Abstract
Background
Internally displaced persons (IDP) represent vulnerable populations whose public health conditions merit special attention. In the China–Myanmar border area, human movement and resettlements of IDP can influence malaria transmission. Comparison of disease incidence and vector densities between IDP camps and surrounding local villages allows for better understanding of current epidemiology and to evaluate the effectiveness of interventions in the region.
Methods
Malaria and vector surveillance was conducted in three IDP camps and three local villages neighbouring the camps along the China–Myanmar border in Myanmar. Clinical malaria cases were collected from seven hospitals/clinics from April 2011 to December 2014. Malaria vector population dynamics were monitored using CDC light traps. The use of malaria preventive measures and information on aid agencies and their activities was obtained through questionnaire surveys.
Results
Malaria was confirmed in 1832 patients. Of these cases, 85.4 % were Plasmodium vivax and 11.4 % were Plasmodium falciparum malaria. Annual malaria incidence rates were 38.8 and 127.0 cases/1000 person year in IDP camps and local villages, respectively. Older children of 5–14 years had the highest incidence rate in the camps regardless of gender, while male adults had significantly higher incidence rates than females in local villages and females child-bearing age had significantly lower risk to malaria in IDP camps compare to local villages. Seasonal malaria outbreaks were observed both in the IDP camps and in the local villages from May to August 2013. The proportion of P. vivax remained unchanged in local villages but increased by approximately tenfold in IDP camps from 2011 to 2014. Anopheles vector density was tenfold higher in local villages compared to IDP camps (2.0:0.2 females/trap/night). Over 99 % of households in both communities owned bed nets. While long-lasting insecticidal nets accounted for 61 % of nets used in IDPs, nearly all residents of local villages owned regular nets without insecticide-impregnation. There were more active aid agencies in the camps than in local villages.
Conclusion
Malaria in IDP camps was significantly lower than the surrounding villages through effective control management. The observation of P. vivax outbreaks in the study area highlights the need for increased control efforts. Expansion of malaria intervention strategies in IDP camps to local surrounding villages is critical to malaria control in the border area.
Similar content being viewed by others
Background
Global efforts to eliminate and eradicate malaria have highlighted the need to target control efforts in international border regions endemic for to malaria [1]. In addition to cross-border migrants seeking economic opportunities, settlements for refugees and internally displaced person (IDP) along international borders as a result of internal conflicts can influence malaria transmission [2]. Refugees and IDPs generally settle in over-crowded suboptimal living conditions, placing them at an increased risk of infectious diseases, particularly water-borne enteric disease and vector-borne disease [3–9]. Disease morbidity in camps and settlements can be greatly reduced if appropriate preventive and treatment measures are implemented in a timely manner [10–17].
Malaria remains one of the most concerning infectious diseases among displaced populations [4, 5, 18–22]. In Southeast Asia, malaria is a significant problem in Myanmar as well as its neighbouring countries through border migrants and refugees [14, 23–26]. Myanmar has the highest malaria burden among other Southeast Asian countries with approximately 200,000 cases per year [26, 27]. Large-scale human movement has led to intensive transmission of malaria in the IDP settlement along the Myanmar–Thailand border [28–30]. Along the China–Myanmar border, despite high malaria incidence in the surrounding villages in Myanmar, malaria in IDPs as well as the impact of human movement on malaria transmission are unclear [25, 31].
Following the conflict between the Kachin Independence Army (KIA) of the Kachin State and the Myanmar government armed forces in 2011, hundreds of thousands of civilians have fled their homes and moved to the IDP camps along the China–Myanmar border. These IDP camps, established in July 2011, were administered by the United Nations High Commissioner for Refugees (UNHCR) with assistance from several non-governmental organizations (NGOs). While there have been extensive studies on malaria epidemiology and vector ecology in the local area [25, 31–40], the malaria situation and its long-term impact on public health in the IDP camps along the China–Myanmar border remains largely unknown.
To assess the burden of malaria in the China–Myanmar border area, clinical malaria was monitored prospectively, clinical malaria incidence rates were compared between IDP camps and local villages surrounding the IDP camps. Malaria vector population was monitored, and malaria control and prevention measures in both the IDP camps and local villages were investigated with the goal to explain differences in malaria incidence rates.
