Abstract
Background
Health clinics in rural Africa are typically resource-limited. As a result, many patients presenting with fever are treated with anti-malarial drugs based only on clinical presentation. This is a considerable issue in Uganda, where malaria is routinely over-diagnosed and over-treated, constituting a wastage of resources and an elevated risk of mortality in wrongly diagnosed patients. However, rapid diagnostic tests (RDTs) for malaria are increasingly being used in health facilities. Being fast, easy and inexpensive, RDTs offer the opportunity for feasible diagnostic capacity in resource-limited areas. This study evaluated the rate of malaria misdiagnosis and the accuracy of RDTs in rural Uganda, where presumptive diagnosis still predominates. Specifically, the diagnostic accuracy of “gold standard” methods, microscopy and PCR, were compared to the most feasible method, RDTs.
Methods
Patients presenting with fever at one of two health clinics in the Kabarole District of Uganda were enrolled in this study. Blood was collected by finger prick and used to administer RDTs, make blood smears for microscopy, and blot Whatman FTA cards for DNA extraction, polymerase chain reaction (PCR) amplification, and sequencing. The accuracy of RDTs and microscopy were assessed relative to PCR, considered the new standard of malaria diagnosis.
Results
A total of 78 patients were enrolled, and 31 were diagnosed with Plasmodium infection by at least one method. Comparing diagnostic pairs determined that RDTs and microscopy performed similarly, being 92.6 and 92.0 % sensitive and 95.5 and 94.4 % specific, respectively. Combining both methods resulted in a sensitivity of 96.0 % and specificity of 100 %. However, both RDTs and microscopy missed one case of non-falciparum malaria (Plasmodium malariae) that was identified and characterized by PCR and sequencing. In total, based on PCR, 62.0 % of patients would have been misdiagnosed with malaria if symptomatic diagnosis was used.
Conclusions
Results suggest that diagnosis of malaria based on symptoms alone appears to be highly inaccurate in this setting. Furthermore, RDTs were very effective at diagnosing malaria, performing as well or better than microscopy. However, only PCR and DNA sequencing detected non-P. falciparum species, which highlights an important limitation of this test and a treatment concern for non-falciparum malaria patients. Nevertheless, RDTs appear the only feasible method in rural or resource-limited areas, and therefore offer the best way forward in malaria management in endemic countries.
Similar content being viewed by others
Background
In Africa, 70 % of fevers are initially managed at home, with traditional remedies and bed rest used to alleviate symptoms [1, 2]. Only when symptoms continue to worsen is medical attention sought, and even at this stage, patients may not receive proper diagnosis—especially at remote or rural health facilities that are often resource-limited [3]. The symptoms of malaria overlap with a number of other illnesses, making accurate diagnosis difficult, even among experienced practitioners [4–7]. In addition, anti-malarial medications can be prescribed by primary care workers who vary in experience and training. As a result, most patients with a recent history of fever are diagnosed with and treated for malaria, despite the fact that a number of other illnesses, including pneumonia, typhoid fever, respiratory tract infections, transient viral illnesses and meningitis may be causing clinical disease [8–10]. Therefore, the sensitivity of malaria diagnosis at clinics may be as high as 100 % since few patients with clinical malaria are missed, but the specificity is often extremely low [5, 6, 11]. Indeed, a study conducted in Tanzanian hospitals showed that less than half of all individuals symptomatically diagnosed for malaria were actually positive for the parasite by microscopic examination of blood smears [12]. Individuals treated for malaria but not actually harbouring the infection were often not prescribed antibiotics, and were more likely to die than individuals with malaria [12]. These results suggest that symptomatic or presumptive diagnosis of malaria is costly not only in terms of wasted anti-malarial drugs, but also in the morbidity and mortality associated with misdiagnosis.
