Abstract
Background
The use of saliva in diagnosis of infectious diseases is an attractive alternative to procedures that involve blood drawing. It promises to reduce risks associated with accidental needle pricks and improve patient compliance particularly in malaria survey and drug efficacy studies. Quantification of parasitaemia is useful in establishing severity of disease and in assessing individual patient response to treatment. In current practice, microscopy is the recommended technique, despite its limitations. This study measured the levels of Plasmodium falciparum lactate dehydrogenase (PfLDH) in saliva of malaria patients and investigated the relationship with blood parasitaemia.
Methods
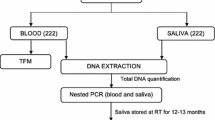
Matched pre-treatment blood and saliva samples were collected from patients at Msambweni District Hospital, Kenya. Parasitaemia was determined and only those confirmed to be Plasmodium falciparum mono-infected were recruited. PfLDH was quantified in saliva using a commercial ELISA kit. A total of 175 samples were collected. Relationship between blood parasitaemia and concentration of PfLDH in saliva was determined using Pearson correlation statistics. F test was used to determine whether there is a significant difference between levels of PfLDH in saliva of patients with moderate to high parasitaemia and those with low parasitaemia.
Results
One-hundred and seventy-five patient samples were positive for malaria by microscopy. Of these, 62 (35%) tested positive for PfLDH in saliva, 113 (65%) were false negatives. For those that tested positive, (53) 85% were from patients with moderate to high parasitaemia while 9 (15%) were from patients with low parasitaemia. A correlation co-efficient of 0.18 indicated a weak positive relationship between the concentration of PfLDH in saliva and blood parasitaemia. There was a marginal difference between levels of PfLDH in saliva of patients with moderate to high parasitaemia and those with low parasitaemia [F (1, 59) = 1.83, p = 0.1807].
Conclusion
The results indicate that there is a weak correlation between levels of PfLDH in saliva and blood parasitaemia. This is weak association could be as a result of low sensitivity of the assay used as well as presence of inhibitors and proteases in saliva. Further studies should be focused towards reducing the number of false negatives and developing a customised assay that is specific for detection of PfLDH in saliva.
Similar content being viewed by others
Background
Malaria continues to be a public health challenge particularly in resource-limited settings in sub-Saharan Africa, 3.3 billion people are at risk of developing the disease [1, 2]. Accurate diagnosis of malaria is a critical component in integrated approach for control and elimination of the disease [3]. Lack of diagnostic support particularly in field and resource-limited settings has led to over-reliance on clinical assessment. This practice has led to over-diagnosis of populations at risk posing a risk of rapid development of anti-malarial drug resistance [4, 5]. Microscopy remains the gold standard method for diagnosis of malaria. This is largely due to its ability of differentiate species and quantify parasitaemia. However, the technique is limited due to infrastructural and technical requirements that are not always available in resource-limited settings. In such settings, the adoption of malaria rapid diagnostic test (MRDT) has greatly improved access to diagnosis. However, its usefulness is limited due to its inability to quantify parasitaemia. In recent years, there have been significant efforts towards developing non-invasive malaria diagnostic tools. Plasmodium falciparum proteins HRP and LDH (PfLDH) have been detected in the saliva of malaria patients [6, 7]. Studies have demonstrated that levels of PfLDH in blood correlate with levels of parasitaemia [8, 9]. This is the first time PfLDH has been quantified in saliva of malaria patients and compared to parasitaemia. A positive correlation would provide a useful non-invasive assay that can be used to predict parasite load in malaria surveys of healthy populations, population-wide monitoring of the impact of anti-malarial interventions as well as in drug efficacy studies where repeated sampling of study cohorts is required.
The hypothesis was that there is a positive correlation between PfLDH concentration in saliva and blood parasitaemia which can be a useful tool in malaria studies.
