Abstract
Background
Despite the success of indoor residual insecticide spraying (IRS) in Africa, particularly in Benin, some gaps of information need to be filled to optimize the effectiveness of this intervention in the perspective of the country’s effort to eliminate malaria. In anticipation to the 2018 IRS campaign in two targeted regions of northern Benin, this study aimed, to collect baseline information on vector composition, spatio-temporal variation and peak malaria transmission in the Alibori and Donga, two targeted regions of northern Benin. Information collected will help to better plan the implementation and later on the impact assessment of this IRS campaign.
Methods
The study was carried out in four districts of the two IRS targeted regions of northern Benin. Human landing catches and pyrethrum spray catches protocols were used to assess the biting rate (HBR) and, biting/resting behaviour of malaria vector populations. After morphological identification of collected Anopheles, the heads and thoraxes of Anopheles gambiae sensu lato (s.l.) were analysed by the ELISA CSP tests to estimate the sporozoite index (SI). The entomological inoculation rate was calculated as the product of mosquito biting rate (HBR) and the SI.
Results
The biting rates of An. gambiae s.l., the major vector in this study sites, varied significantly from region to region. It was higher: in rural than in urban areas, in rainy season than in dry season, indoors than outdoors. Overall, SI was comparable between sites. The highest EIRs were observed in the Donga region (16.84 infectious bites/man/month in Djougou district and 17.64 infectious bites/man/month in Copargo district) and the lowest in the Alibori region (10.74 infectious bites/man/month at Kandi district and 11.04 infectious bites/man/month at Gogounou district).
Conclusion
This study showed the heterogeneous and various nature of malaria epidemiology in Northern Benin. Indeed, the epidemiological profile of malaria transmission in the Alibori and Donga regions is made of a single season of transmission interrupted by a dry season. This period of transmission is relatively longer in Donga region than in Alibori. This information can be used to guide the extension of IRS in the Alibori and in the Donga, by primarily targeting areas with short periods of transmission, and easy to cover.
Similar content being viewed by others
Background
Indoor residual spraying (IRS) and insecticide-treated nets (ITNs) are two key and effective strategies designed to interrupt malaria transmission [1,2,3]. IRS has greatly contributed to reduce or eliminate malaria from many areas of the world, particularly in situations where mosquito vectors feed and rest indoors and where the transmission of malaria is seasonal [4,5,6,7]. In Benin, after 6 years of intervention, IRS has proved to be an effective vector control intervention [8]. Started in 2008 in the Oueme region (southern Benin), then relocated to the Atacora region (North Benin) from 2011 to 2015, the intervention was effective in reducing the level of malaria transmission [8,9,10]. The same trend has been observed in other sub-Saharan countries with this intervention: Swaziland, Botswana, South Africa, Zimbabwe and Mozambique [11], Madagascar [12], Equatorial Guinea (Bioko Island) [13,14,15], in Uganda [16], Kenya [17] and Tanzania [18]. Unfortunately, IRS effectiveness is being jeopardized by the spread and intensification of insecticide resistance, including to pyrethroids [19,20,21,22,23,24] and more recently to bendiocarb [25,26,27]. Density and distribution of Anopheles, vectors of malaria vary according to the region and the time of year, and these variations can modify malaria transmission levels [28,29,30,31]. Several studies have shown that malaria infection is influenced by environmental factors, such as temperature, precipitation, and relative humidity that vary from region to region [32]. However, in most parts of Africa, there are still gaps in information regarding the dynamics of malaria transmission resulting in the implementation of vector control interventions without sufficient decision-making basis [33,34,35].
This was the case of Benin where, from 2008 to 2009, a single round of IRS instead of two was implemented in the Oueme region to cover the period of malaria transmission [9]. In 2017, the IRS campaign, with pirimiphos methyl (Actellic 300CS), has targeted all eligible households in the Alibori and Donga regions. These two regions being located in two different eco-geographical areas despite their proximity, it was hypothesized that variations in vectors ecology may affect the micro-epidemiology of malaria. It is in this context that this study was initiated with the aim of obtaining useful information for a better planning and assessment of IRS intervention.
Methods
Study site
Entomological data were collected from two regions with two districts each: Alibori (Gogounou, Kandi) and Donga (Copargo, Djougou) (Fig. 1). The region of Alibori is characterized by a Sudanese climate and the Donga by a Sudano-guinean climate, with a single dry season (December to May) and a single rainy season (June to November). The annual average rainfall varies between 700–1200 mm and 1200–1300 mm, respectively in Alibori and Donga regions. The average monthly temperature varies between 23 and 40 °C. The region of Donga has more rivers than the region of Alibori. The major economic activity is farming of cotton, maize and millet [36, 37].