In many malaria endemic regions, safe, inexpensive anti-malarials such as chloroquine have become ineffective due to the emergence of drug-resistant parasites. These drugs are being replaced by more toxic and expensive alternatives, including sulfadoxine-pyrimethamine [13]. Prescribing anti-malarials only following diagnostic testing is perhaps the most appropriate way to dispense drugs, but can be challenging in locations with already overburdened health infrastructure. For instance, the traditional standard of malaria diagnosis is microscopic examination of peripheral blood smears. However, this requires high quality reagents, clean equipment, functioning microscopes, workspace, and skilled personnel [10]. To circumvent poor diagnostic access, antigen-based malaria rapid diagnostic tests (RDTs) have been implemented with success in field conditions, especially in regions without prior access to microscopic diagnostics [3, 14, 15]. Being relatively affordable, easy to use, and quick, RDTs offer a promising method of minimizing over-diagnosis. As a result, there has been a tremendous increase in RDT usage at public health facilities throughout sub-Saharan Africa [2]. However, still only 65 % of suspected malaria cases were diagnostically confirmed by RDT in 2014, and the majority of presumptive diagnoses still occur in sub-Saharan Africa [16].
Uganda is currently among the few countries where cases of malaria have recently increased [16]. Indeed, malaria transmission is endemic across 95 % of the country [17]. This is despite diagnostic testing being free of charge in the public sector, and the recent implementation of the Uganda Malaria Reduction Strategic Plan (2014–2020), which recommends parasite-based diagnosis at all scales and for all patients [16, 18]. One considerable limitation of this plan has been consistent RDT stockouts, which have limited diagnostic capacity, especially in rural areas [19]. Therefore, the objective of this study was to assess the rate of malaria misdiagnosis in a rural area of Uganda when RDT usage was just beginning to circumvent presumptive diagnosis. To establish an accurate estimation of misdiagnosis and assess the efficacy of RDTs, malaria infection was confirmed using two standard diagnostic methods, microscopy and PCR, and the results of RDTs were compared to these methods.
Methods
Blood sample collection and processing
Two rural health clinics located within the Kabarole District of Uganda were enlisted in this study. Patients visiting these clinics in June and July 2011 with a fever (an axillary temperature of 37.5 °C or higher), who were not pregnant, and who had not treated their symptoms with standard anti-malarials, were asked to participate. Following informed consent, blood samples were collected via finger prick by the clinic’s health practitioner. One drop of blood was used in an RDT that detected histidine-rich protein II (HRP-II) antigen of Plasmodium falciparum, which is the recommended RDT for malaria diagnosis in Uganda. Health practitioners read, interpreted, and recorded the result of the test after 15 min. Results were recorded as positive or negative, with faint lines being interpreted as positive. An additional two drops of blood were used to make thick and thin blood smears for microscopic diagnosis. Finally, one to five additional blood drops were collected on Whatman FTA Classic Cards for subsequent molecular analyses. All patients were offered complimentary treatment based on RDT results and the health practitioner’s diagnosis. Blood samples were shipped to North America for microscopic examination and molecular diagnostics.
Microscopy
Thin and thick smears were stained with Giemsa and viewed under 100× oil immersion objective magnification. Each slide was read by two independent microscopists; in the event of a discrepancy, the slides were read by a third microscopist. Thick smears were used to confirm the presence of blood parasites, while thin smears were used to confirm speciation and determine infection intensity (parasitaemia). For each thin smear, a field containing a representative monolayer of red blood cells (RBCs) was identified and all RBCs in that field were counted. This was repeated five times to calculate an average number of RBCs per field. One hundred fields, or the maximum number of viewable fields on the slide, were scanned for intra-erythrocytic parasites morphologically consistent with Plasmodium. Parasitaemia of positive smears was calculated following a previously described formula [20].
DNA extraction, PCR, and Sanger sequencing
DNA was extracted from Whatman FTA Classic Cards following modified manufacturer’s instructions. Briefly, a 4-mm sample disc was punched from each FTA card and placed in a PCR amplification tube. FTA purification reagent (200 µl) was added to each tube and incubated for 5 min at room temperature. The FTA purification agent was removed by pipette, and this process repeated an additional three times. 200 µl of TE buffer (10 mM Tris-HCl, 0.1 mM EDTA, pH 8.0) was then added to each PCR tube and incubated for 5 min at room temperature. TE buffer was removed by pipette and this process was repeated once more. The sample disc was allowed to dry completely at room temperature before the disc was used as template in PCR amplification. This method resulted in consistently higher, better quality DNA yields than extraction methods that eluted DNA, such as prepGEM (ZyGEM NZ Ltd, Hamilton, New Zealand) or QIAamp DNA Investigator (QIAGEN, Hilden, Germany) kits.
