean (standard deviation [SD]) gestational age in the 1st and 3rd trimester was 11.7 (3) and 33 (3.2) weeks gestation, respectively. For uRDT and cRDT, kappa coefficients ranged from 96% to 99%; both RDTs were then considered as positive if at least one out of four readings was positive. Agreement between the initial qPCR and the quality check PCR was 92.6%. Discrepant results were probably due to a difference in the detection limit between both PCRs [18].
The overall positivity rate detected by uRDT (16.2%) was slightly lower than with qPCR (18.3%), but higher than with cRDT (11.6%) (Table 3). Irrespective of the diagnostic test, infection rates were consistently the highest in the 1st trimester of pregnancy. The overall proportion of sub-microscopic infection was 16.7%, with the highest prevalence in the first trimester of pregnancy (32%). Most infections detected during pregnancy or in peripheral blood at delivery (88.2%, 172/195) were asymptomatic.
Performance of RDTs
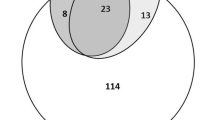
The uRDT identified all specimens—except two—with positive cRDT results, plus 46 additional specimens with negative cRDT results (30.0%, 46/153) (Fig. 2). Of these 46 specimens, 28 were also positive by qPCR; mean (95%CI) parasite density was 20.7 p/µL (10.8–39.6). Two specimens were cRDT positive and uRDT negative; both were negative by HRP2.
The uRDT sensitivity (60.5% (52.7−67.8)) was significantly higher than that of the cRDT (44.2% (36.6–51.9)) (Table 4). The difference in sensitivity between uRDT and cRDT was particularly high in the 1st trimester, in multigravidae and in asymptomatic women (Table 5). The uRDT specificity was marginally lower than the one of the cRDT (93.6% vs. 95.7%, respectively) (Table 4), with statistically significant differences for samples collected in the 1st trimester, in primi/secundigravidae, in multigravidae and in asymptomatic women (Table 5). The positive predictive value was slightly lower for uRDT as compared to cRDT (68.0% vs. 69.7%), but uRDT had a higher negative predictive value (91.4% vs. 88.5%). Finally, positive and negative likelihood ratios were similar between uRDT and cRDT (Table 4).
Parasite density and HRP2 concentration
Among the 941 specimens with HRP2 data as well as concomitant RDTs and qPCR results, the geometric mean (95%CI) HRP2 concentration in positive specimens (238/941) was 2139 (1486–3079) pg/mL. Forty specimens were qPCR positive but negative for HRP2 (Table 6); all of them tested negative by both RDTs and had low parasite densities (geometric mean (95%CI): 18.6 p/µL (7.3–47.5)). A total of 106 specimens were qPCR negative but positive for HRP2; the concentration of HRP2 was higher when both RDTs were positive. Among the HRP2 positive samples, the proportion of HRP2 positive/qPCR negative samples was higher in the 3rd trimester (44% (22/50)) and at delivery (58% (22/38) in maternal peripheral blood and 85% (35/41) in placental blood) than in the first trimester (24% (27/111)). Among the 5 women at delivery who were qPCR negative in peripheral blood, but positive by uRDT and had detectable levels of HRP2 (in peripheral blood), all of them were negative by qPCR in placental blood. Figure 3 presents the distribution of specimens, and their positivity regarding uRDT and cRDT, according to parasite density and HRP2 concentration. The proportion of infections that were detected by uRDT but not by cRDT increased with decreases in HRP2 levels. However, at very low concentrations of the antigen, both the uRDT and cRDT tended to be negative. The same trend was observed with decreasing parasite densities.
Distribution of specimens by parasite density (a) and HRP2 concentration (b), according to Das et al. [19]. a The outer clear bars represent the specimens that were positive by qPCR only; the gray bars are the number of specimens positive by uRDT and the black bars are the number of specimens positive by cRDT. b The outer clear bars represent the specimens that were positive by quantitative HRP2 assay, but not by RDTs; the gray bars are the number of specimens positive by uRDT and the black bars are the number of specimens positive by cRDT
Clinical impact of uRDT-detected malaria infections
Poor maternal and birth outcomes were more likely in women with uRDT-detected infections (see Additional file 2). In multivariate analysis, women with uRDT infections had a 3.4-times increased risk of anaemia during pregnancy than uninfected women (aOR (95%CI) = 3.44 (1.29–9.19), p = 0.01) (Table 7). This excess risk was higher than the one associated with cRDT infections (aOR (95%CI) = 2.03 (1.01–4.01), p = 0.05), but the difference was not statistically significant (p = 0.64). There was no association between qPCR positive and RDT negative infections and maternal anaemia. Similar results were found with maternal Hb level considered as a continuous variable (see Additional file 3).
