Abstract
Background
This study was initiated from the observation that prevalence of malaria obtained with rapid diagnostic test (RDT) (CareStart™Malaria HRP2/pLDH Combo Test) was higher than in microscopy in a malaria low transmission area of Senegal. PCR was then performed to evaluate the performance of the RDT compared to microscopy in clinical settings.
Methods
The study included 215 patients suspected of malaria in two peri-urban area of Dakar. Finger-pick blood samples were tested using RDT (CareStart™Malaria HRP2/pLDH Combo Test). Venous blood samples were collected for light microscopy and PCR (gold standard). Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated as performance characteristics.
Results
Considering PCR as the gold standard, CareStart™RDT showed high sensitivity (97.3%) and specificity (94.1%) with PPV and NPV of 97.3 and 94.1%, respectively, while microscopy had a sensitivity and specificity of 93.2 and 100%, respectively, and PPV and NPV of 100 and 87.2%, respectively.
Conclusions
Malaria CareStart™RDT test demonstrated a superior sensitivity compared to microscopy, which is the gold standard for malaria diagnosis. CareStart™RDT could be a useful tool in individuals suspected of malaria even in areas where prevalence is low.
Similar content being viewed by others
Background
Light microscopy detecting human malarial parasites in Giemsa-stained thick and thin blood films still remains the gold standard for malaria diagnosis [1]. Although with good sensitivity in diagnosing malaria, its reliability relies upon good-quality slide preparation and well-trained staff in parasite detection and differentiation, especially at low parasite densities [2]. In many peripheral health centres of endemic countries, microscopy is not available [3] due to limited resources or lack of expertise [4]. Simpler and rapid methods of identification of parasites may be useful for prompt diagnosis and appropriate treatment especially in peripheral health posts where microscopy is not available [5].
Malaria rapid diagnostic tests (RDTs), based on immunochromatographic parasite antigen detection, are of great value in endemic regions. Since 2010, WHO has recommended either RDT or microscopy confirmation of suspected malaria cases before treatment [3]. However, it remains unclear whether RDTs are useful in field conditions in low transmission areas [6] since their reliability is limited by the lack of detection of low-density parasitaemia [7]. Recently, an anecdotal observation indicated that there were more positive results with RDT (CareStart™Malaria HRP2/pLDH Combo Test) than with microscopy in a peri-urban region of Dakar where malaria transmission is low. CareStart™RDT Pf/Pan is one of the RDT being used in Senegal accordingly to the National Malaria Control Programme (NMCP).
The objective of this study was to compare CareStart™ RDT to microscopy performed by a WHO-certified level 1 microscopist and determine the test characteristics using PCR as gold standard.
Methods
Study design
The study was carried out in two sub-urban sites of Dakar (Pikine and Rufisque) from October to November 2016. Malaria transmission in Dakar is low, with parasitaemia prevalence estimate of 1.3% and a typical urban malaria pattern [8]. Pikine and Rufisque are characterized by unorganized urban growth. Malaria transmission in Dakar is heterogeneous in space and highly focal. Short rains occur from August to November. During this period breeding sites appear in tyres, step tracks, puddles, ditches, and garbage cans, or in debris on construction sites [9].
The study patients were enrolled if they presented with clinically suspected malaria on the basis of fever or history of fever in the previous 48 h to the health clinics in the study areas. Blood samples were collected for RDT and microscopy, and a filter paper collected for PCR. Inclusion criterion for this study was individuals with fever (suspicion of malaria) and without clinical sign of severe malaria.
Microscopy evaluations
All parasitological examinations were carried out in Aristide Le Dantec Hospital Laboratory, the national reference laboratory of Senegalese National Malaria Control Programme. The laboratory hosts the external competency assessment (ECA) centre for African Francophone countries. Thick and thin blood smears were prepared from collected venous blood samples. Both thick and thin films were made in the same slide. The thin films were fixed in methanol. The slides were stained in 10% Giemsa solution for 15 min. Stained slides were read by two WHO-certified level 1 microscopists in the laboratory. The number of parasites was counted against 200 leucocytes when more than 100 parasites were counted, and 500 leucocytes were required when fewer than 100 parasites were counted. Quantification of parasite density was estimated by assuming 8000 leucocytes/μL of blood. The result was considered negative if no parasite was detected after examining 200 microscopic fields at 1000× magnification. The technician was blinded to the results of the RDT. In case of discordant results between the two readers, a third expert reader was used.