Methods
Study population
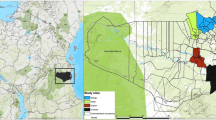
The study was initiated in April 2011 in the China–Myanmar border area of the Kachin state, Myanmar, as part of the International Centers of Excellence for Malaria Research (ICEMR) Southeast Asia project [25, 31]. Shortly thereafter, civil war prompted fleeing populations to resettle in camps by the border areas. In July–August 2011, about 1 month after the camps were established, study areas were expanded to include three IDP camps, located approximately 1.5–4 km away (Figs. 1, 2). By August 2012, catchment population sizes were approximately 11,000 and 1200 in the three IDP camps and four local villages, respectively (Additional file 1). The seven sites are the only residential areas (except Laiza town) on the Myanmar side along the Myanmar–China border, they are not randomly selected sites (Fig. 1), and clinical malaria incidence rate is very low on the China side of the border area [25].
All study sites are located in the same valley area with an elevation between 240 and 280 m above sea level, they have similar landscape and ecological settings [39]. All IDP camps have clinics located within them that provide routine diagnosis and treatment to address the population’s health needs free of charge. The neighbouring study villages have no health clinics and villagers need to travel to nearby health care facilities to seek treatment, usually in Laiza town or less commonly the small military clinic (between Mung Seng Yang village and Ja Htu Kawng village on Fig. 1) before the establishment of IDP camps (Fig. 1). Clinics in IDP camps also provide free diagnosis and treatment for local villagers should they come.
Clinical malaria surveillance
Passive case surveillance (PCS) was conducted prospectively at all IDP camp clinics and at all hospitals in the Laiza area where residents from the local villages sought treatment. All patients are traced back to the village/camp where they are originally from based on clinical case report and questionnaire surveys. Final data analysis was restricted to consenting PCS cases residing in the study camps and villages (note: in this study, all patients with malaria symptoms were asked to sign the consent form before been recruited, all of them have actually signed the consent form). After obtained consents or ascents (for minors <18 years) from the patients or parents/guardians, finger-prick blood samples were obtained from individuals who had malaria-like symptoms, and thick and thin smears were prepared to identify malaria parasites, parasite density and species. Clinical malaria was defined as fever (axillary temperature ≥37.5 °C), chills, severe malaise, and headache or vomiting at the time of examination, or 1–2 days prior to the examination, with a Plasmodium positive blood smear [25, 41]. Malaria parasites were identified microscopically by two experienced technicians. For quality control purposes, approximately 5 % of the slides were randomly selected for parasite identification by a third microscopist. Individuals found positive for malaria infection and having malaria symptoms were treated by local doctors the same day when possible, if not, the following day according to the World Health Organization guidelines, i.e., chloroquine for the treatment of vivax malaria, dihydroartemisinin–piperaquine for the treatment of uncomplicated falciparum malaria and quinine for falciparum with complications [36, 37]. Primaquine 8-day treatment (0.375 mg/kg/day) was given as radical treatment for vivax [37], however, primaquine single dose was not given as a gametocytocidal for falciparum malaria [36]. Both local villages and IDP camps used the same treatment regimens. However, primaquine treatment of vivax was supervised for patients from IDP camps but not for local villagers due to the difficulties for follow-ups.
Case report forms were administrated to collect the following information from patients: demographic characteristics, occupation, education level, clinical symptoms, history of malaria in the preceding 12 months, history of travel within the 2 weeks preceding the clinic visit, history of fever (days with fever before seeking treatment), and use of measures to prevent malaria. Patient reported revisits of the same case or clinical case follow-up was recorded as one case, however, relapse or recrudescent cases were not confirmed in laboratory.
Malaria vector population sampling
Longitudinal adult mosquito surveys were initiated in April 2012 in two villages and one IDP camp. Twice a month, at least 36 houses were systematically selected at each site (camp or village) to maximize the coverage for alternate monthly adult mosquito sampling. On average, 147 trap-nights were conducted in each site each month. Mosquitoes were collected using unbaited CDC light traps. Anopheles species were morphologically identified. Mosquitoes were pooled monthly and mosquito density was calculated as Anopheles females per trap per night (f/t/n).
All potential habitats were identified through thoroughly search over the study area. Mosquito larvae were surveyed using a standard dipper (size of 350 mL). Water was dipped up to 20 times. When a habitat was too small to make 20 dips, water was dipped as many times as possible. Tire tracks, hoof prints and container habitats were not sampled. A subset of larvae was further analysed with the ribosomal DNA (rDNA) polymerase chain reaction (PCR) method [39]. Larvae density was calculated as Anopheles larvae per dip per day (l/d/day). Larval sampling was conducted monthly during major malaria transmission seasons, i.e., from April to August, 2012 at all sites and from May to August, 2014 in IDP camps only.