Malaria prevalence is generally higher in Donga region than in Alibori [38]. Long-lasting insecticide-treated mosquito nets (LLINs) distributed every 3 years throughout the mass campaign distribution are the main tools used to prevent human–vector contact in the four districts. In each district, two sites, one in urban and one in rural setting, were selected for mosquito collections. These sites are:
-
In Djougou district:
Zountori: a urban area of Djougou (09°42′10.1″ N latitude and 01°40′55.4″ E longitude);
Barienou: a rural village of Djougou (09°42′58.4″ N latitude and 01°46′06.5″ E longitude) (Fig. 1).
-
In Copargo district:
Parakounan: a urban area of Copargo (09°50′19.3″ N latitude and 01°32′39.5″ E longitude);
Kataban: a rural village of Copargo (09°54′34.3″ N latitude and 01°31′26.9″ E longitude);
-
In Kandi district:
Kossarou: a urban area of Kandi (11°07′29.32″ N latitude and 2°56′9.57″ E longitude);
Sonsoro: a rural village of Kandi (11°4′58.91″ North latitude and 2°13′37.60″ East longitude);
-
In Gogounou district:
Bantansoue: a urban area of Gogounou (10°50′30.6″ N latitude and 2°50′20.3″ E longitude);
Gounarou: a rural village of Gogounou (10°52′20.8″ N latitude and 2°50′51.2″ E longitude) (Fig. 1).
Sampling of Anopheles vectors of malaria was conducted from May 2016 to February 2017.
Ethical considerations
The protocol of this study has been reviewed and approved by the Institutional Ethics Committee of the Center for the Research in Entomology of Cotonou (IECC). Verbal consent was obtained from local mosquito collectors before being involved in the study. They subsequently received a vaccine against yellow fever as a prophylactic measure. An agreement with health facilities close to sites was also obtained for the free anti-malarial treatment of mosquito collectors who would suffered from malaria.
Anopheles adult collection
Malaria transmission dynamics were assessed in the 08 identified and described sites in the study area. This is why two classical methods were used for the sampling of Anopheles mosquitoes. The first sampling method is termed as Human Landing Catch (HLC), and was carried out from 9:00 p.m. to 5:00 a.m. in 2 nights per month per district, enabled the evaluation of the frequency of human–vector contact (human biting rate). For this purpose, four volunteers (02 inside and 02 outside) captured mosquitoes in 02 randomly selected houses per site, making a total of eight collectors per district per night. The collectors are rotated in the different houses to avoid biases related to their ability or their individual attractiveness. The second sampling method is pyrethrum spray catch (PSC) which was carried out through two morning sessions (between 6 a.m. and 8:30 a.m.) per month in 40 houses per district. This method allowed the collection of resting mosquitoes inside houses.
Mosquito processing
These mosquitoes as well as those caught on human bait were examined for Plasmodium falciparum infectivity. After each collection, collected mosquitoes were counted and morphologically identified using the taxonomic key of Gillies and De Meillon [39]. Females of An. gambiae s.l. (refered to as An. gambiae thereafter in the text) caught on human bait were then dissected to assess their physiological age [40]. Each specimen was finally stored in a labelled Eppendorf tube containing silicagel for further laboratory analysis.
The heads and thorax of females of An. gambiae collected at each site were analysed by enzyme-linked immunosorbent assay (ELISA) according to the method described by Wirtz et al. [41]. This allows the detection of P. falciparum infection and the calculation of infectivity rates.
Estimation of entomological parameters
The human biting rate (HBR) for identified vector species was calculated as the number of An. gambiae caught per person per night of sampling effort. The sporozoite index (SI) is the proportion of An. gambiae s.l. with circumsporozoite protein of P. falciparum: SI = (Thorax +/Thorax analysed) × 100. The parity rate is a percentage of An. gambiae that have laid eggs at least once (parous) out of the total number of An. gambiae dissected for the examination of the physiological status of their ovaries. It indicates the proportion of older mosquitoes within the population during the survey. The entomological inoculation rate (EIR), a key variable expressing the malaria transmission level and which is defined as the number of infective bites received/man/night. It is the product of An. gambiae biting rate and SI data acquired from HLCs and the ELISA tests, respectively.
Data analysis
Data were analysed with the R statistics software, version 2.8. The Poisson method was used to estimate the confidence intervals [42] of HBRs and EIRs of An. gambiae. The binomial method for calculation of confidence intervals [42] was used to estimate the confidence intervals of parity and infectivity rates of An. gambiae. The unconditional maximum likelihood estimation method Wald, or median unbiased estimation (mid-p) of the risk ratio (RR) followed by their confidence intervals obtained using the normal approximation or the exact method p-values obtained by Khi2 or mid-p.exact [43] was used to compare EIR. Proportion comparison tests were used to compare SI and parity rates. A difference is considered as significant when the p-value is less than 0.05.