Rapid diagnostic test (RDT)
The same patients were tested for malaria parasites using CareStart™Malaria HRP2/pLDH Combo Test in the fields (Lot Number: MR15A06). CareStart™RDT is an immunochromatographic test coated with monoclonal antibodies in two separate bands, one recognizing the specific histidine-rich protein-2 (HRP-2) associated with the presence of Plasmodium falciparum and the other detecting presence of pan malaria-specific antigen (pLDH) of all malarial parasites species. Five microlitre of blood was drawn using a loop provided with the device. The test preparation and interpretation was done following manufacturer’s instructions. Positive results indicate the presence of two or three bands (including control test line). For negative results, only the control line appears. Results were observed and number of visible line was recorded.
PCR assay
From the collected venous blood, two drops of blood were spotted onto filter paper, individually stored in a plastic bag and sent to the Parasitology Laboratory of Le Dantec University Hospital for PET-PCR assay. DNA was extracted using the QIAamp blood kit (QIAGEN™) according to the manufacturer’s instructions and stored at 4 °C until processed. The Plasmodium PET-PCR reaction was performed as previously described [10, 11]. Samples with a CT value of 40.0 or below were considered positive.
Data analysis
Data were processed in Excel (version 15.27) and analysed using STATA. Diagnostic performance was determined by calculating the test sensitivity (Se), specificity (Sp), predictive values (PPV and NPV), with 95% CI. PET-PCR was used as the reference method. McNemar Chi square analysis was used to determine the significance differences between Se, Sp, NPV and PPV. The p values less than 0.05 were considered statistical significance.
Ethical consideration
Oral informed consent was obtained from the selected patients. Ethical approval with the reference number was obtained from Senegalese National Ethic Committee of Ministry of Health.
Results
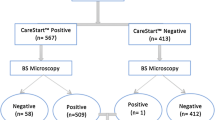
A total of 215 blood samples collected from malaria suspected patients were analysed. The sex ratio was 1.56:1. The mean age was 23 years (range 4–77 years). Among the 215 samples tested, the PPV was 137 (63.7%), 146 (67.9%) and 144 (68.4%) using microscopy, CareStart™RDT and PCR, respectively. Overall, CareStart™RDT detected more positive samples than microscopy and PCR. Species of malarial parasites identified in all study participants were P. falciparum. The parasite density ranged from 16 to 341,090 parasites/μL of blood with a mean of 36,823 parasites/μL by microscopy.
When compared to PCR, CareStart™RDT test showed better Se (97.3%) than microscopy (93.2%) (p = 0.0143) and better NPV, 94.1 versus 87.2% (p = 0.0251). However, better Sp (100%) and PPV (100%) were noted in microscopy versus RDT (94.1 and 97.3%, respectively) (p = 0.0455 and p = 0.0487, respectively) (see Table 1). PCR was used to resolve the discrepancies between microscopy and CareStart™RDT. There were 201 (93.5%) agreement results between all three methods. Thus, discrepancies were noted in 14 samples (6.5%). Microscopy was not able to detect 10 samples (4.7% of false negative microscopy). Among those 10 samples, CareStart™RDT also failed to detect four samples (false negative RDT) but correctly detected six samples. The four remaining samples were correctly read as negative by microscopy but were positive by RDT (false positive RDT). On false positive RDT, only HRP2 bands appeared. There was no false positive result with microscopy. In summary, CareStart™RDT showed less false negative but more false positive results compared to microscopy (see Table 2).
Among the 147 RDT positive samples, only 47 presented LDH band (plus HRP2 band), giving an overall LDH positivity rate of 32.0%. This positivity rate was even less (17.4%) when parasite densities were less than 1000 (see Table 3).
