Abstract
Background
Recently published data suggest that artemisinin derivatives and synthetic peroxides, such as the ozonides OZ277 and OZ439, have a similar mode of action. Here the cross-resistance of OZ277 and OZ439 and four additional next-generation ozonides was probed against the artemisinin-resistant clinical isolate Plasmodium falciparum Cam3.I, which carries the K13-propeller mutation R539T (Cam3.IR539T).
Methods
The previously described in vitro ring-stage survival assay (RSA0–3h) was employed and a simplified variation of the original protocol was developed.
Results
At the pharmacologically relevant concentration of 700 nM, all six ozonides were highly effective against the dihydroartemisinin-resistant P. falciparum Cam3.IR539T parasites, showing a per cent survival ranging from <0.01 to 1.83%. A simplified version of the original RSA0–3h method was developed and gave similar results, thus providing a practical drug discovery tool for further optimization of next-generation anti-malarial peroxides.
Conclusion
The absence of in vitro cross-resistance against the artemisinin-resistant clinical isolate Cam3.IR539T suggests that ozonides could be effective against artemisinin-resistant P. falciparum. How this will translate to the human situation in clinical settings remains to be investigated.
Similar content being viewed by others
Background
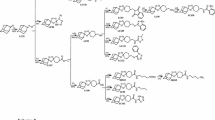
Malaria is one of the most important tropical diseases resulting in 214 million new cases and an estimated 438,000 malaria deaths worldwide in 2015 [1]. The discovery of artemisinin in the 1970s was an important step forward in anti-malarial drug therapy and was recognized with the Nobel Prize in Physiology or Medicine in 2015 [2, 3]. Artemisinin and its semi-synthetic derivatives, such as dihydroartemisinin (DHA) (Fig. 1), artesunate and artemether, contain a unique sesquiterpene lactone peroxide (1,2,4-trioxane) structure and artemisinin-based combination therapy (ACT) represents the current first-line treatment of uncomplicated Plasmodium falciparum malaria [4–6]. Since the starting material artemisinin is a natural product, its production is limited to the availability of the plant [4, 7], although several total syntheses of artemisinin have been described [8]. In 2004, Vennerstrom et al. reported the discovery of a completely synthetic peroxide anti-malarial containing a 1,2,4-trioxolane (ozonide) pharmacophore named OZ277 (arterolane) (Fig. 1) with anti-malarial activity comparable to the artemisinin derivatives [9, 10]. In combination with piperaquine, arterolane was registered for anti-malarial combination therapy in India in 2011 [11–14]. The next-generation ozonide, OZ439 (artefenomel) (Fig. 1), exhibits an increased pharmacokinetic half-life and good safety profile and is now being tested in phase IIb clinical trials [12, 14–17].
The iron-dependent alkylation hypothesis is one of the proposed modes of action of artemisinin and synthetic peroxides [18–21] where the peroxide is thought to be activated by the reductive cleavage in the presence of ferrous haem (or free Fe(II) derived from haem) released as a by-product of haemoglobin digestion in the food vacuole [20, 22–27]. Thereby carbon-centred radicals are generated, which then alkylate haem and parasite proteins [28–33]. The interaction of the artemisinin derivatives or ozonides with parasite targets is irreversible [31, 34]. Although the semi-synthetic artemisinins are highly effective, prolonged parasite clearance times were first reported along the Thai–Cambodian border in 2006, suggesting an emerging artemisinin resistance phenotype [35]. Today, delayed parasite clearance following treatment with artemisinin derivatives has been observed across Southeast Asia [36–41]. It was found that mutations in the Kelch 13 propeller domain are associated with ring-stage parasites entering a quiescent state with delayed parasite clearance after exposure to artemisinins [41–45]. When 50% inhibitory concentrations (IC50) were measured using conventional methods such as the [3H] hypoxanthine incorporation assay [46], no difference was observed between artemisinin-resistant and -susceptible strains after treatment with artemisinin or its derivatives [47–50]. In an effort to correlate the delayed parasite clearance observed in vivo with in vitro parasite survival, Witkowski et al. [48, 49] developed a ring-stage survival assay (RSA0–3h) that exploited the differences in susceptibility observed between wild-type and K13 mutants at the early ring stage of the asexual blood cycle following a short pulse of artemisinin treatment. In the RSA0–3h, synchronized young ring stage parasites (0–3 h old) are exposed to drugs for 6 h, and then cultured in drug free culture medium for 66 h before relative growth is determined by microscopic analysis [48, 49]. Since the structural analogies between artemisinins and ozonides (Fig. 1) suggest that they share similar modes of action, and thus some level of cross resistance [9, 10, 51, 52], the per cent survival of an artemisinin-resistant clinical isolate (Cam3.IR539T) treated with DHA, OZ277, OZ439, and four additional next-generation ozonides (Fig. 1) using the RSA0–3h as described by Witkowski et al. [48, 49] was evaluated. Additionally, a sub-set of these compounds was tested in the RSA0–3h described by Xie et al. [53] that also uses tightly synchronized ring-stage cultures, but allows the assay to be performed routinely within a convenient time-frame.