Methods
Study site and enrolment
This study was conducted in Kwale County, Msambweni, Kenya, in a Kenya Medical Research Institute (KEMRI) established site at Msambweni District Hospital. This area is endemic to malaria with prevalence of 8%, as of the 2015 Kenya malaria indicator survey report [10]. Informed consent was obtained from the patients upon recruitment into the study.
Initial screening
Patients aged between 2 and 65 years presenting with clinical symptoms of malaria were screened using RDTs (Malaria Ag Pf/Pan Standard diagnostics Inc).
Diagnosis by microscopy
The infection status of patients found to be positive by RDT was confirmed by microscopy. At least two smears per patient were prepared and read by two independent microscopists. Six μl of blood was spread in a circular pattern of 12 mm diameter; 100 high-power fields of the thick film were examined to detect the presence of malaria parasites at 100 × oil immersion. Parasite density was calculated and recorded as number of parasites/μl blood using the following formula.
where discrepancies were found a third reading was done. Only those found to be falciparum malaria (mono-infection) were recruited (Table 1).
Collection of saliva samples
A matched saliva sample was collected from patients confirmed to be falciparum malaria (mono-infection). Saliva samples were collected using saliva collection aid (Salimetrics, USA) and immediately stored at − 80 °C without processing. Patients were instructed to allow saliva to passively flow into the saliva collection aid; approximately 1 ml of saliva was collected from each patient.
Plasmodium falciparum LDH enzyme-linked immunosorbent assay (PfLDH ELISA)
PfLDH in saliva was detected and quantified using a commercial malaria antigen ELISA kit (Standard Diagnostics Inc). The kit is designed for qualitative detection of malaria Plasmodium species PLDH antigen in human whole blood and has a sensitivity of 98% for P. falciparum. The ELISA assays were run as per manufacturer’s instructions. Prior to analysis, the samples were thawed on ice. Fifty μl of each test sample (saliva) was mixed with 50 μl of phosphate-buffered saline supplemented with 0.05% Tween 20 (PBS/T) and allowed to react for 15 min with 100 μl of PLDH detector antibody solution. The reaction mixture was transferred into the ELISA test plate, pre-coated with the capture antibody provided in the kits. The plates were incubated for 60 min at 37 °C to allow effective binding of the analyte sandwiched between the corresponding capture and detector antibodies. The wells were washed and 100 μl chromogen (TMB) substrate, supplied in the kit, was added. The relative absorbance values were read using a microplate reader at 450 nm. The amount of bound sandwiched target analyte was measured as optical density (OD). The cut-off value was 0.1 and was determined as per manufacturer’s instructions (Table 1).
Comparison of PfLDH levels in saliva and blood
The difference in concentration of PfLDH in saliva and blood was determined using a commercial malaria antigen ELISA kit (Standard Diagnostics Inc). The ELISA assays were run as per the manufacturer’s instructions. Prior to analysis the blood and saliva samples were thawed on ice. The ELISA assay was run as previously described. Negative samples of patients confirmed to be negative for malaria were also included in the ELISA assay (Table 1).
Statistical analysis
The F test was used to determine whether there is a significant difference between levels of PfLDH in saliva of patients with moderate to high parasitaemia > 1000 parasite/μl and those with low parasitaemia < 1000 parasites/μl. A comparison between the PfLDH ELISA and parasite density was done using Pearson correlation statistics.
Results
Detection and quantification of PfLDH in saliva of malaria patients
A total of 175 patients were confirmed to be P. falciparum-positive by microscopy and recruited into the study. Thirty-five per cent of the patients recruited into study tested positive for PfLDH ELISA in saliva. The remaining 65% were false negatives.
The concentration of PfLDH in saliva of malaria patients was several folds lower than the concentration of PfLDH in blood of malaria patients with similar range of parasitaemia. The occurrence of false negatives was observed in both low and high parasitaemia samples. The ELISA assay generally gave weak positives for PfLDH in saliva (Fig. 1).