Malaria preventive questionnaire survey
At each site, we conducted a baseline census in August 2011, followed by 2–3 subsequent surveys per year to update demographic data. In 2013, after obtaining written informed consent, 100 households (519 individuals) from the villages and 300 households (1629 individuals) from the camps were randomly selected and interviewed to gather information on the usage of malaria preventive measures, including bed net ownership, type, number, and usage, indoor residual spray, and travel history during the previous 2 weeks. The sample size was chosen to maximize the representatives of the study populations.
Aid agencies play an important role in IDP camps, providing various supplies and services, as well as disease prevention measures such as indoor residual spraying (IRS) and insecticide-treated bed nets (ITNs). NGO and local government supported projects/services in IDP camps and local villages were investigated in November 2013 through questionnaire surveys. Services were tracked back to 2011. At each site, we interviewed five people, including village/agency staff/head/manager or school head/teacher, and asked them to complete the questionnaire to the best of their knowledge.
Statistical analysis
Malaria incidence rate was calculated as the number of clinical episodes per 1000 person-years (or months). Population size used to determine incidence rate was based on the 11 demographic surveys, and assumed a constant rate of change in population size between surveys. Statistical significance of differences in monthly malaria incidence rates and vector densities between the IDP camps and the local villages were assessed using a one-way ANOVA post hoc Tukey HSD test with repeated measures. Age- and sex-specific incidence rates were compared between IDP camps and local villages using χ2 test and odds ratio was calculated.
Scientific and ethical statement
Scientific and ethical clearance was obtained from the ethical review boards of Kunming Medical University, China (IRB # KMC2011-01); University of California, Irvine (IRB HS # 2012-9123) and Pennsylvania State University (IRB # 34319), USA. Written informed consent/assent (for minors under age 18) for study participation was obtained from all consenting heads of households and each individual who was willing to participate in the study.
Results
Malaria incidence rate
During the survey period, there were 1462 confirmed malaria cases in the IDP camps and 441 cases in the local villages. The annual clinical malaria incidence rate in the villages was on average 127 cases per 1000 person-years, which was significantly higher than that in the camps (38.8 cases per 1000 person-years, adjusted relative risk ratio 3.9, 95 % CI [1.9, 15.9], P < 0.0001) (Table 1). In the local villages, 75.1 % of the confirmed cases were P. vivax, 22.9 % P. falciparum, and 1.8 % mixed infections of P. falciparum and P. vivax, while in the IDP camps, 90.4 % were P. vivax, 7.9 % P. falciparum, and 1.4 % mixed infections. In contrast to villages, the IDP camps exhibited a fourfold higher P. vivax infection incidence than P. falciparum (odds ratio OR = 3.9, 95 % CI [2.9, 5.2], P < 0.0001) (Table 2). Plasmodium malariae (n = 5) and Plasmodium ovale (n = 1) infections were uncommon.
The monthly dynamics of malaria incidence rates varied significantly between the IDP camps and local villages over time (Fig. 3). Clinical malaria incidence rates in the camps were low in the first 21 months after the camps were established, with an average monthly incidence rate of 1.7 cases per 1000 person-years (P. falciparum 21.2 % and P. vivax 71.6 %) (Table 2). Between May and August 2013, the IDP camps experienced an epidemic with an overall monthly incidence rate of 16.3 cases per 1000 person-years (P. falciparum 4.5 % and P. vivax 95.3 %) (Table 2), tenfold higher compared to previous years (Fig. 3), and the increase in P. vivax was more pronounced than that in P. falciparum (Table 2). Subsequently, it dropped to an average monthly incidence of 2.9 cases per 1000 person-years from September 2013 to the end of 2014. By contrast, the local villages consistently exhibited high malaria incidence rates during the months of May–August every year, and the peak incidence rates varied from year to year with the highest in 2013 (Fig. 3). A large proportion of the malaria cases in the IDP camps was due to P. vivax, and the ratio of P. vivax and P. falciparum cases increased over time, from 2.1 in 2011 to 24.5 in 2014. On the contrary, the ratio of P. vivax and P. falciparum cases remained constant in the villages over the four years (Table 2).
Males had significantly higher malaria incidence rates than females in the local villages, but not in the IDP camps (Table 1). In IDP camps, older children (5–14 years) had the highest malaria incidence rate among all age groups; while adults (≥15 years) had the highest incidence rate in local villages (Table 1). Overall, women of child-bearing age (15–45 years) were at considerably higher risk of malaria compared to other females. There was slight difference in age- and gender-specific incidence rates between P. falciparum and P. vivax (Additional file 2). For example, for P. falciparum malaria, male adults were the most vulnerable group in both IDP camps and local villages, while gender was not a risk factor for vivax malaria in IDP camps (Additional file 2). Prompt diagnostic and treatment of P. falciparum clinical cases is the main factor that influences transmission. In local villages, the mean days with malaria symptoms before seeking treatment for uncomplicated falciparum malaria was 3.22 d (95 % CI 3.22 ± 0.63, median 3, range 1–10 days), which is not significantly different from that in IDP camps (mean 3.00 ± 0.25, median 3, range 1–7 days) (two-samples t test assuming unequal variances t = 0.66, df = 46, P = 0.26).