Methods
Parasite cultivation
The artemisinin-resistant P. falciparum isolate Cam3.IR539T from Battambang, Cambodia was obtained from BEI Resources [54] with the accession number MRA-1240. The drug-sensitive P. falciparum strain NF54 (airport strain from The Netherlands) was provided by F. Hoffmann-La Roche Ltd. Parasites were cultivated in standard cultivation medium, consisting of hypoxanthine (50 mg/l), RPMI (10.44 g/l) supplemented with HEPES (5.94 g/l), albumax (5 g/l), sodium bicarbonate (2.1 g/l) and neomycin (100 mg/l) [55].
Ring-stage survival assays (RSA0–3h)
Ring-stage survival assays (RSA0–3h) were carried out essentially as previously described by Witkowski et al. [48], but with a few modifications in the drug-washing procedure to ensure that no residual peroxide was present during the 66-h post-treatment period [56]. Briefly, zero to 3 h post-invasion ring stages were adjusted to 1% parasitaemia and 2.5% haematocrit by adding uninfected erythrocytes, transferred in a total volume of 1 ml into 48-well plates and exposed for 6 h to a range of concentrations (700, 350, 175, 88, and 49 nM) of DHA or one of the six ozonides tested in this study. The synthesis of the four next-generation ozonides, OZ493, OZ609, OZ655 and OZ657, will be reported in due course by the laboratory of Prof. Jonathan Vennerstrom (pers. comm.). After 6 h, cultures were transferred to 15 ml conical tubes, centrifuged at 1400 rpm (400g) for 2 min and carefully washed two times with 12 ml of culture medium. The complete removal of compound after washing was verified by incubating the supernatant recovered after the last washing step with fresh cultures of NF54 parasites, ensuring that no growth inhibition was detected. After washing, blood pellets were resuspended in complete drug-free culture medium, transferred into new wells and cultured for 66 h under standard conditions.
Thin blood smears were prepared, methanol-fixed and stained with 10% Giemsa. Per cent survival was assessed using light microscopy, counting the number of parasitized cells in ≥10,000 red blood cells (RBCs) and comparing survival to that of the drug-free dimethylsulfoxide incubation. Microscopy analysis was performed independently by two microscopists, one having more than 15 years of work experience.
Alternative parasite synchronization method
Parasites were synchronized according to Xie et al. [53] with 5% D-sorbitol. After 30 and 43 h, parasites were synchronized a second and third time, respectively, resulting in zero to 1-h old ring-stage parasites. The RSA0–3h was initiated 2 h later.
Standard [3H] hypoxanthine incorporation assay
The in vitro anti-malarial activity was measured using the [3H]-hypoxanthine incorporation assay [55]. Results were expressed as the concentration resulting in 50% inhibition (IC50).
Results
The per cent survival of parasites exposed to a concentration range of DHA and six different ozonides (Fig. 1) was determined using the artemisinin-resistant P. falciparum Cambodian isolate Cam3.IR539T. As expected, DHA exposure gave a high survival rate ranging from 74 to 33% at concentrations of 49 and 700 nM, respectively (Fig. 2), which is comparable to the observed survival value of 40% at 700 nM published previously [44]. In contrast, when tested at 700 nM, the two ozonides OZ277 and OZ439 showed an approximate 18- to 45-fold increase in potency compared with DHA (Fig. 2). Full and equal potency was observed when DHA, OZ277 and OZ439 were tested in parallel in the RSA0–3h using the artemisinin-sensitive strain NF54 (Additional file 1: Table S1). At the lowest concentration (49 nM), OZ277 had poor activity, showing a similar per cent survival to that of DHA, whereas OZ439 was still about fivefold more potent. A possible explanation for OZ439 being more potent than OZ277 could be related to its improved stability in blood as previously described [15]. In those studies, OZ277 or OZ439 were incubated at 37 °C in P. falciparum-infected human blood. After 2 h more than 90% of OZ277 was degraded, whereas OZ439 was found to be about 10–20× more stable. A similar and more recent study found similar differences in stability for OZ277 and OZ439 [56]. The same compounds were also tested in a more convenient variation of the standard RSA0–3h that uses synchronized ring-stage cultures that can be easily produced during normal working hours [53]. As shown in Table 1, this alternative synchronization method gave results that were comparable to those obtained using the standard RSA0–3h.