Results
Vector species composition
A total of 3876 specimens of Anopheles mosquitoes from six different species were collected in the regions of Alibori and Donga. The most abundant species was An. gambiae, which accounted for 97.72% (3788/3876) of the collected vectors, followed by Anopheles funestus (1.88%, 73/3876). The biting behaviour of these two vector species was generally higher in the Donga compared to the Alibori. It should be noted that An. funestus was mainly collected in the district of Djougou which provided about 82.2% (60/73) of the specimens (Table 1). Among Anopheles species which were collected, there were only 9 Anopheles coustani (0.23%), 3 Anopheles pharoensis (0.077%), 2 Anopheles ziemanni (0.05%) and 1 Anopheles paludis (0.025%) (Table 1).
Variability of human biting rates
The biting rate of An. gambiae varied significantly from region to region: 6.55–7.51 bites/man/night (b/m/n) in the Donga against 4.4–4.78 bites/man/night in the Alibori (p < 0.05) (Table 2). The HBR was four times higher in rural areas compared to urban areas (RR = 4.27; p < 0.0001). The data also show that the average biting rate of An. gambiae was higher indoors (6.37 bites/man/night) than outdoors (5.25 bites/man/night). In the dry season, it was 2.27 bites/man/night compared to 9.95 bites/man/night in the rainy season, an increase of more than four times (RR = 4.38; p < 0.0001) (Table 2). In both regions, the highest biting rates were observed between June and October with a peak in October and August, respectively in Alibori and Donga (Table 2).
Sporozoite index of Anopheles gambiae
Overall, out of a total of 3788 head-thoraxes of An. gambiae assessed by ELISA, approximately 305 were found to be positive for the circumsporozoitic antigen of P. falciparum, which equates to a mean SI of 8.05% [7.20–8.96]. The SI were similar between urban (6.35% [4.63–8.46]) and rural (8.42% [7.6–9.45]) areas (p = 0.086). The highest infection rates were observed in Gounarou (11.65% [8.48–15.46]) and Kossarou (11.36% [5.58–19.90]) and the lowest in Bantansoue (4.44% [2.38–7.46]) (Table 3).
Overall, mosquito infectivity was 7.48% [5.81–9.43], 8.37% [6.35–10.78], 8.56% [6.96–10.38] and 7.82% [6.36–9.50], respectively in the districts of Kandi, Gogounou, Djougou and Copargo (Table 3). By cumulating data by region, SI was also similar (7.86% [6.55–9.33] in the Alibori versus 8.18% [7.08–9.38] in the Donga, p = 0.769) (Table 3).
Cumulative data from both regions show similar average of infectivity rates in the rainy season (8.07% [7.14–9.07]) and in the dry season (7.95% [5.95–10.34]) (p = 0.981). In Kandi, Gogounou and Djougou, mosquito infectivity was respectively 8.89% [3.91–16.76], 4.38% [1.43–9.93] and 11.26% [7.92–15.37] in the dry season versus 7.32% [5.58–9.37], 9.23% [6.90–12.01] and 7.53% [5.77–9.60] in the rainy season (p > 0.05). Conversely, in Copargo, the SI was significantly higher in the rainy season (8.45% [6.84–10.28]) than in the dry season (2.44% [0.63–7.50]) (p = 0.0297) (Table 3).
Entomological inoculation rate of Anopheles gambiae
Tables 4 and 5 show the spatio-temporal variation in entomological inoculation rates (EIR). A variation of the EIR across districts was significantly higher in the rainy season (June, July, August and October) than in the dry season (May, January and February) (p < 0.05) (Table 4).
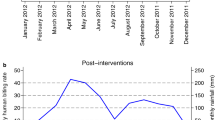
Overall, the lowest infectivity was observed in Kandi (10.74 infective bites/man/month) and Gogounou (11.04 infective bites/man/month) in Alibori region than in Djougou (16.84 infective bites/man/month) and Copargo (17.64 infectious bites/man/month) in Donga region (Table 4). In the four districts, the infectivity was higher in rural areas than in urban areas (p < 0.05). Cumulative data revealed an average EIR of 23.79 infectious bites/man/month in rural areas versus 4.15 infectious bites/man/month in urban areas (p < 0.0001) (Table 5). In Alibori’s districts (Kandi and Gogounou), the period of malaria transmission was relatively shorter than in Donga’s districts where it extended from May to February at Djougou and, from June to January at Copargo (Fig. 2). In Alibori region, the peak of transmission was recorded in October at Kandi (42.06 infectious bites/man/month) and Gogounou (42.86 infectious bites/man/month). Conversely, in Donga region, it was observed in August at Djougou (45.6 infectious bites/man/month) and Copargo (57.6 infectious bites/man/month) (Fig. 2 and Table 5).