Malaria vector population density
The predominant vector species was Anopheles minimus (85 % in local villages and 81 % in IDP camps). Other major vector species included Anopheles maculatus s.l. (3.4 %), Anopheles culicifacies s.l. (2.8 %), Anopheles jeyporiensis (1.8), Anopheles vagus (1.4 %) and Anopheles sinensis (1.0 %), and eight other malaria vector species with <1 % to total vector population. On average, Anopheles adult density was tenfold higher in local villages than in the IDP camps (2.00:0.18 f/t/n, Tukey HSD test P < 0.001). The vector density in both IDP camps and local villages decreased from 2012 to 2014 (Fig. 4). In IDP camps, vector density decreased about fivefold from 0.37 f/t/n in 2012 to 0.14 f/t/n in 2013 and 0.08 f/t/n in 2014. However, the decrease in vector densities was not statistically significant in both settings (ANOVA with repeated measure, for IDP camps, F 2,30 = 1.51, P = 0.23; for local villages, F 2,30 = 0.82, P = 0.45).
Larval survey indicated that there was no significant difference in average number of breeding habitats (18.0:18.5), habitat infestation rate (34.7%:36.1 %) and mean larval density (0.19:0.32 larvae/dip) between IDP camps and local villages in 2012. However, different from the decreasing trend in adult density, larval density in the IDP camps was significantly higher in 2014 than that in 2012 (larval density 0.70:0.19 larvae per dip, t = 3.44, df = 11, P < 0.01). In addition, more breeding habitats have been found in IDP camps in 2014, monthly average of 26.3 habitats, than that in 2012.
Disease preventive methods used
Among the 400 households surveyed (100 in local villages and 300 in IDP camps), 100 % used at least one malaria prevention method (including ITNs, IRS, and repellents), and only one household (0.3 %) in the IDP camps did not own an ITN. Bed net usage, defined as the percentage of individuals who slept under nets, was 76.4 % in IDP camps and 75.9 % in local villages (P = 0.81). However, the IDP camps and local villages used different types of bed nets. In the IDP camps, 60.9 % of bed nets were long-lasting insecticide-treated nets (LLIN) whereas 0.4 % used LLIN in local villages.
More than ten NGOs and local agencies have provided various services in the IDP camps, while fewer aid agencies have provided limited services in the local villages (Additional file 3). Some local governmental agencies and NGOs resided in the camps, but all aid agencies in local villages only stayed temporally, usually 1–3 months. In all study villages and camps, free ITNs and insecticide spray were provided, but insecticide spray was more frequent and reliable in camps than in local villages (Additional file 4). In local villages, insecticide spray was done once a year in summer, whereas in IDP camps, indoor spray was routine almost monthly and ground and outdoor spray was done four times a year. Spray team in IDP camps was ready to treat anywhere at any time when needed. Disease diagnosis and treatment were also free for everyone in all clinics and hospitals, i.e., local villagers can also visit hospitals in the camps free of charge. While clinics and doctors/nurses were located in the camps, there was no clinic and doctor/nurse in the local villages (Additional file 4). Local villagers have to go to either hospitals in the camps or elsewhere far away from home.
Discussion
The observations from this study indicate that the recently established IDP camps had a significantly lower burden of malaria compared to local villages in the same area. Prompt establishment of health care clinics, resource mobilization by international and non-governmental agencies in response to the disaster, and pro-active malaria control activities such as indoor and outdoor residual spray and adequate case management could all be factors affecting the risk of malaria transmission in the camps. Early diagnosis and treatment potentially played more vital role in malaria control than ITNs in such context like this study, where the major vector An. minimus can feed on both humans and animals, and inhabit/feed both indoors and outdoors [42, 43]. For patients with falciparum infections, there was no difference in duration of fever days before seeking treatment between local villagers and IDPs. No primaquine was used in both areas for falciparum malaria treatment, and the current treatment regimens are rather effective [36]. The decreased trend and lower P. falciparum incidence rate in IDP camps (but not the case in local villages) may be explained by less frequent human movement in the camp area compared to residents in the villages. Moreover, effective vector interventions may also reduce the overall P. falciparum transmission in the camps. Although primaquine has been administrated as radical treatment for vivax in both local villages and IDP camps, only patients from IDP camps were closely supervised for such treatment through follow-up visits. This practice may have major effects in alleviating relapse of vivax malaria in IDP camps.