A semi-nested PCR targeting the cytochrome b (cytb) gene of Plasmodium was conducted using primers CytB3384F (5′-GTAATGCCTAGACGTATTCCTG-3′) and CytB4595R (5′-GTTTGCTTGGGAGCTGTAATC-3′) in the external reaction, and CytB3706F (5′-GTTTGCTTGGGAGCTGTAATC-3′) and CytB4595R in the internal reaction. This procedure generated amplicons of 1254 bp (external) and 932 bp (internal) predicted size [20]. Both external and internal reactions were performed in 25 µl volumes using the FailSafe system (EpiCenter Biotechnologies, Madison, WI, USA), with reactions containing 1× FailSafe PCR PreMix with Buffer E, and 1 unit of FailSafe Enzyme Mix, 2.5 pmol of each primer. For external reactions, sample discs were used as template. For internal reactions, 1 µl of purified external PCR product were used as template. Reactions were cycled using previously described profiles [20]. A Plasmodium gallinaceum-positive sample that underwent the same extraction method was used as a positive control; exogenous DNA added post-DNA extraction was used as internal negative control (Qiagen, Hilden, Germany). All samples were run in duplicate at separate intervals to ensure diagnostic accuracy.
For samples identified as positive for Plasmodium by PCR but negative by microscopy and/or RDT, amplicons were Sanger sequenced in both directions using internal primers CytB3706F and CytB4595R on ABI 3730xl DNA Analyzers (Applied Biosystems, Carlsbad, CA) at the University of Wisconsin Biotechnology Center DNA Sequence Facility. Sequences were hand-edited using Sequencher v4.9 (Gene Codes Corporation, Ann Arbor, MI). To identify parasite species, newly generated DNA sequences were compared to reference sequences in Genbank using BLASTn [21].
Analysis
The sensitivity and specificity of RDTs and microscopy were compared to PCR, considered the primary reference standard because it is established to outperform microscopy in sensitivity and specificity [22–24]. Parallel testing was also conducted, which combined the results of RDTs and microscopy. This determined if the combination of these two methods, which may be feasible to perform in larger healthcare facilities, can match the diagnostic accuracy of PCR, which is not realistic in most developing country healthcare settings.
Results
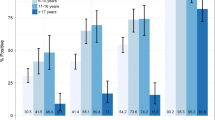
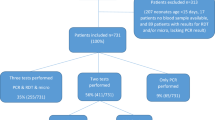
A total of 78 patients were enrolled in this study (38 women and 40 men). All patients were tested by RDT. Blood samples for PCR were collected from 71 of these patients, and blood samples for microscopy were collected from 67 patients. Parasitaemia ranged from 0.003 to 3.399 % of total RBCs in Plasmodium-positive samples (as determined by microscopy). Average parasitaemia was 0.45 % (±1.00 SD) in females and 0.31 % (±0.43 SD) in males, and was 1.26 % (±1.43 SD) in children aged five or younger and 0.16 % (±0.31 SD) in patients older than five. Females presenting with fever were more frequently positive for malaria than males (41.2 % prevalence in females, 35.1 % in males by PCR; Table 1). In contrast, patients aged five or less were diagnosed with malaria at roughly equal frequency to those older than five (40.0 % prevalence in patients ≤5, 39.3 % prevalence in patients >5 by PCR; Table 1). Overall, the prevalence of malaria-positive patients was 38.0 % by the gold standard method, PCR (Table 1).
In order to assess the efficacy of RDTs, the most promising diagnostic method in resource-limited regions, the sensitivity and specificity of RDTs and microscopy were compared to PCR. PCR identified 27 patients (out of 71) as positive for Plasmodium. Microscopic examination of peripheral blood smears identified 28 patients (out of 67) as positive for P. falciparum, and RDTs identified 32 patients as positive (out of 78; Table 1). Compared to PCR, RDTs were 92.6 % sensitive and 95.5 % specific, while microscopy was 92.0 % sensitive and 94.4 % specific (Table 2). Combining the results of RDTs and microscopy yielded 96 % sensitivity and 100 % specificity.