Seasonal variation of the parity of Anopheles gambiae
Ovaries dissection of 1640 females of An. gambiae collected through HLCs were performed to determine parity rates. 1185 (72.3%) were found parous. Vectors’ parity rate was 81.90% [73.19–88.73], 91.38% [81.01–97.14] and 89.13% [76.43–96.37] during the dry season against 71.14% [66.39–75.56], 70.59% [65.90–74.96] and 68.58% [62.57–74.16] during the rainy season (p < 0.05), respectively in Djougou, Copargo and Gogounou. In Kandi, no significant difference was observed between parous rates of both seasons, probably due to the low number of mosquitoes dissected in the dry season (Table 6). Overall, cumulative data showed an average parous rate estimated at 86.70% [81.45–90.90] in the dry season against 70.04% [67.58–72.41] in the rainy season (p < 0.001).
Discussion
The study of the dynamics of malaria transmission is a prerequisite to not only understand the epidemiology of this disease, but also to establish effective and targeted control of mosquito, vectors of diseases [44]. The entomological monitoring that we carried out revealed that An. gambiae and An. funestus were the main malaria vectors in Alibori and Donga regions, which confirms the results of previous studies conducted in West Africa, and specifically in Benin [45,46,47]. Anopheles gambiae appeared as the major vector (97.72%) in the two regions. This finding corroborates previous data published by Aikpon et al. [48] in Atacora and Gnanguenon et al. [47] in Kandi and Malanville, two districts of the region of Alibori. According to Akogbéto et al. [49], in Alibori and Donga regions, An. gambiae is composed of two sibling species (An. gambiae and Anopheles coluzzii) whose proportions vary depending on the region. In Alibori region, species composition showed An. coluzzii (62.2%) and An. gambiae (37.8%). In Donga, An. gambiae was the most abundant (64.7%). The two species were present throughout the transmission season [49]. Anopheles gambiae showed endophagic tendency. This behaviour of An. gambiae which feeds on man, preferentially inside houses, is justified by the fact that An. gambiae populations rest exclusively indoors during the rainy season, a period of vector abundance. However, this strong endophagy could also be facilitated by the alteration of the repulsive and lethal properties of the LLINs distributed in 2014 within the communities in both regions. In fact, according to Darriet [50], a new Olyset net reduces the entry rate of An. gambiae in the huts by 44% compared to an untreated net, whereas when it is 3 years old, its repellent effect is halved and this effect does no longer exist when it is washed. In addition, the dosage of the amount of insecticide carried out by Azondékon et al. [51] on LLINs fibers surface revealed a decrease in chemical efficacy only 6 months after the distribution of the LLINs Furthermore, the endophagic nature of An. gambiae in the study area is an asset for a preventive control based on IRS.
Regarding An. funestus, it represents the secondary vector encountered in the study area as it was found in a very low density compared to An. gambiae. This very low density has already been reported by Aikpon et al. [48] in the region of Atacora and by Gnanguenon et al. [47] in the Alibori (particularly in Kandi). The low abundance of An. funestus in Kandi and Gogounou may be due to the absence of its typical larval habitat (permanent or semi-permanent shaded freshwater streams, swamps, ponds and lakes) in these areas in view of the length of the drought period.
However, Aikpon et al. [52] reported a marked seasonal trend of An. funestus in Copargo (Donga region) with high abundance in the dry season. Conversely, the relatively high density of An. funestus in Djougou district, compared to other districts, is due to the existence of a small semi-permanent river, with surrounding vegetation, located not far from one of the study sites (Barienou).
The proportion of An. gambiae tested positive to circumsporozoitic antigen of P. falciparum was very high in the Alibori and in the Donga (SI = 8.05%), which stresses the need for the implementation of an effective malaria vectors control strategy in these two regions. A similar SI was previously reported in the Atacora, a northern region in Benin (SI = 6.63%) [48], in the Ouidah-Kpomasse-Tori region (southern Benin) (SI = 9.63%) [53] and in western Kenya (SI = 8.2%) [54]. This SI is lower than those observed in Eastern Gambia (SI = 17.73%) [55] and in Guinea-Bissau (SI = 12%) [56, 57]. This relatively high infection rate could be due to an increase in human–vector contact facilitated by anthropogenic behaviours (late hours at which people go to bed, non-usage of LLINs and others) and some factors that affect physical integrity (usage of sharp objects and lighted candles) and chemical effectiveness (high washing frequency) of LLINs. Other environmental factors, including high ambient temperature, prompt people to sleep outside without any protection against mosquito bites. This observation underscores the need to support IRS campaign with appropriate information, education and communication campaigns to combat this sleeping are misbehaviour in sprayed areas.