In addition to anti-malarials, vector intervention may have contributed in part to the low incidence rate in IDP camps. In this study, An. minimus was the predominant vector species [39]. Since An. minimus bites and rests both indoors and outdoors [42, 43], both ITNs and outdoor vector control interventions such as insecticide spray are important measures. In all study villages and camps, free ITNs were provided. However, ITNs in local villages were conventional ITN and were not re-treated routinely, whereas a high proportion of ITNs used in the IDP camps were LLINs that provided long-lasting protection. In addition, indoor and outdoor insecticide sprays were also provided free of charge, but this was performed more frequently in camps than in local villages. Insecticide spray was scheduled every few months in IDP camps. However, it was done only once a year during the summer in local villages, which was likely insufficient to suppress vector populations. Apart from insecticide spraying, management and treatment of mosquito breeding habitats, i.e., treating habitats with insecticides and/or draining the aquatic habitats, was also a routine work in the IDP camps. For example, more than 20 large water ponds (of which many were fish ponds) were observed in early 2013, and these ponds harboured a high density of mosquito larvae. Few months later in August, about half of these ponds were drained (Zhou, personal observation), and this has resulted in decreased adult density in IDP camp from 2013 to 2014. On the other hand, larval habitats have never been treated in local villages. The contrast in larval habitat management and indoor/outdoor preventive measures may explain the difference in malaria transmission between the IDP camp areas and local villages [10, 13, 14, 44–48]. The use of CDC light trap for vector surveillance may have biased to some species and underestimated the true vector density [49]. However, this is not a major problem because the same collection methods were used in all study sites for mosquito composition comparison. The higher malaria incidence rate in local residents may also be associated with the inaccessibility to health services in local residents. Although clinics in the IDP camps provide free treatment to local village residents, seeking treatment is not easy for them because of the distance and rough road conditions. Commute is especially difficult during rainy season from June to August, the onset of the high transmission season.
A decreased P. falciparum incidence rate in IDP camps but not in local villages, together with the increased trend in P. vivax over P. falciparum indicated effective treatment of falciparum malaria in IDP camps. Yet, despite these efforts, the camps experienced a P. vivax outbreak in 2013, a month after the peak in the villages. This is unlikely due to diagnostic errors given all infected cases diagnosed by microscopy and rapid diagnostic tests (RDT) were confirmed by PCR [38] method. Previous study in the same area showed that microscopic diagnoses had a sensitivity of about 75 % and specificity of 95 % for both vivax and falciparum infections compared to PCR. RDTs had a similar sensitivity to detect falciparum infections, but only 60 % of P. vivax infections [38]. The vivax epidemic might be due to the introduction of novel strains of P. vivax from the villages into the IDP camps whose residents have no prior immunity [40]. In contrast, the villages experienced a consistently high burden of malaria because no effective control measures or resources were available for this population. Lack of convenient access to health care facilities likely further accentuated this problem. Relief agencies and donors should expand healthcare services and malaria control measures to neglected communities beyond the resettled populations. Insufficient resource mobilization to areas that are difficult to access is a significant hurdle to disease control. The findings highlight the need of close monitoring and better healthcare in under-served and indigent populations residing in proximity to the IDP camps.
This study found that in local villages women of child-bearing age are at a higher risk of malaria compared to other women. The well-known adverse impacts of malaria during pregnancy warrant urgent attention to this vulnerable group [15–17, 50–53]. Globally, efforts to control and prevent malaria have primarily focused on P. falciparum in part because infections with this species pose a higher risk of mortality [54–56]. The emergence of a P. vivax outbreak in the IDP camps in 2013, despite sustained control measures and health care, highlights both the importance of P. vivax control in order to achieve malaria elimination [57, 58].
Conclusion
Malaria in IDP camps can be significantly reduced with effective management. Despite sustained control measures and health care delivery, P. vivax outbreaks are unpredictable, which emphasizes both the difficulties and importance of P. vivax control in order to achieve malaria elimination. Expansion of malaria intervention strategies in IDP camps to local surrounding villages is critical to malaria control in the border area in particular and malaria elimination in China in general.