Women with uRDT infection at delivery had children with a higher risk of LBW (adjusted OR (95%CI) = 5.27 (0.46–57.14), p = 0.17) compared with uninfected women, but this association did not reach statistical significance (Table 7). A similar result was found when birthweight was considered as a continuous variable (see Additional file 3).
Discussion
For the first time, the performance of a malaria RDT with a significantly improved analytical sensitivity as compared to commonly used RDTs [19,20,21] was evaluated in pregnant women living in a high malaria transmission area. Using qPCR as the reference standard, this uRDT had a significantly better sensitivity (60.5% (52.7−67.8)) than the studied cRDT (44.2% (36.6−51.9)). uRDT specificity was slightly lower (93.6% (91.7–95.3)) than for cRDT (95.7% (94.0–97.0)). The gain in sensitivity was particularly high and statistically significant in the 1st trimester of pregnancy, in multigravidae and in asymptomatic women (range, 29%–33%). In addition, our results indicate that infections which can be detected by uRDT but not by cRDT, were associated with maternal anaemia, suggesting that the use of more sensitive malaria RDT in the context of pregnancy might provide clinical benefits for the pregnant women, even in a context where IPTp-SP is made available.
Overall, 18.3% of specimens were qPCR positive, compared to 16.2% and 11.6% with uRDT and cRDT, respectively. The highest positivity rates were found for samples collected in the first trimester of pregnancy, before IPTp administration. In contrast, persistence of HRP2 (with qPCR negative) was observed in the 3rd trimester probably due to the clearance of parasites by IPTp. At delivery, positivity rate by qPCR was particularly low in placental blood compared to peripheral blood, possibly due to placental inhibitors or a high concentration of chelex in placental blood. Also, it was low compared with positivity rate by RDT. It is plausible that all infections detected in the peripheral blood were not necessarily of the placental type, and the RDTs did not make the difference by covering a wider parasite biomass.
The uRDT sensitivity ranged between 40.0% and 83.3% depending on the trimester of pregnancy, presence of symptoms and gravidity. The highest sensitivity rates were found for peripheral specimens collected at delivery (83.3%) and in symptomatic women (66.7%), but confidence intervals were large due to small sample sizes and low numbers of positive specimens in these two groups. The overall uRDT sensitivity in our study (60.5% (52.7–67.8)) was lower than the one reported by Vasquez et al. in Colombian pregnant women (85.7% (70.6–93.7)) [11]. The gain in performance between the cRDT and the uRDT is expected to vary from settings to settings, depending on the proportion of the infection that fall within the added detection window of the more sensitive uRDT [19, 22]. In this study, the relative gain in sensitivity was about 1.3 fold, similarly to previously reported data in the study by Vasquez et al. [11]. This improvement was particularly evident for samples collected in the first trimester of pregnancy. Such a high gain in sensitivity probably reflected the high proportion of sub-microscopic infections at this period.
Only 28 (41%) out of the 68 samples which were positive by qPCR but negative by uRDT had detectable levels of HRP2. However, the HRP2 concentrations were very low, explaining the negative uRDT result. Compared to samples which were negative both by cRDT and uRDT, higher HRP2 levels were detected in samples which were positive by uRDT but not by cRDT (5.5-fold increase), and positive by both RDTs (146-fold increase). HRP2 was detected in 48 of the 49 samples that were positive by uRDT but negative by qPCR. The discordance in the 40 samples which were HRP2 negative and qPCR positive could be due to the presence of gametocytes or pfhrp2 deletions, which could not be investigated [23]. The presence of HRP2 in qPCR negative samples could be explained either by infections below the qPCR detection limit or by the persistence of the antigen from a recently cleared infection [23]. Indeed, as suggested by the observation that all (n = 5) the women at delivery who were qPCR negative in peripheral blood but positive by uRDT and had detectable levels of HRP2, were also negative by qPCR in placental blood. In regards to qPCR detection limit, uRDT and cRDT sensitivity might have been lower by using an ultrasensitive PCR as gold standard, with probably a higher gain in sensitivity for uRDT compared to cRDT.