Although the infectivity rate was not the same in the four districts the highest rates were obtained in Djougou (16.84 infectious bites/man/month) and Copargo (17.64 infectious bites/man/month) and the lowest in Kandi (10.74 infectious bites/man/month) and Gogounou (11.04 infectious bites/man/month). These results suggest that the intensity of malaria transmission is higher in Donga region than in Alibori. Considering that the entomological inoculation rate is calculated using human biting and SI and, on the other hand, the similarity of SI in the two regions, it is legitimate to infer that the biting rate of An. gambiae was the main factor causing the difference observed between malaria transmission levels of the two regions. This deduction confirms the findings of Garrett-Jones [58], who reported that vector abundance is an important determinant in the malaria transmission level. In the case of the present study, the highest biting frequency of An. gambiae was observed in Donga region compared to Alibori (p < 0.0001), which could be due to some environmental characteristics (rainfall and soil humidity higher in the Donga region than in that of Alibori, thus promoting vector proliferation). Variation in malaria transmission levels between the two investigated regions may also be related to differences in topographies. In Alibori region, Kandi and Gogounou districts are located in areas of sloping plateaus that favour the runoff of water towards the south of the country after the rains. This situation prevents the on-site formation of a large number of breeding sites, resulting in lower vector abundance in Alibori region compared to Donga region. These results are consistent with findings of Omukunda et al. [59] who investigated similar bioecological areas in western Kenya.
The higher infection rates in rural areas compared to urban areas (p = 0) in the four districts confirm the spatial polymorphism in malaria epidemiology as previously observed by Sovi et al. [60] in the region of the Plateau, southeast of Benin. High EIRs obtained in rural areas were likely due to a high biting rate of An. gambiae resulting from an exponential proliferation in breeding sites meeting optimum conditions for development, in contrast to urban areas where the larval habitats are generally quite polluted and, therefore, more conducive to the development of Culicines [61]. Similar results have been observed in Dar es Salaam, Tanzania [62], in Ouagadougou, Burkina Faso [63], in Tori-bossito, southern Benin [64], in Kandi, northeastern Benin [31] and in the north–south transect of Benin [47]. In the study area, the risk of malaria infection was very high during the rainy season, but too low during the dry season despite the high parity rate in An. gambiae during this period compared to the rainy season. The period of malaria transmission is relatively longer in Donga region than in Alibori with a peak, respectively in August and October. These results are typical of tropical facies to which belong the two regions and characterized by a seasonal transmission of malaria interrupted by a dry season covering a non-negligible time in a year. A similar epidemiological facies was found in Zimbabwe, Kenya, Tanzania, northern areas of Nigeria, Benin, Ghana, Côte d’Ivoire and Guinea [65]. This observation should be taken into account to schedule spraying operations for a better impact of the intervention. Indeed, it is more appropriate to start the IRS campaign before the transmission period. In the four district covered by this study, June appeared to be the best period to start the IRS intervention. Since the CS formulation of pirimiphos methyl to be used for the next IRS campaign has a 4-month persistence period in field conditions of Atacora region [8], the short duration of malaria transmission in the Alibori region could be considered as an advantage.
Conclusion
The Alibori and Donga regions are characterized by one transmission season relatively longer in Donga region than in Alibori. Spatio-temporal variation in entomological inoculation rates was also observed with higher rates in rural areas and during the rainy season. Given the duration of persistence of pirimiphos methyl selected for the IRS operations, the month of June would be the ideal period to start the implementation of the intervention. The information collected in this study provides a reference for the monitoring and evaluation of the IRS intervention in four districts of the study area.
Abbreviations
- EIR:
-
entomological inoculation rate
- HBR:
-
human biting rate
- HLC:
-
human landing catch
- SI:
-
sporozoite index
- IRS:
-
indoor residual spraying
- ITN:
-
insecticide treated net
- LLIN:
-
long lasting insecticidal nets
- PSC:
-
pyrethrum spray catch
- CS:
-
micro-encapsulated formulation
References
WHO. Global Malaria Programme. Indoor residual spraying: use of indoor residual spraying for scaling up global malaria control and elimination. Geneva: World Health Organization; 2006.
WHO. Global Malaria Programme. Insecticide-treated mosquito nets: a position statement. Geneva: World Health Organization; 2007.
Roll Back Malaria. The global malaria action plan 2012. 2013. http://www.rollbackmalaria.org/gmap. Accessed 26 Feb 2018.
Schiff C. Integrated approach to malaria control. Clin Microbiol Rev. 2002;15:278–93.
Lengeler C, Sharp B. Indoor residual spraying and insecticide-treated nets. In: Murphy C, Ringheim K, Woldehanna S, Volmink J, editors. Reducing malaria’s burden: evidence of effectiveness for decision makers. Washington, DC: Global Health Council; 2003. p. 17–24.
Roberts D, Curtis C, Tren R, Sharp B, Shiff C, Bate R. Malaria control and public health. Emerg Infect Dis. 2004;10:1170–1.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
Akogbeto MC, Aikpon R, Azondekon R, Padonou G, Osse R, Agossa FR, et al. Six years of experience in entomological surveillance of indoor residual spraying against malaria transmission in Benin: lessons learned challenges and outlooks. Malar J. 2015;14:242.