RDTs and microscopy both missed one patient sample that was positive by PCR. Interestingly, DNA sequencing revealed that this sample was 100 % identical to two published Plasmodium malariae cytochrome b sequences (Genbank Accession Numbers AB489194 (unpublished) and AB354570 [25]; BLASTn). Another sample was positive by PCR and microscopy (parasitaemia = 0.013 %) but negative by RDT. Sequencing identified this sample as 99 % identical to sequences from a published set of P. falciparum cytochrome b sequences from India [26]. Similarly, an additional sample positive by PCR and RDT but negative by microscopy was also determined to be P. falciparum, sharing 100 % sequence identity with sequences from the aforementioned Indian population set [26]. Lastly, two patients were negative by PCR but positive by RDT and microscopy.
Discussion
This study evaluated the rate of malaria misdiagnosis occurring in rural Uganda, and assessed the accuracy of RDTs, the most promising diagnostic method for limiting over-diagnosis in resource-limited settings. All individuals in this study were symptomatically diagnosed with malaria by health practitioners, based on the presence of fever. However, results of the gold standard diagnostic test suggest that only 38.0 % of patients were positive for Plasmodium. Thus, the majority of patients (62.0 %) were misdiagnosed and would have been treated with anti-malarials if not for diagnostic intervention. These results corroborate findings of malaria over-diagnosis elsewhere. For example, a cross-sectional study evaluating Uganda’s policy of treating febrile illness with anti-malarials reported rates of over-diagnosis ranging from 45.3 to 80.9 % [27]. Indeed, malaria over-diagnosis is a well-known and widespread issue occurring throughout malaria endemic regions, with overestimates typically being greater than 30 % [1].
Results indicate that RDTs performed as well as the traditional gold standard, microscopy. Both were over 90 % sensitive and specific, although RDTs were marginally better at detecting infection when present and returning negative results when the parasite was absent. That RDTs are as effective or better than microscopy is consistent with the findings of others [28]. However, it should be noted that microscopy was performed under ideal conditions and by expert microscopists, which was necessary to establish accurate estimates of malaria misdiagnosis. A study that compared the diagnostic efficacy of RDTs to microscopy conducted in field conditions reported that RDTs had significantly higher sensitivity but lower specificity than microscopy [29].
Combining RDT and microscopic diagnosis resulted in perfect specificity, which indicates that malaria-negative patients are unlikely to be misdiagnosed as positive by combining these methods. However, one malaria-positive patient was still missed, even when RDTs and microscopy were combined. This patient was only detected by PCR, and sequencing revealed infection with P. malariae. In this study, RDTs specific for P. falciparum were selected based on this species’ ubiquity in the study area, and their recommended usage for malaria diagnosis in Uganda [30]. However, results from this study raise an important concern moving forward with malaria eradication efforts. Specifically, non-falciparum malaria patients face an elevated risk of misdiagnosis, even when recommended RDT diagnostic testing is applied. While microscopy is considered a better diagnostic tool for detecting non-falciparum malaria [28], this study’s results indicate that even a combinatorial approach does not result in infallible accuracy, especially when dealing with non-falciparum infection. Furthermore, the Uganda Malaria Strategic Reduction Plan (2014–2020) estimate the prevalence of non-falciparum species (2 % for P. malariae and Plasmodium vivax, <1 % for Plasmodium ovale) based on surveys published in the 1960s [31]. Needless to say, up-to-date surveys of non-falciparum malaria are necessary to estimate how frequently misdiagnosis of non-falciparum malaria might arise.
A surprising result in this study was that PCR failed to detect two samples that were confirmed positive by RDT and microscopy. Other studies have suggested that PCR false positives can be the result of recently cured malaria [24]. However, given that parasites were detected by microscopy in addition to RDT makes it possible that instead, PCR failed to detect parasites from these samples. Given that the percentage parasitaemia identified by microscopy were all within reasonable PCR detection limits, these two false-negatives may be a consequence of very limited blood quantities for DNA extraction, which highlights the necessity to fully saturate Whatman cards for accurate diagnosis. Since this issue is most likely to occur in young children where blood quantities from finger pricks is limited, RDTs (which require a single drop of blood) may in fact offer a performance advantage over PCR in this demographic.