Association between levels of blood parasitaemia and PfLDH levels in saliva of malaria patients
A correlation coefficient of 0.18 indicated a slight linear positive relationship between the concentration of PfLDH in saliva and parasitaemia in blood. There was a slight difference between levels of PfLDH in saliva of patients with moderate to high parasitaemia and those with low parasitaemia [F (1, 59) = 1.83, p = 0.1807] (Fig. 2).
Discussion
The use of saliva as an alternative non-invasive diagnostic matrix for infectious diseases continues to elicit interest. However, despite numerous reports on the diagnostic potential of saliva, there is still a limited number of saliva-based assays in use in clinical settings compared to its plasma counterparts. This can be attributed partly to the fact that the target analytes are usually present in many folds higher concentration in blood that in saliva, making plasma-based assays more sensitive. This has limited the development of saliva-based, malaria, point-of-care diagnostic tools. However, saliva remains an attractive alternative for diagnosis as it is less bio-hazardous, non-invasive and relatively easy to collect from patients. Compliance to blood drawing may not be an issue for malaria patients in clinical settings, however, an investigator may face challenges convincing healthy patients in a malaria survey study or drug efficacy study to comply with multiple blood drawing. It is thus worthwhile to further advance the quest for saliva diagnostics. This study sought to investigate the potential use of saliva in predicting parasite load by investigating the relationship between blood parasitaemia and concentration of PfLDH in saliva of malaria patients. A positive correlation would provide a useful diagnostic tool that can be used to enhance malaria surveys studies as well as drug efficacy studies.
PfLDH in malaria
Plasmodium lactate dehydrogenase (PLDH) is an intracellular glycolytic enzyme that catalyses oxidation of lactate to pyruvate. Each Plasmodium species has a variant isomer of the enzyme. The enzyme is expressed in high levels during the asexual stage of the parasites and clears within 24 h of effective malaria treatment [11]. PfLDH was selected for this study based on studies that have demonstrated its presence in saliva [7] and its correlation with the parasite density in plasma of malaria patients [11, 12]. The measurement of concentration of PfLDH in saliva of malaria patients and relating it to parasitaemia could offer an alternative non-invasive assay that can be used to predict parasite load and potentially be used in assessing patient response to treatment. This paper reports for the first time the detection and quantification of PfLDH in saliva of malaria patients on day 0 prior to treatment and its correlation to parasitaemia.
A significant number of false negatives were observed and there were variations in concentration of PfLDH in individuals with comparable parasitaemia. A weak positive correlation between lactate dehydrogenase concentration in saliva and parasite density was observed. There was a marginal difference between levels of PfLDH in saliva of patients with moderate to high parasitaemia and those with low parasitaemia.
In the current study a commercial kit Malaria Antigen ELISA kit (Standard Diagnostics Inc) was used to detect PfLDH in saliva. Other studies have reported qualitative detection of Plasmodium antigen HRP in saliva using commercial kits designed for detection of Plasmodium antigens in plasma. However, the detection was limited due to requirement of low detection levels when it comes to saliva samples [9]. Detection of PLDH in saliva of malaria patients using RDTs has also been reported [7]. This is the first time PfLDH in saliva of malaria patients has been detected and quantified using ELISA. Other studies have reported improved detection of Plasmodium antigens (HRP) in saliva when a customized in-house ELISA assay is developed [9]. This study further corroborates the viability of saliva in malaria diagnostics. The assay was limited to the limit of detection (LOD) of a commercial kit designed for use with plasma samples. This could partly explain the occurrence of false negatives. Variations in concentration of PfLDH in saliva of individuals with similar ranges of parasitaemia suggests that there are inherent factors in saliva that affect the assay [13, 14]. The stability of PLDH in saliva is not equivalent to its stability in plasma. Saliva has proteases that may contribute to the breakdown of the enzyme (PLDH) in saliva. Noteworthy is the clarity of the saliva samples which varied between individuals despite the request for patients to give unstimulated drool; it was also difficult to get clear saliva samples from children who were dehydrated. Future work should be focused on standardizing saliva collection as well and standardization of saliva samples prior to running the ELISA assay. In addition the development of an in-house ELISA assay for the detection of PLDH in saliva samples may also help in reducing the number of false negatives.