To investigate further the level of cross-resistance between DHA and the ozonides, four additional next-generation ozonides (OZ493, OZ609, OZ655, OZ657) (Fig. 1) were tested against the Cam3.IR539T parasites. While all six ozonides had a similar IC50 value using a conventional 72-h [3H] hypoxanthine incorporation assay (Additional file 1: Table S2), the RSA0–3h showed that OZ493, OZ609 and OZ655 were highly potent and completely inhibited the growth of the artemisinin-resistant isolate at the two highest concentrations tested (Fig. 2). At the lowest concentration, potency was comparable to that for OZ439. The overall potency of OZ657 was comparable to that of OZ277.
The RSA0–3h was recently developed to provide an in vitro correlate of the longer in vivo parasite clearance times observed after artemisinin treatment in Southeast Asia, which is widely interpreted as a sign of potential artemisinin resistance [57, 58]. Provided that the RSA0–3h does indeed predict the potency of compounds against artemisinin-resistant parasites in malaria patients, the here described data suggest that all of the tested ozonides are highly potent against isolates such as P. falciparum Cam3.IR539T. These data are in line with the recent clinical observation that the parasite clearance rate following OZ439 treatment is not significantly affected by resistance-associated mutations in the Kelch 13 propeller region [17] and the recent data published by Siriwardana et al. [59], which showed no reduced susceptibility of OZ439 in a different delayed clearance phenotype parasite (Cam3.II) in vitro.
Conclusion
In the traditional RSA0–3h, as well as a more convenient variation of the original method, all of the tested ozonides, were highly potent against the artemisinin-resistant isolate P. falciparum Cam3.IR539T in contrast to results for DHA. These data indicate that artemisinin-resistant P. falciparum infections could be successfully treated with ozonide anti-malarial drugs.
Abbreviations
- OZ277:
-
arterolane
- OZ439:
-
artefenomel
- DHA:
-
dihydroartemisinin
- ACT:
-
artemisinin combination therapy
- RBC:
-
red blood cell
References
WHO. World Malaria Report 2015. Geneva: World Health Organization; 2015.
Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217–20.
Su XZ, Miller LH. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci China Life Sci. 2015;58:1175–9.
Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–55.
White NJ. Artemisinin: current status. Trans R Soc Trop Med Hyg. 1994;88:3–4.
White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–4.
Kumar S, Srivastava S. Establishment of artemisinin combination therapy as first line treatment for combating malaria: Artemisia annua cultivation in India needed for providing sustainable supply chain of artemisinin. Curr Sci. 2005;89:1097–102.
Avery MA, Chong WKM, Jennings- White C. Stereoselective total synthesis of (+)- artemisinin, the antimalarial constituent of Artemisia annua L. J Am Chem Soc. 1992;114:974–9.
Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FC, Chollet J, et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–4.
Tang Y, Dong Y, Vennerstrom JL. Synthetic peroxides as antimalarials. Med Res Rev. 2004;24:425–48.
Anthony MP, Burrows JN, Duparc S, Moehrle JJ, Wells TN. The global pipeline of new medicines for the control and elimination of malaria. Malar J. 2012;11:316.
Mäser P, Wittlin S, Rottmann M, Wenzler T, Kaiser M, Brun R. Antiparasitic agents: new drugs on the horizon. Curr Opin Pharmacol. 2012;12:562–6.
Patil CY, Katare SS, Baig MS, Doifode SM. Fixed dose combination of arterolane and piperaquine: a newer prospect in antimalarial therapy. Ann Med Health Sci Res. 2014;4:466–71.
Wells TN, van Huijsduijnen RH, Van Voorhis WC. Malaria medicines: a glass half full? Nat Rev Drug Discov. 2015;14:424–42.
Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci USA. 2011;108:4400–5.