Women infected with malaria detected only by uRDT and not by cRDT, were at higher risk of anaemia compared to uninfected women, highlighting the clinical relevance of detecting low density malaria infections in pregnancy. The systematic treatment of microscopic infections during RECIPAL study may have reduced the overall effect of cRDT infections on Hb level. Also, the analysis suggested that uRDT infections at delivery were associated with a five times higher risk of LBW, but this association was not statistically significant. Because of the small numbers of LBW babies and women infected with malaria at delivery, the analysis was probably underpowered to detect any significant association. In any case, this result has to be taken with caution for the two following reasons. First, malaria at delivery was the main exposure, which may not reflect malaria history during pregnancy because of a high IPTp coverage and the systematic treatment of microscopic infections in RECIPAL study. Second, one cannot exclude that women with a positive uRDT infection at delivery had a cRDT positive infection during pregnancy.
Conclusion
In conclusion, these results are complementary to those previously reported in a low malaria transmission area. They confirm the higher overall performance of uRDT, compared to the studied conventional RDT, to detect P. falciparum infections in pregnant women. Indeed, the gain in sensitivity associated with uRDT exceeded its loss in specificity, with undetected infections possibly associated with poor pregnancy outcomes. The higher detection of infections using uRDT compared to cRDT was particularly evident in the first trimester of pregnancy, when sub-microscopic infections are the most prevalent. Since IPTp-SP is recommended from the 2nd trimester onwards, using uRDT for identifying those women who are infected with malaria in the first trimester of pregnancy may be especially relevant. Although uRDT will still miss almost 50% of the infected women at this period, women detected as infected with malaria using uRDT may be the ones at risk of poor outcomes. Overall, these encouraging results call for further prospective studies assessing uRDT performance and clinical relevance under field conditions.
Availability of data and materials
The dataset generated and analysed during the current study will be available in Open Science Framework repository only after acceptance of the manuscript for publication.
Change history
07 September 2020
An amendment to this paper has been published and can be accessed via the original article.
References
WHO. World malaria report 2019. Geneva, World Health Organization, 2019.
Rogerson SJ, Desai M, Mayor A, Sicuri E, Taylor SM, van Eijk AM. Burden, pathology, and costs of malaria in pregnancy: new developments for an old problem. Lancet Infect Dis. 2018;18:e107–18.
Cottrell G, Moussiliou A, Luty AJF, Cot M, Fievet N, Massougbodji A, et al. Submicroscopic Plasmodium falciparum infections are associated with maternal anemia, premature births, and low birth weight. Clin Infect Dis. 2015;60:1481–8.
Hounkonnou CP, Briand V, Fievet N, Accrombessi M, Yovo E, Mama A, et al. Dynamics of submicroscopic Plasmodium falciparum infections throughout pregnancy: a preconception cohort study in Benin. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciz748.
Adegnika AA, Verweij JJ, Agnandji ST, Chai SK, Breitling LP, Ramharter M, et al. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg. 2006;75:798–803.
Malhotra I, Dent A, Mungai P, Muchiri E, King CL. Real-time quantitative PCR for determining the burden of Plasmodium falciparum parasites during pregnancy and infancy. J Clin Microbiol. 2005;43:3630–5.
Accrombessi M, Yovo E, Fievet N, Cottrell G, Agbota G, Gartner A, et al. Effects of malaria in the first trimester of pregnancy on poor maternal and birth outcomes in Benin. Clin Infect Dis. 2019;69:1385–93.
Walker PGT, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health. 2014;2:e460–7.
Accrombessi M, Fievet N, Yovo E, Cottrell G, Agbota G, Massougbodji A, et al. Prevalence and associated risk factors of malaria in the first trimester of pregnancy: a preconceptional cohort study in Benin. J Infect Dis. 2018;217:1309–17.
Das S, Peck RB, Barney R, Jang IK, Kahn M, Zhu M, et al. Performance of an ultra-sensitive Plasmodium falciparum HRP2-based rapid diagnostic test with recombinant HRP2, culture parasites, and archived whole blood samples. Malar J. 2018;17:118.
Vásquez AM, Medina AC, Tobón-Castaño A, Posada M, Vélez GJ, Campillo A, et al. Performance of a highly sensitive rapid diagnostic test (HS-RDT) for detecting malaria in peripheral and placental blood samples from pregnant women in Colombia. PLoS ONE. 2018;13:e0201769.
Vásquez AM, Zuluaga L, Tobón A, Posada M, Vélez G, González IJ, et al. Diagnostic accuracy of loop-mediated isothermal amplification (LAMP) for screening malaria in peripheral and placental blood samples from pregnant women in Colombia. Malar J. 2018;17:262.
Accrombessi M, Yovo E, Cottrell G, Agbota G, Gartner A, Martin-Prevel Y, et al. Cohort profile: effect of malaria in early pregnancy on fetal growth in Benin (RECIPAL preconceptional cohort). BMJ Open. 2018;8:e019014.