Conclusion
This study has demonstrated that there is a weak correlation between the levels of PfLDH in saliva and blood parasitaemia in malaria-positive patients. For the assay to be useful, the sensitivity of the test has to be improved. In this study, the sensitivity was limited due to use of a commercial assay designed for plasma samples. Future work should be focused on the development of a customized saliva assay that would take into consideration the complex saliva matrix and mitigate the effects of proteases and mucins that may have contributed to low sensitivity.
References
WHO. Treatment of severe malaria. Guidelines for the treatment of malaria. Geneva: World Health Organization. 2015; p. 71–88.
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
Shiff C. Integrated approach to malaria control. Clin Microbiol Rev. 2002;15:278–93.
Bloland PB. Drug resistance in malaria. Geneva: World Health Organization. WHO/CDS/CSR/DRS/2001/4; 2001.
Harchut K, Standley C, Dobson A, Klaassen B, Rambaud-Althaus C, Althaus F, et al. Over-diagnosis of malaria by microscopy in the Kilombero Valley, Southern Tanzania: an evaluation of the utility and cost-effectiveness of rapid diagnostic tests. Malar J. 2013;12:159.
Wilson NO, Adjei AA, Anderson W, Baidoo S, Stiles JK. Detection of Plasmodium falciparum histidine-rich protein II in saliva of malaria patients. Am J Trop Med Hyg. 2008;78:733–5.
Gbotosho GO, Happi CT, Folarin O, Keyamo O, Sowunmi A, Oduola AMJ. Rapid detection of lactate dehydrogenase and genotyping of Plasmodium falciparum in saliva of children with acute uncomplicated malaria. Am J Trop Med Hyg. 2010;83:496–501.
Verma P, Biswas S, Mohan T, Ali S, Rao DN. Detection of histidine rich protein & lactate dehydrogenase of Plasmodium falciparum in malaria patients by sandwich ELISA using in-house reagents. Indian J Med Res. 2013;138:977–87.
Fung AO, Damoiseaux R, Grundeen S, Panes JL, Horton DH, Judy JW, et al. Quantitative detection of PfHRP2 in saliva of malaria patients in the Philippines. Malar J. 2012;11:175.
National Malaria Control Programme. Malaria indicator survey 2015. Nairobi, Kenya; 2015.
Oduola AMJ, Omitowoju GO, Sowunmi A, Makler MT, Falade CO, Kyle DE, et al. Plasmodium falciparum: evaluation of lactate dehydrogenase in monitoring therapeutic responses to standard antimalarial drugs in Nigeria. Exp Parasitol. 1997;87:283–9.
Piper R, LeBras J, Wentworth L, Hunt-Cooke A, Houzé S, Chiodini P, et al. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am J Trop Med Hyg. 1999;60:109–18.
Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204.
Nunes LAS, Mussavira S, Bindhu OS. Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem Med (Zagreb). 2015;25:177–92.
Authors’ contributions
EN, FK and JK conceived and designed the experiments; WB and JL provided advise on protocols development EN, WC, MO, SK, and ET performed the experiments; EN, FK and JH analysed the data; EN wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was funded by grant no S5 0425-01 from Grand Challenges Canada and supported by KEMRI IRG. The authors thank the entire team at Msambweni District Hospital that assisted immensely in sample collection. We would like to thank KEMRI for the administrative support.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The protocol SSC 2639 was approved by the Ethics Review Board (SERU) in KEMRI.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nambati, E.A., Kiarie, W.C., Kimani, F. et al. Unclear association between levels of Plasmodium falciparum lactate dehydrogenase (PfLDH) in saliva of malaria patients and blood parasitaemia: diagnostic implications?. Malar J 17, 9 (2018). https://doi.org/10.1186/s12936-017-2151-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-017-2151-y