Abbreviations
- IDP:
-
internally displaced persons
- CDC:
-
Center for Diseases Prevention and Control
- KIA:
-
Kachin Independence Army
- UNHCR:
-
United Nations High Commissioner for Refugees
- ICEMR:
-
International Centers of Excellence for Malaria Research
- NGO:
-
non-governmental organization
- PCS:
-
passive case surveillance
- ACS:
-
active case surveillance
- PCR:
-
polymerase chain reaction
- IRS:
-
indoor residual spraying
- ITN:
-
insecticide-treated bed nets
- ANOVA:
-
analysis of variance
- GEE:
-
generalized estimating equations
- OR:
-
odds ratio
- LLIN:
-
long-lasting insecticide-treated nets
References
Roll Back Malaria Partnership. The global malaria action plan: for a malaria-free world. http://www.rollbackmalaria.org/gmap/toc.html. Accessed 10 June 2015.
Spiegel PB. The state of the world’s refugees: the importance of work, cash assistance, and health insurance. JAMA. 2015;314:445–6.
Polonsky JA, Ronsse A, Ciglenecki I, Rull M, Porten K. High levels of mortality, malnutrition, and measles, among recently-displaced Somali refugees in Dagahaley camp, Dadaab refugee camp complex, Kenya, 2011. Confl Health. 2013;7:1.
Grandesso F, Sanderson F, Kruijt J, Koene T, Brown V. Mortality and malnutrition among populations living in South Darfur, Sudan: results of 3 surveys, September 2004. JAMA. 2005;293:1490–4.
Turner C, Turner P, Carrara V, Burgoine K, Tha Ler Htoo S, Watthanaworawit W, et al. High rates of pneumonia in children under two years of age in a South East Asian refugee population. PLoS One. 2013;8:e54026.
Anderson J, Doocy S, Haskew C, Spiegel P, Moss WJ. The burden of malaria in post-emergency refugee sites: a retrospective study. Confl Health. 2011;5:17.
Hershey CL, Doocy S, Anderson J, Spiegel P, Moss WJ. Incidence and risk factors for malaria, pneumonia and diarrhea in children under 5 in UNHCR refugee camps: a retrospective study. Confl Health. 2011;5:24.
Decludt B, Pecoul B, Biberson P, Lang P, Imivithaya S. Malaria surveillance among the displaced Karen population in Thailand April 1984 to February 1989, Mae Sot, Thailand. Southeast Asian J Trop Med Public Health. 1991;22:504–8.
Spiegel PB, Hering H, Paik E, Schilperoord M. Conflict-affected displaced persons need to benefit more from HIV and malaria national strategic plans and global fund grants. Confl Health. 2010;4:2.
Kirkby K, Galappaththy GN, Kurinczuk JJ, Rajapakse S, Fernando SD. Knowledge, attitudes and practices relevant to malaria elimination amongst resettled populations in a post-conflict district of northern Sri Lanka. Trans R Soc Trop Med Hyg. 2013;107:110–8.
Richards AK, Banek K, Mullany LC, Lee CI, Smith L, Oo EK, et al. Cross-border malaria control for internally displaced persons: observational results from a pilot programme in eastern Burma/Myanmar. Trop Med Int Health. 2009;14:512–21.
Carrara VI, Lwin KM, Phyo AP, Ashley E, Wiladphaingern J, Sriprawat K, et al. Malaria burden and artemisinin resistance in the mobile and migrant population on the Thai-Myanmar border, 1999–2011: an observational study. PLoS Med. 2013;10:e1001398.
Spencer S, Grant AD, Piola P, Tukpo K, Okia M, Garcia M, et al. Malaria in camps for internally-displaced persons in Uganda: evaluation of an insecticide-treated bednet distribution programme. Trans R Soc Trop Med Hyg. 2004;98:719–27.
Graham K, Mohammad N, Rehman H, Nazari A, Ahmad M, Kamal M, et al. Insecticide-treated plastic tarpaulins for control of malaria vectors in refugee camps. Med Vet Entomol. 2002;16:404–8.
Mullany LC, Lee CI, Paw P, Shwe Oo EK, Maung C, Masenior N, et al. The MOM project: delivering maternal health services among internally displaced populations in eastern Burma. Reprod Health Matter. 2008;16:44–56.
Mullany LC, Lee TJ, Yone L, Lee CI, Teela KC, Paw P, et al. Impact of community-based maternal health workers on coverage of essential maternal health interventions among internally displaced communities in eastern Burma: the MOM project. PLoS Med. 2010;7:e1000317.
McGready R, Boel M, Rijken MJ, Ashley EA, Cho T, Moo O, et al. Effect of early detection and treatment on malaria related maternal mortality on the north-western border of Thailand 1986–2010. PLoS One. 2012;7:e40244.
Chaparro P, Padilla J, Vallejo AF, Herrera S. Characterization of a malaria outbreak in Colombia in 2010. Malar J. 2013;12:330.