Akogbeto M, Padonou GG, Bankole HS, Gazard DK, Gbedjissi GL. Dramatic decrease in malaria transmission after large-scale indoor residual spraying with bendiocarb in Benin, an area of high resistance of Anopheles gambiae to pyrethroids. Am J Trop Med Hyg. 2011;85:586–93.
Ossè R, Aikpon R, Padonou GG, Oussou O, Yadouléton A, Akogbéto M. Evaluation of the efficacy of bendiocarb in indoor residual spraying against pyrethroid resistant malaria vectors in Benin: results of the third campaign. Parasit Vectors. 2012;5:163.
Mabaso ML, Sharp B, Lengeler C. Historical review of malarial control in southern African with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–56.
Brutus L, Le Goff G, Rasolomaina LG, Rajaonarivelo V, Raveloson A, Cot M. Lutte contre le paludisme dans le moyen-ouest de Madagascar : Comparaison de l’efficacité de la lambda-cyhalothrine et du DDT en aspersions intradomiciliaires. I. Etude entomologique. Parasite. 2001;8:309–16.
Kleinschmidt I, Sharp B, Benevente L, Schwabe C, Torrez M, Kuklinski J, et al. Reduction in infection with Plasmodium falciparum one year after the introduction of malaria control interventions on Bioko Island, Equatorial Guinea. Am J Trop Med Hyg. 2006;74:972–8.
Sharp BL, Kleinschmidt I, Streat E, Maharaj R, Barnes KI, Durrheim DN, et al. Seven years of regional malaria control collaboration—Mozambique, South Africa and Swaziland. Am J Trop Med Hyg. 2007;76:42–7.
Overgaard HJ, Reddy VP, Abaga S, Matias A, Reddy MR, Kulkarni V, et al. Malaria transmission after five years of vector control on Bioko Island, Equatorial Guinea. Parasit Vectors. 2012;5:253.
Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS ONE. 2012;7:e42857.
Gimnig JE, Otieno P, Were V, Marwanga D, Abong’o D, Wiegand R, et al. The effect of indoor residual spraying on the prevalence of malaria parasite infection, clinical malaria and anemia in an area of perennial transmission and moderate coverage of insecticide treated nets in Western Kenya. PLoS ONE. 2016;11:0145282.
Protopopoff N, Wright A, West PA, Tigererwa R, Mosha FW, Kisinza W, et al. Combination of insecticide treated nets and indoor residual spraying in Northern Tanzania provides additional reduction in vector population density and malaria transmission rates compared to insecticide treated nets alone: a randomised control trial. PLoS ONE. 2015;10:e0142671.
Chandre F, Manguin S, Brengues C, Dossou Yovo J, Darriet F, Diabate A, et al. Current distribution of pyrethroid resistance gene (kdr) in Anopheles gambiae complex from West Africa and further evidence for reproductive isolation of Mopti form. Parassitologia. 1999;41:319–22.
Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, et al. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–16.
Yadouleton A, Asidi A, Djouaka R, Braïma J, Agossou C, Akogbeto M. Development of vegetable farming. A cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103.
Djogbenou L, Pasteur N, Bio-Bangana S, Baldet T, Irish SR, Akogbeto M, et al. Malaria vectors in the Republic of Benin: distribution of species and molecular forms of the Anopheles gambiae complex. Acta Trop. 2010;114:116–22.
Djegbe I, Boussari O, Sidick A, Martin T, Ranson H, Chandre F, et al. Dynamics of insecticide resistance in malaria vectors in Benin: first evidence of the presence of L1014S kdr mutation in Anopheles gambiae from West Africa. Malar J. 2011;10:261.
Sovi A, Djegbe I, Soumanou L, Tokponnon F, Gnanguenon V, Azondekon R, et al. Microdistribution of the resistance of malaria vectors to deltamethrin in the region of Plateau (southeastern Benin) in preparation for an assessment of the impact of resistance on the effectiveness of long lasting insecticidal nets (LLINs). BMC Infect Dis. 2014;14:103.
Aïzoun N, Aïkpon R, Gnanguenon V, Oussou O, Agossa F, Padonou GG, et al. Status of organophosphate and carbamate resistance in Anopheles gambiae sensu lato from the south and north Benin, West Africa. Parasit Vectors. 2013;6:274.
Aïkpon R, Agossa F, Ossè R, Oussou O, Aïzoun N, Oké-Agbo F, et al. Bendiocarb resistance in Anopheles gambiae s.l. populations from Atacora department in Benin, West Africa: a threat for malaria vector control. Parasit Vectors. 2013;6:192.
Gnanguenon V, Agossa FR, Badirou K, Govoetchan R, Anagonou R, Oke-Agbo F, et al. Malaria vectors resistance to insecticides in Benin: current trends and mechanisms involved. Parasit Vectors. 2015;8:223.
Cox J, Craig MH, Le Sueur D, Sharp BL. Mapping malaria risk in the highlands of Africa. Technical report. London/Durban: MARA/HIMAL; 1999.