Swysen C, Vekemans J, Bruls M, Oyakhirome S, Drakeley C, Kremsner P, et al. Development of standardized laboratory methods and quality processes for a phase III study of the RTS, S/AS01 candidate malaria vaccine. Malar J. 2011;10:223.
Tran TM, Aghili A, Li S, Ongoiba A, Kayentao K, Doumbo S, et al. A nested real-time PCR assay for the quantification of Plasmodium falciparum DNA extracted from dried blood spots. Malar J. 2014;13:393.
Diallo A, Ndam NT, Moussiliou A, Dos Santos S, Ndonky A, Borderon M, et al. Asymptomatic carriage of Plasmodium in urban Dakar: the risk of malaria should not be underestimated. PLoS ONE. 2012;7:e31100.
Martiáñez-Vendrell X, Jiménez A, Vásquez A, Campillo A, Incardona S, González R, et al. Quantification of malaria antigens PfHRP2 and pLDH by quantitative suspension array technology in whole blood, dried blood spot and plasma. Malar J. 2020;19:12.
Frickmann H, Wegner C, Ruben S, Behrens C, Kollenda H, Hinz R, et al. Evaluation of the multiplex real-time PCR assays RealStar malaria S&T PCR kit for the differentiation of Plasmodium species in clinical samples. Travel Med Infect Dis. 2019;31:101442.
Das S, Jang IK, Barney B, Peck R, Rek JC, Arinaitwe E, et al. performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg. 2017;97:1540–50.
Landier J, Haohankhunnatham W, Das S, Konghahong K, Christensen P, Raksuansak J, et al. Operational performance of a Plasmodium falciparum ultrasensitive rapid diagnostic test for detection of asymptomatic infections in Eastern Myanmar. J Clin Microbiol. 2018;56:e00565-18.
Hofmann NE, Gruenberg M, Nate E, Ura A, Rodriguez-Rodriguez D, Salib M, et al. Assessment of ultra-sensitive malaria diagnosis versus standard molecular diagnostics for malaria elimination: an in-depth molecular community cross-sectional study. Lancet Infect Dis. 2018;18:1108–16.
Hofmann NE, Antunes Moniz C, Holzschuh A, Keitel K, Boillat-Blanco N, Kagoro F, et al. Diagnostic performance of conventional and ultrasensitive rapid diagnostic tests for malaria in febrile outpatients in Tanzania. J Infect Dis. 2019;219:1490–8.
Poti KE, Sullivan DJ, Dondorp AM, Woodrow CJ. HRP2: transforming malaria diagnosis, but with caveats. Trends Parasitol. 2020;36:112–26.
Acknowledgements
We are extremely grateful to all women who took part in this study, the midwifes, nurses and community-health workers for recruiting and following them, and the whole RECIPAL team, including engineers and technicians, who were key in obtaining high-quality data and samples.
Funding
This work was supported by the Bill and Melinda Gates Foundation [OPP1169555]. The RECIPAL project was supported by the French Agence Nationale de la Recherche [ANR-13-JSV1-0004] and the Fondation Simone Beer under the auspices of the Fondation de France [00074147]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
VB, NF, AM and XD conceived and designed the study; MA, AM, NF and VB supervised the field work; BK, GC and VB analyzed the data; BV, EG, DS, NF and XD performed/supervised the realisation of the RDTs; XM and AM performed the HRP2 tests and analysis; NT, AM, SH and NF supervised the realisation of the qPCRs; VB, AM, XM, NF, GC and XD drafted and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Ethics committee of the Institut des Sciences Biomédicales Appliquées in Benin (n°39 04/01/2018 & n°108 19/02/2018) and by the Ministry of Health in Benin (n°0300/MS/DC/SGM/DRFMT/SA). Women were included in the present study after providing a signed written informed consent (or orally and recorded consent, for two of them).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1
. Type and volume of samples that were used for uRDT and cRDT testing, HRP2-assay and qPCR. RECIPAL, 2014–2017.
Additional file 2
. Maternal and birth outcomes, according to type of concomitant malaria infection. RECIPAL, 2014–2017.
Additional file 3
. Association between malaria and maternal and child outcomes (maternal Hb level during pregnancy and birthweight). RECIPAL, 2014–2017.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Briand, V., Cottrell, G., Tuike Ndam, N. et al. Prevalence and clinical impact of malaria infections detected with a highly sensitive HRP2 rapid diagnostic test in Beninese pregnant women. Malar J 19, 188 (2020). https://doi.org/10.1186/s12936-020-03261-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-020-03261-1