Abeku T. Response to malaria epidemics in Africa. Emerg Infect Dis. 2007;13:681–6.
Koros D. Malaria outbreak in Kakuma refugee camp, Kenya: the IRC responds. Global Health Link. 2006;11:17.
Rowland M, Nosten F. Malaria epidemiology and control in refugee camps and complex emergencies. Ann Trop Med Parasitol. 2001;95:741–54.
Lee TJ, Mullany LC, Richards AK, Kuiper HK, Maung C, Beyrer C. Mortality rates in conflict zones in Karen, Karenni, and Mon states in eastern Burma. Trop Med Int Health. 2006;11:1119–27.
Chen G-W, Li H-X, Lin Y-X. Horizontal survey on the epidemiological characteristics of malaria in Laiza city of the second special administrative region of Kachin State of Myanmar, a China–Myanmar border area. Chin J Vector Biol Control. 2012;23:352–6 (in Chinese).
Zhang L, Feng J, Xia ZG. Malaria situation in the People’s Republic of China in 2013. Chin J Parasitol Parasit Dis. 2014;32:407–13.
Zhou G, Sun L, Xia R, Duan Y, Xu J, Yang H, et al. Clinical malaria along the China–Myanmar border area in Yunnan, China. Emerg Infect Dis. 2014;20:675–8.
Coker RJ, Hunter BM, Rudge JW, Liverani M, Hanvoravongchai P. Emerging infectious diseases in Southeast Asia: regional challenges to control. Lancet. 2011;377:599–609.
WHO. World Malaria Report 2013. Geneva: World Health Organization. 2013. http://www.who.int/malaria/publications/world_malaria_report_2013/report/en/. Accessed 19 Sep 2015.
Delacollette C, D’Souza C, Christophe IE, Thimasarn K, Abdur R, Bell D, et al. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian J Trop Med Public Health. 2009;40:674–91.
Kumar A, Chery L, Biswas C, Dubhashi N, Dutta P, Dua VK, et al. Malaria in South Asia: prevalence and control. Acta Trop. 2012;121:246–55.
Archavanitkul K, Vajanasara K. Health of migration workers from Myanmar, Cambodia and Laos. In: Kanchanachitra C et al., editors. Thai health 2010: capitalism in crisis, opportunity for society? Bangkok: Amarin Printing; 2010. p. 31–32.
Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, Duan J, Lee MC, Yan G, Matthews SA, Cui L, Wang Y. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China–Myanmar border. Malar J. 2013;12:361.
Wang X, Zhou G, Zhong D, Wang X, Wang Y, Yang Z, et al. Life-table studies revealed significant effects of deforestation on the development and survivorship of Anopheles minimus larvae. Parasit Vectors. 2016;9:323.
Zhong D, Wang X, Xu T, Zhou G, Wang Y, Lee MC, et al. Effects of microclimate condition changes due to land use and land cover changes on the survivorship of malaria vectors in China–Myanmar border region. PLoS One. 2016;11:e0155301.
Lo E, Nguyen J, Oo W, Hemming-Schroeder E, Zhou G, Yang Z, et al. Examining Plasmodium falciparum and P. vivax clearance subsequent to antimalarial drug treatment in the Myanmar–China border area based on quantitative real-time polymerase chain reaction. BMC Infect Dis. 2016;16:154.
Hu Y, Zhou G, Ruan Y, Lee MC, Xu X, Deng S, et al. Seasonal dynamics and microgeographical spatial heterogeneity of malaria along the China–Myanmar border. Acta Trop. 2016;157:12–9.
Wang Y, Yang Z, Yuan L, Zhou G, Parker D, Lee MC, et al. Clinical efficacy of dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria at the China–Myanmar border. Am J Trop Med Hyg. 2015;93:577–83.
Yuan L, Wang Y, Parker DM, Gupta B, Yang Z, Liu H, et al. Therapeutic responses of Plasmodium vivax malaria to chloroquine and primaquine treatment in northeastern Myanmar. Antimicrob Agents Chemother. 2015;59:1230–5.
Yan J, Li N, Wei X, Li P, Zhao Z, Wang L, et al. Performance of two rapid diagnostic tests for malaria diagnosis at the China–Myanmar border area. Malar J. 2013;12:73.
Wang Y, Zhong D, Cui L, Lee M-C, Yang Z, Yan G, et al. Population dynamics and community structure of Anopheles mosquitoes along the China–Myanmar border. Parasit Vectors. 2015;8:445.