Kleinschmidt I, Sharp B, Mueller I, Vounatsou P. Rise in malaria incidence rates in South Africa: small area spatial analysis of variation in time trends. Am J Epidemiol. 2002;155:257–64.
Munga S, Yakob L, Mushinzimana E, Zhou G, Ouna T, Minakawa N, et al. Land use and land cover changes and spatiotemporal dynamics of Anopheline larval habitats during a four-year period in a highland community of Africa. Am J Trop Med Hyg. 2009;81:1079–84.
Govoetchan R, Gnanguenon V, Azondékon R, Agossa RF, Sovi A, Oké-Agbo F, et al. Evidence for perennial malaria in rural and urban areas under the Sudanian climate of Kandi, Northeastern Benin. Parasit Vectors. 2014;7:79.
Kazembe LN, Kleinschmidt I, Holtz TH. Spatial analysis and mapping of malaria risk in Malawi using point-referenced prevalence of infection data. Int J Health Geograph. 2006;5:41.
Le Sueur D, Binka F, Lengeler C, de Savigny D, Snow B, Teuscher T, et al. An atlas of malaria in Africa. Afr Health. 1997;19:23–4.
Carter R, Mendis KN, Roberts D. Spatial targeting of interventions against malaria. Bull World Health Organ. 2000;78:1401–11.
Killeen GF, Okumu FO, N’Guessan R, Coosemans M, Adeogun A, Awolola S, et al. The importance of considering community-level effects when selecting insecticidal malaria vector products. Parasit Vectors. 2011;4:160.
INSAE, RGPH4 2013. Cahiers des villages et quartiers de ville du département de l’Alibori; 2016. http://www.insae-bj.org/recensement-population.html/enquêtes-recensements/rgph/Cahier_village_2013/Alibori.pdf. Accessed 20 Apr 2018.
INSAE, RGPH4 2013. Cahiers des villages et quartiers de ville du département de la Donga; 2016. http://www.insae-bj.org/recensement-population.html/enquêtes-recensements/rgph/Cahier_village_2013/Donga.pdf. Accessed 20 Apr 2018.
Ministère de la Santé. Annuaire des statistiques sanitaires 2016. Cotonou: Direction de la Programmation et de la Prospective; 2017.
Gillies MT, De Meillon B. The Anophelinae of Africa south of the Sahara. South Afri Inst Med Res. 1968;54:1–343.
Detinova TS, Gillies MT. Observations on the determination of the Age composition and epidemiological importance of populations of Anopheles gambiae Giles and Anopheles funestus Giles in Tanganyika. Bull World Health Organ. 1964;30:23–8.
Wirtz R, Zavala F, Charoenvit Y, Campbell G, Burkot T, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39.
Rothman KJ. Epidemiology: an introduction. Oxford: Oxford University Press; 2012.
Jewell NP. Statistics for epidemiology. Boca Raton: CRC Press; 2003.
Fontenille D, Simard F. Unraveling complexities in human malaria transmission dynamics in Africa through a comprehensive knowledge of vectors populations. Comp Immunol Microbiol Infect Dis. 2004;27:357–75.
Akogbeto M, Di Deco M. Distribution of members of the Anopheles gambiae complex and their chromosomal variants in Benin and Togo, West Africa. Afr Zool. 1995;109:443–54.
Kelly-Hope LA, Mckenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:19.
Gnanguenon V, Govoetchan R, Agossa FR, Osse R, Oke-Agbo F, Azondekon R, et al. Transmission patterns of Plasmodium falciparum by Anopheles gambiae in Benin. Malar J. 2014;13:444.
Aïkpon R, Osse R, Govoetchan R, Sovi A, Oke-Agbo F, Akogbeto MC. Entomological baseline data on malaria transmission and susceptibility of Anopheles gambiae to insecticides in preparation for indoor residual spraying (IRS) in Atacora, (Benin). J Parasitol Vector Biol. 2013;5:102–11.
Akogbéto MC, Salako SA, Dagnon F, Aïkpon R, Kouletio M, Sovi A, et al. Blood feeding behaviour comparison and contribution of Anopheles coluzzii and Anopheles gambiae, two sibling species living in sympatry, to malaria transmission in Alibori and Donga region, northern Benin, West Africa. Malar J. 2018;17:307.
Darriet F. Moustiquaires imprégnées et résistance des moustiques aux insecticides. Paris: Éditions IRD; 2007.
Azondekon R, Gnanguenon V, Oke-Agbo F, Houevoessa S, Green M, Akogbeto M. A tracking tool for long-lasting insecticidal (mosquito) net intervention following a 2011 national distribution in Benin. Parasit Vectors. 2014;7:6.