Discussion
RDTs have been developed especially for their ease of use in remote settings in endemic countries [1, 12]. However, many drawbacks have been reported with RDTs, especially relating to their sensitivity. In the literature, limit of detection of RDTs is estimated to be around 100 parasites/μL [4].
Several RDTs are commercially available with various quality of performance [12,13,14]. Thus, WHO requires all RDTs to reliably detect at least 100 parasites/μL and to present a minimum sensitivity of 95% and minimum specificity of 90% compared to traditional microscopy. Many studies have reported low sensitivity of RDTs when parasitaemia becomes lower than 200 parasites/μL [15]. Such low parasitaemia usually occur in malaria low transmission areas.
In a clinical health centre of sub-urban of Dakar (where low transmission of malaria occurs), it was reported that CareStart™RDT gave more positive results than microscopy performed by average microscopists. The aim of this study was to compare CareStart™RDT to microscopy using PCR as gold standard. The results of the study confirmed the observation noted by health-centre microscopists. In fact, CareStart™RDT showed better sensitivity than microscopy although specificity was better with the latter.
The CareStart™Malaria HRP2/pLDH (Pf/pan) Combo Test is a three-band RDT that detects HRP2 and pan-pLDH antigens [4]. CareStart™RDT has been evaluated previously in field settings using PCR-corrected microscopy as reference, showing better sensitivity for microscopy [16]. In a few studies the microscopy standard was not corrected by PCR [17]. High sensitivity (94%) were noted when parasitaemia were above 100 parasites/μL. The sensitivity increased with the increasing parasitaemia, reaching 99% when parasitaemia was up to 1000 parasites/μL, while sensitivity was only 88% when parasitaemia was fewer than 100 parasites/μL [4]. In the study described here, there were 8 samples with parasitaemia under 100 parasites/μL, and 23 samples had parasitaemia fewer than 1,000 parasites/μL. Since all positive microscopy were also positive in CareStart™RDT and confirmed by PCR, the sensitivity against microscopy was 100% irrespective to range of parasitaemia. However, at the PCR level, sensitivity of CareStart™RDT was significantly higher. A previous study reported superior sensitivity of RDT over microscopy (including expert microscopy) [5]. In a field study performed in unstable malaria transmission, performance of CareStart™RDT has shown a sensitivity of 85.6% and specificity of 92.5% when compared to gold standard microscopy; the sensitivity increased with increasing parasite densities, achieving 95.8% when parasite density was higher than 5000 parasites/μL [18, 19]. However, in another study, CareStart™RDT showed better sensitivity (99.4%) and specificity (96.0%) while it did not show change in sensitivity with decreasing parasitaemia [20]. Unlike when compared to microscopy, RDTs showed lower sensitivity against PCR in a holo-endemic area where malaria transmission occurs throughout the year [19].
Despite CareStart™RDT detecting more true positive samples than microscopy in this study, there were also some false positive records. These false positives may be due to the persistence of the HRP2 antigen after treatment [21]. However, it has been shown that CareStart™RDT false positivity decreases quickly after successful treatment [20]. Moreover, it has been suggested that rheumatoid factor can produce false positive due to binding IgG [22]. Although the use of IgM is supposed to reduce the issue of rheumatoid factor cross-reactions [15], it is not known which type of antibody was used to coat CareStart™RDT.
Many other factors can affect the performance of RDTs. False-negative can be observed when parasites fail to express the target antigen (gene deletion) or when the parasite express a variant of the protein (polymorphism) which can affect the antigen–antibody binding. PfHRP2 gene sequence of parasites varies depending on the geographic locations [15]. In Senegal, until recently, PfHRP2 polymorphism did not affect the performance of HRP2-based RDTs [23] and no PfHRP2 gene deletion was observed. However, in Mali, a neighbouring country of Senegal, parasites lacking PfHRP2 have been reported to cause false-negative results [24] suggesting a need for a larger investigation.
Unlike PfHRP2, the pLDH protein does not persist in the blood after effective treatment with anti-malarial [20]. Thus, the protein has been proposed as a target for monitoring parasite responses to treatment and for predicting treatment failure [15, 20]. Moreover, the pLDH protein seems not to be subject of antigenic variation [15]. Taken together, pLDH-based RDTs should be an alternative approach in case of PfHRP2 gene deletion.