Moehrle JJ, Duparc S, Siethoff C, van Giersbergen PL, Craft JC, Arbe-Barnes S, et al. First-in-man safety and pharmacokinetics of synthetic ozonide OZ439 demonstrates an improved exposure profile relative to other peroxide antimalarials. Br J Clin Pharmacol. 2013;75:535–48.
Phyo AP, Jittamala P, Nosten FH, Pukrittayakamee S, Imwong M, White NJ, et al. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect Dis. 2016;16:61–9.
Kaiser M, Wittlin S, Nehrbass-Stuedli A, Dong Y, Wang X, Hemphill A, et al. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160). Antimicrob Agents Chemother. 2007;51:2991–3.
Creek DJ, Charman WN, Chiu FCK, Prankerd RJ, Dong Y, Vennerstrom JL, et al. Relationship between antimalarial activity and haem alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob Agents Chemother. 2008;52:1291–6.
Meunier B, Robert A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc Chem Res. 2010;43:1444–51.
Tilley L, Straimer J, Gnädig NF, Ralph SA, Fidock DA. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol. 2016;32:682–96.
Klonis N, Crespo-Ortiz MP, Bottova I, Abu-Bakar N, Kenny S, Rosenthal PJ, Tilley L. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci USA. 2011;108:11405–10.
Crespo MD, Avery TD, Hanssen E, Fox E, Robinson TV, Valente P, Taylor DK, Tilley L. Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology. Antimicrob Agents Chemother. 2008;52:98–109.
Hartwig CL, Rosenthal AS, D’Angelo J, Griffin CE, Posner GH, Cooper RA. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem Pharmacol. 2009;77:322–36.
Robert A, Claparols C, Witkowski B, Benoit-Vical F. Correlation between Plasmodium yoelii nigeriensis susceptibility to artemisinin and alkylation of heme by the drug. Antimicrob Agents Chemother. 2013;57:3998–4000.
Klonis N, Creek DJ, Tilley L. Iron and heme metabolism in Plasmodium falciparum and the mechanism of action of artemisinins. Curr Opin Microbiol. 2013;16:722–7.
Uhlemann AC, Wittlin S, Matile H, Bustamante LY, Krishna S. Mechanism of antimalarial action of the synthetic trioxolane RBX11160 (OZ277). Antimicrob Agents Chemother. 2007;51:667–72.
Asawamahasakda W, Ittarat I, Pu YM, Ziffer H, Meshnick SR. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–8.
Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–60.
Tang Y, Dong Y, Wang X, Sriraghavan K, Wood JK, Vennerstrom JL. Dispiro-1,2,4-trioxane analogs of a prototype dispiro-1,2,4-trioxolane: mechanistic comparators for artemisinin in the context of reaction pathways with iron (II). J Org Chem. 2005;70:5103–10.
Fügi MA, Wittlin S, Dong Y, Vennerstrom JL. Probing the antimalarial mechanism of artemisinin and OZ277 (arterolane) with nonperoxidic isosteres and nitroxyl radicals. Antimicrob Agents Chemother. 2010;54:1042–6.
Hartwig CL, Lauterwasser EMW, Mahajan SS, Hoke JM, Cooper RA, Renslo AR. Investigating the antimalarial action of 1,2,4-trioxolanes with fluorescent chemical probes. J Med Chem. 2011;54:8207–13.
Tilley L, Charman S, Vennerstrom JL. Semisynthetic artemisinin and synthetic peroxide antimalarials. In: Palmer MJ, Wells TNC, editors. RSC Drug Discovery Series No. 14. London: Neglected Diseases and Drug Discovery; 2011. p. 33–64.
Abiodun OO, Brun R, Wittlin S. In vitro interaction of artemisinin derivatives or the fully synthetic peroxidic anti-malarial OZ277 with thapsigargin in Plasmodium falciparum strains. Malar J. 2013;12:43.
Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. Artemisinin resistance in Cambodia 1 (ARC1) study consortium evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–20.
Noedl H, Socheat D, Satimai W. Artemisinin-resistant malaria in Asia. N Engl J Med. 2009;361:540–1.
Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;38:455–67.
Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–8.
Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–6.
Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23.
Paloque L, Ramadani AP, Mercereau-Puijalon O, Augereau JM, Benoit-Vical F. Plasmodium falciparum: multifaceted resistance to artemisinins. Malar J. 2016;15:149.
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5.
Mok S, Ashley EA, Ferreira PE, Zhu L, Lin Z, Yeo T, et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–5.