Lo EYY, Zhou G, Oo W, Lee M-C, Baum E, Felgner PL, et al. Molecular inference of sources and spreading patterns of Plasmodium falciparum malaria parasites in internally displaced person settlements in Myanmar–China border area. Infect Genet Evol. 2015;33:189–96.
Zhou G, Afrane YA, Malla S, Githeko AK, Yan G. Active case surveillance, passive case surveillance and asymptomatic malaria parasite screening illustrate different age distribution, spatial clustering and seasonality in western Kenya. Malar J. 2015;14:41.
Nájera JA, González-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969). PLoS Med. 2011;8:e1000412.
Smithuis FM, Kyaw MK, Phe UO, van der Broek I, Katterman N, Rogers C, et al. The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J. 2013;12:363.
Akogbéto MC, Aïkpon RY, Azondékon R, Padonou GG, Ossè RA, Agossa FR, et al. Six years of experience in entomological surveillance of indoor residual spraying against malaria transmission in Benin: lessons learned, challenges and outlooks. Malar J. 2015;14:242.
Pinder M, Jawara M, Jarju LB, Salami K, Jeffries D, Adiamoh M, et al. Efficacy of indoor residual spraying with dichlorodiphenyltrichloroethane against malaria in Gambian communities with high usage of long-lasting insecticidal mosquito nets: a cluster-randomised controlled trial. Lancet. 2015;385:1436–46.
Smith DL, Perkins TA, Tusting LS, Scott TW, Lindsay SW. Mosquito population regulation and larval source management in heterogeneous environments. PLoS One. 2013;8:e71247.
Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database Syst Rev. 2013;8:CD008923.
Fillinger U, Lindsay SW. Larval source management for malaria control in Africa: myths and reality. Malar J. 2011;10:353.
Sriwichai P, Karl S, Samung Y, Sumruayphol S, Kiattibutr K, Payakkapol A, et al. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai-Myanmar border. Parasit Vectors. 2015;8:636.
Schantz-Dunn J, Nour NM. Malaria and pregnancy: a global health perspective. Rev Obstet Gynecol. 2009;2:186–92.
WHO. Global Malaria Programme: pregnant women and infants. Geneva: World Health Organization. http://apps.who.int/malaria/pregnantwomenandinfants.html. Accessed 30 July 2015.
Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104.
Menéndez C, Ferenchick E, Roman E, Bardají A, Mangiaterra V. Malaria in pregnancy: challenges for control and the need for urgent action. Lancet Global Health. 2015;3:e433–4.
Galinski MG, Barnwell JW. Plasmodium vivax: who cares? Malar J. 2008;7(Suppl 1):S9.
Price RN, Tjitra E, Guerra CA, Yeung S, White NJ, Anstey NM. Vivax malaria: neglected and not benign. Am J Trop Med Hyg. 2007;77(Suppl):79–87.
Feachem RGA, Phillips AA, Hwang J, Cotter C, Wielgosz B, Greenwood BM, et al. Shrinking the malaria map: progress and prospects. Lancet. 2010;376:1566–78.
Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11.
Price RN, von Seidlein L, Valecha N. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–91.
Authors’ contributions
GY, CL and GZ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: GY, CL and GZ. Data collection: GZ, YW, DZ, XW and ZY. Data management: ML and SM. Analysis and interpretation of data: GZ and EL. Draft of the manuscript: GZ, EL, and GY. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank the field team for their technical assistance in the field and laboratory. We are grateful to the communities and hospitals for support and willingness to participate in this research.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets supporting the conclusions of this article can be found publicly within the main text and its additional files of this article.
Consent for publication
All authors read and approved the final manuscript.
Funding information
This project was funded by the National Institute of Health (U19 AI089672 and D43 TW009527) and the National Natural Science Foundation of China (U1202226 and 31260508 to Z.Y). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Scientific and ethical statement
Scientific and ethical clearance was obtained from the ethical review boards of Kunming Medical University, China (IRB # KMC2011-01); University of California, Irvine (IRB HS # 2012-9123) and Pennsylvania State University (IRB # 34319), USA. Written informed consent/assent (for minors under age 18) for study participation was obtained from all consenting heads of households and each individual who was willing to participate in the study.
Author information
Authors and Affiliations
Corresponding authors
Additional files
12936_2016_1512_MOESM4_ESM.docx
Additional file 4. Diseases prevention and control provided and medical services availabilities in different villages and camps.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, G., Lo, E., Zhong, D. et al. Impact of interventions on malaria in internally displaced persons along the China–Myanmar border: 2011–2014. Malar J 15, 471 (2016). https://doi.org/10.1186/s12936-016-1512-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-016-1512-2