Aïkpon R, Ossè R, Padonou G, Anagonou R, Salako A, Ahogni I, et al. Involvement of both Anopheles gambiae and Anopheles funestus (Diptera: Culicidae) in the perennial malaria transmission through a seasonal abondance in savannah area in Benin. Int J Mosq Res. 2017;4:107–12.
Djènontin A, Bio-Bangana S, Moiroux N, Henry M-C, Bousari O, Chabi J, et al. Culicidae diversity, malaria transmission and insecticide resistance alleles in malaria vectors in Ouidah-Kpomasse-Tori district from Benin (West Africa): a preintervention study. Parasit Vectors. 2010;3:83.
Taylor KA, Koros JK, Nduati J, Copeland R, Collins FH, Brandling-Bennett AD. Plasmodium falciparum infection rates in Anopheles gambiae, An. arabiensis, and An. funestus in Western Kenya. Am J Trop Med Hyg. 1990;43:124–9.
Thomson MC, D’Alessandro U, Bennett S, Connor SJ, Langerock P, Jawara M, et al. Malaria prevalence is inversely related to vector density in The Gambia, West Africa. Trans R Soc Trop Med Hyg. 1994;88:638–43.
Snounou G, Pinheiro L, Gonçalves A, Fonseca L, Dias F, Brown KN, et al. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg. 1993;87:649–53.
Jaenson TG, Gomes MJ, Barreto dos Santos RC, Petrarca V, Fortini D, Evora J, et al. Control of endophagic Anopheles mosquitoes and human malaria in Guinea Bissau, West Africa by permethrin-treated bed nets. Trans R Soc Trop Med Hyg. 1994;88:620–4.
Garrett-Jones C. Prognosis for interruption of malaria transmission through the assessment of mosquito vectorial capacity. Nature. 1964;204:1173–5.
Omukunda E, Githeko A, Ndong’a MF, Mushinzimana E, Atieli H, Wamae P. Malaria vector population dynamics in highland and lowland regions of western Kenya. J Vector Borne Dis. 2013;50:85–92.
Sovi A, Govoétchan R, Tokponnon F, Hounkonnou H, Aïkpon R, Agossa F, et al. Impact of land-use on malaria transmission in the Plateau region, southeastern Benin. Parasit Vectors. 2013;6:352.
Omumbo JA, Guerra CA, Hay SI, Snow RW. The influence of urbanization on measures of Plasmodium falciparum infection prevalence in East Africa. Acta Trop. 2005;93:11–21.
Wang SJ, Lengeler C, Mtasiwa D, Mshana T, Manane L, Maro G, et al. Rapid urban malaria appraisal (RUMA) II: epidemiology of urban malaria in Dar es Salaam (Tanzania). Malar J. 2006;5:29.
Wang SJ, Lengeler C, Smith TA, Vounatsou P, Diadie DA, Pritroipa X, et al. Rapid urban malaria appraisal (RUMA) I: epidemiology of urban malaria in Ouagadougou. Malar J. 2006;4:43.
Pierrat C. Des moustiques et des hommes: les territoires du paludisme à Tori-Bossito (sud du Bénin). Thèse de Doctorat de Géographie, Université Paris I Panthéon-Sorbonne; 2010.
Mouchet J, Carnevale P, Coosemans M, Fontenille D, Ravaonjanahary C, Richard A, Robert V. Typologie du paludisme en Afrique. Santé: Cahiers d'Etudes et de Recherches Francophones. 1993;3(4):220–38
Authors’ contributions
ASS, IA, CK, FT and MCA conceived the study. ASS and MCA have participated in the design of the study. ASS, IA, CK, AAS and VG carried out the field activities and the laboratory analysis. ASS and MCA drafted the manuscript. RA, AS, LI, FT, FD and MCA critically revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to the President’s Malaria Initiative which supported financially this study. We thank Bruno AKINRO for statistical analysis and also the populations of Sonsoro, Kossarou, Gounarou, Bantansouè, Bariénou, Zountori, Kataban and Kparakouna centre for their collaboration. We acknowledge Monica Patton, Peter Thomas and Raymond Beach of US Centers for Disease Control and Prevention for providing technical assistance and proofreading the manuscript.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data used and/or analysed in this study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The protocol of this study was reviewed and approved by the Institutional Ethics Committee of CREC (IECC). Before mosquito collectors were involved in this study, they gave their consent to participate. They were vaccinated against yellow fever, regularly checked up by a medical doctor and taken care in case of confirmed malaria case.
Funding
This study was financially supported by the US President’s Malaria Initiative (PMI) thru the United States Agency for International Development (USAID) Africa Indoor Residual Spraying Project (AIRS).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Salako, A.S., Ahogni, I., Kpanou, C. et al. Baseline entomologic data on malaria transmission in prelude to an indoor residual spraying intervention in the regions of Alibori and Donga, Northern Benin, West Africa. Malar J 17, 392 (2018). https://doi.org/10.1186/s12936-018-2507-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-018-2507-y