Straimer J, Gnädig NF, Witkowski B, Amaratunga C, Duru V, Ramadani AP, et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347:428–31.
Mbengue A, Bhattacharjee S, Pandharkar T, Liu H, Estiu G, Stahelin RV, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–7.
Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–8.
Witkowski B, Lelièvre J, Barragán MJ, Laurent V, Su XZ, Berry A, Benoit-Vical F. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–7.
Witkowski B, Khim N, Chim P, Kim S, Ke S, Kloeung N, et al. Reduced artemisinin susceptibility of Plasmodium falciparum ring stages in western Cambodia. Antimicrob Agents Chemother. 2013;57:914–23.
Witkowski B, Amaratunga C, Khim N, Sreng S, Chim P, Kim S, et al. Novel phenotypic assays for the detection of artemisinin-resistant Plasmodium falciparum malaria in Cambodia: in vitro and ex vivo drug-response studies. Lancet Infect Dis. 2013;13:1043–9.
Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis. 2010;202:1362–8.
Jourdan J, Matile H, Reift E, Biehlmaier O, Dong Y, Wang X, et al. Monoclonal antibodies that recognize the alkylation signature of antimalarial ozonides OZ277 (Arterolane) and OZ439 (Artefenomel). ACS Infect Dis. 2015;2:54–61.
Ismail HM, Barton VE, Panchana M, Charoensutthivarakul S, Biagini GA, Ward SA, et al. A click chemistry-based proteomic approach reveals that 1,2,4-trioxolane and artemisinin antimalarials share a common protein alkylation profile. Angew Chem Int Ed Engl. 2016;55:1–6.
Xie S, Dogovski C, Kenny S, Tilley L, Klonis N. Optimal assay design for determining the in vitro sensitivity of ring stage Plasmodium falciparum to artemisinins. Int J Parasitol. 2014;44:893–9.
B E I Resources. https://www.beiresources.org/Catalog/BEIParasiticProtozoa/MRA-1240.aspx. Accessed 26 Nov 2014.
Snyder C, Chollet J, Santo-Tomas J, Scheurer C, Wittlin S. In vitro and in vivo interaction of synthetic peroxide RBx11160 (OZ277) with piperaquine in Plasmodium models. Exp Parasitol. 2007;115:296–300.
Yang T, Xie SC, Cao P, Giannangelo C, McCaw J, Creek DJ, Charman SA, Klonis N, Tilley L. Comparison of the exposure time dependence of the activities of synthetic ozonide antimalarials and dihydroartemisinin against K13 wild-type and mutant Plasmodium falciparum strains. Antimicrob Agents Chemother. 2016;60:4501–10.
Phyo AP, Ashley EA, Anderson TJ, Bozdech Z, Carrara VI, Sriprawat K, et al. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis. 2016;63:784–91.
Meshnick SR. Artemisinin resistance in Southeast Asia. Clin Infect Dis. 2016;63:1527.
Siriwardana A, Iyengar K, Roepe PD. Endoperoxide drug cross resistance patterns for Plasmodium falciparum exhibiting an artemisinin delayed clearance phenotype. Antimicrob Agents Chemother. 2016;60:6952–6.
Authors’ contributions
FB, JJ, CS, BB, BC, PM and SW designed the research. FB and CS performed the research. All authors analysed data. FB, JJ and SW wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are grateful to Jonathan L Vennerstrom, Hugues Matile and Susan A Charman for critically reading the manuscript and making valuable suggestions.
Competing interests
The use of OZ277 and OZ439 against malaria has been patented.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Funding
This work was financially supported by the Swiss National Science Foundation (Grant 310030_149896 to SW), the Medicines for Malaria Venture, and the Swiss Tropical and Public Health Institute.
Author information
Authors and Affiliations
Corresponding author
Additional files
12936_2017_1696_MOESM1_ESM.docx
Additional file 1: Table S1. Mean per cent survival (individual values in brackets) of NF54 after 6 h exposure to 500 nM of DHA, OZ439 or OZ277 using the synchronization protocol from Straimer et al. [44]. Table S2. Mean in vitro IC50 values (single values in brackets) for Plasmodium falciparum isolate Cam3.IR539T and NF54 in the 72-h [3H] hypoxanthine assay.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Baumgärtner, F., Jourdan, J., Scheurer, C. et al. In vitro activity of anti-malarial ozonides against an artemisinin-resistant isolate. Malar J 16, 45 (2017). https://doi.org/10.1186/s12936-017-1696-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-017-1696-0