However, pLDH-based RDTs have several limitations. pLDH-based tests have decreased sensitivity at low parasitaemia [7]. The positivity rate of pLDH in this study was very low even at relatively high parasitaemia when compared to other studies [4]. The storage and transportation conditions could affect the performance of RDTs in field conditions. In fact, the quality of RDTs may show poor performances when they are exposed to heat and humidity [6]. pLDH is especially sensitive to those tropical conditions than HRP2 [7]. Therefore, quality control of procured RDTs is essential to minimize false negative pLDH results.
Conclusions
Although RDT usefulness in low transmission areas or in the detection of low parasite density infections is being questioned, CareStart™RDT showed good sensitivity comparable to that performed by expert microscopist. However, in the wake of reported HRP2 deletion in other countries, microscopy should always accompany the use of RDT. Combination of RDT and microscopy together with the evaluation of malaria RDTs over time should be a powerful tool for diagnosing malaria in endemic countries.
Abbreviations
- CT:
-
threshold cycle
- DNA:
-
deoxyribonucleic acid
- EDTA:
-
ethylenediaminetetraacetic acid
- ECA:
-
external competency assessment
- Ig:
-
immunoglobulin
- LDH:
-
lactate dehydrogenase
- NMCP:
-
National Malaria Control Programme
- NPV:
-
negative predictive value
- PET-PCR:
-
photo-induced electron transfer-polymerase chain reaction
- PfHRP:
-
Plasmodium falciparum histidine-rich protein
- pLDH:
-
Plasmodium lactate dehydrogenase
- PPV:
-
positive predictive value
- RDT:
-
rapid diagnostic test
- WHO:
-
World Health Organization
References
Pakalapati D, Garg S, Middha S, Kochar A, Subudhi AK, Arunachalam BP, et al. Comparative evaluation of microscopy, OptiMAL and 18S rRNA gene based multiplex PCR for detection of Plasmodium falciparum and Plasmodium vivax from field isolates of Bikaner, India. Asian Pac J Trop Med. 2013;6:346–51.
Makler MT, Palmer CJ, Ager AL. A review of practical techniques for the diagnosis of malaria. Ann Trop Med Parasitol. 1998;92:419–33.
Kobayashi T, Gamboa D, Ndiaye D, Cui L, Sutton PL, Vinetz JM. Malaria diagnosis across the International Centers of Excellence for malaria research: platforms, performance, and standardization. Am J Trop Med Hyg. 2015;93(3 Suppl):99–109.
Maltha J, Gillet P, Bottieau E, Cnops L, Van Esbroeck M, Jacobs J. Evaluation of a rapid diagnostic test (CareStart TM Malaria HRP-2/pLDH (Pf/pan) Combo Test) for the diagnosis of malaria in a reference setting. Malar J. 2010;9:171.
Batwala V, Magnussen P, Nuwaha F. Are rapid diagnostic tests more accurate in diagnosis of Plasmodium falciparum malaria compared to microscopy at rural health centres? Malar J. 2010;9:349.
Endeshaw T, Gebre T, Ngondi J, Graves PM, Shargie EB, Ejigsemahu Y, et al. Evaluation of light microscopy and rapid diagnostic test for the detection of malaria under operational field conditions: a household survey in Ethiopia. Malar J. 2008;7:118.
McMorrow ML, Aidoo M, Kachur SP. Malaria rapid diagnostic tests in elimination settings-can they find the last parasite? Clin Microbiol Infect. 2011;17:1624–31.
Giardina F, Gosoniu L, Konate L, Diouf MB, Perry R, Faye O, et al. Estimating the burden of malaria in Senegal: Bayesian zero-inflated binomial geostatistical modeling of the MIS 2008 data. PLoS ONE. 2012;7:e32625.
Machault V, Gadiaga L, Vignolles C, Jarjaval F, Bouzid S, Sokhna C, et al. Highly focused anopheline breeding sites and malaria transmission in Dakar. Malar J. 2009;8:138.
Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, et al. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS ONE. 2013;8:e56677.
Lucchi NW, Karell MA, Journel I, Rogier E, Goldman I, Ljolje D, et al. PET-PCR method for the molecular detection of malaria parasites in a national malaria surveillance study in Haiti, 2011. Malar J. 2014;13:162.
Abba K, Deeks J, Olliaro P, Naing C, Jackson S, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst Rev. 2011;12:CD011431.
Alareqi LMQ, Mahdy MAK, Lau YL, Fong MY, Ghani RA, Ali AA, et al. Field evaluation of a PfHRP-2/pLDH rapid diagnostic test and light microscopy for diagnosis and screening of falciparum malaria during the peak seasonal transmission in an endemic area in Yemen. Malar J. 2016;15:49.
Mahende C, Ngasala B, Lusingu J, Yong TS, Lushino P, Lemnge M, et al. Performance of rapid diagnostic test, blood film microscopy and PCR for the diagnosis of malaria infection among febrile children from Korogwe District, Tanzania. Malar J. 2016;15:391.
Mouatcho JC, Goldring JPD. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–505.
Xiaodong S, Tambo E, Chun W, Zhibin C, Yan D, Jian W, et al. Diagnostic performance of CareStart TM malaria HRP2/pLDH (Pf/pan) combo test versus standard microscopy on falciparum and vivax malaria between China–Myanmar endemic borders. Malar J. 2013;12:6.
Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, et al. Comparison of CareStartTM HRP2/pLDH COMBO rapid malaria test with light microscopy in north-west Ethiopia. Malar J. 2012;11:234.
Ashton RA, Kefyalew T, Tesfaye G, Counihan H, Yadeta D, Cundill B, et al. Performance of three multi-species rapid diagnostic tests for diagnosis of Plasmodium falciparum and Plasmodium vivax malaria in Oromia Regional State, Ethiopia. Malar J. 2010;9:297.
Wanja EW, Kuya N, Moranga C, Hickman M, Johnson JD, Moseti C, et al. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar J. 2016;15:456.
Gerstl S, Dunkley S, Mukhtar A, De Smet M, Baker S, Maikere J. Assessment of two malaria rapid diagnostic tests in children under 5 years of age, with follow- up of false-positive pLDH test results, in a hyperendemic falciparum malaria area, Sierra Leone. Malar J. 2010;9:28.
Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, Okia M, et al. Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malar J. 2008;7:202.
Stauffer W, Cartwright C, Olson D, Boulware D. Superior diagnostic performance of malaria tests as compared to blood smears in U.S. clinical practice. Clin Infect Dis. 2009;49:908–13.
Deme AB, Park DJ, Bei AK, Sarr O, Badiane AS, Gueye PEHO, et al. Analysis of pfhrp2 genetic diversity in Senegal and implications for use of rapid diagnostic tests. Malar J. 2014;13:34.
Koita OA, Doumbo OK, Ouattara A, Tall LK, Konaré A, Diakité M, et al. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am J Trop Med Hyg. 2012;86:194–8.
Authors’ contributions
MAD, ASB and KD performed the microscopy examinations. MAD drafted the manuscript; AG collected blood samples and performed the RDT tests. AD performed the PET-PCR assay; ASB, KD and DN critically commented on the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Naomi Lucchi for reviewing the English in this manuscript. We are thankful to Younouss Diedhiou and Amadou Mactar Mbaye for their help to collect and transport samples.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Consent for publication
Written informed consent was obtained from all participants for publication.
Ethics approval
The study received ethical clearance from the Ethic Committee of the Senegalese Ministry of Health. Informed consent was obtained from all participants.
Funding
MAD was funded by Laboratory of Parasitology-Mycology/Cheikh Anta Diop University (UCAD).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Diallo, M.A., Diongue, K., Ndiaye, M. et al. Evaluation of CareStart™ Malaria HRP2/pLDH (Pf/pan) Combo Test in a malaria low transmission region of Senegal. Malar J 16, 328 (2017). https://doi.org/10.1186/s12936-017-1980-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-017-1980-z