Despite limitations in detecting non-falciparum malaria, the new generation of RDTs satisfy all criteria required for implementation in rural settings, being easy to use and durable over the long-term in tropical conditions [32]. Furthermore, a recent analysis estimated that RDTs are more cost-effective than microscopy per case correctly diagnosed and treated (at US$5.00, as compared to microscopy at US$9.61) [33]. Therefore, RDTs overall offer a promising approach to alleviate the costs of presumptive diagnosis in Africa’s high and medium–high transmission regions.
Conclusions
Despite global efforts to eradicate malaria, the burden of this disease is still high. In rural Uganda, 38.0 % of patients visiting peripheral health clinics with fever were positive for malaria. However, this means that 62.0 % of patients symptomatically diagnosed with malaria were negative for the parasite, suggesting that over-diagnosis of this disease remains a critical problem. Combining both microscopy and RDT testing yielded high sensitivity and specificity. However, only PCR detected non-falciparum parasites, and is the only method that offers species-level diagnoses. Nevertheless, the cost and expertise required for this method render it impractical in nearly all developing healthcare settings. On the other hand, the ease and rapidity of RDTs confirm this method is perhaps the best approach for reducing malaria over-diagnosis, even in resource-limited settings.
Abbreviations
- PCR:
-
polymerase chain reaction
- RDT:
-
rapid diagnostic test, refers specifically to test for Plasmodium falciparum malaria
References
Amexo M, Tolhurst R, Barnish G, Bates I. Malaria misdiagnosis: effects on the poor and vulnerable. Lancet. 2004;364:1896–8.
Mbonye AK, Bygbjerg IC, Magnussen P. Prevention and treatment practises and implications for malaria control in Mukono District, Uganda. J Biosoc Sci. 2008;40:283–96.
Ansah EK, Narh-Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, et al. Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ. 2010;340:c930.
Olaleye B, Williams L, D’Alessandro U, Weber M, Mulholland K, Okorie C, et al. Clinical predictors of malaria in Gambian children with fever or a history of fever. Trans R Soc Trop Med Hyg. 1998;92:300–4.
Rooth I, Björkman A. Fever episodes in a holoendemic malaria area of Tanzania: parasitological and clinical findings and diagnostic aspects related to malaria. Trans R Soc Trop Med Hyg. 1992;86:479–82.
Chandramohan D, Jaffar S, Greenwood B. Use of clinical algorithms for diagnosing malaria. Trop Med Int Health. 2002;7:45–52.
Kilian AHD, Metzger WG, Mutschelknauss EJ, Kabagambe G, Langi P, Korte R, et al. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop Med Int Health. 2000;5:3–8.
Uneke C. Concurrent malaria and typhoid fever in the tropics: the diagnostic challenges and public health implications. J Vector Borne Dis. 2008;45:133–42.
Källander K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia–policy implications for home management strategies. Acta Trop. 2004;90:211–4.
Bell D, Wongsrichanalai C, Barnwell JW. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat Rev Microbiol. 2006;4:682–95.
Oliver M, Develoux M, Abari A, Loutan L. Presumptive diagnosis of malaria results in a significant risk of mistreatment of children in urban Sahel. Trans R Soc Trop Med Hyg. 1991;85:729–30.
Reyburn H, Mbatia R, Drakeley C, Carneiro I, Mwakasungula E, Mwerinde O, et al. Overdiagnosis of malaria in patients with severe febrile illness in Tanzania: a prospective study. BMJ. 2004;329:1212.
Redd SC, Luby SP, Hightower AW, Kazembe PN, Nwanyanwu O, Ziba C, et al. Clinical algorithm for treatment of Plasmodium falciparum malaria in children. Lancet. 1996;347:223–7.
Wilson ML. Malaria rapid diagnostic tests. Clin Infect Dis. 2012;54:1637–41.
Masanja MI, McMorrow M, Kahigwa E, Kachur SP, McElroy PD. Health workers’ use of malaria rapid diagnostic tests (RDTs) to guide clinical decision making in rural dispensaries, Tanzania. Am J Trop Med Hyg. 2010;83:1238–41.
WHO. World Malaria Report. Geneva: World Health Organization; 2015. p. 280.
Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25.
WHO. Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015. p. 313.
Batwala V, Magnussen P, Nuwaha F. Are rapid diagnostic tests more accurate in diagnosis of Plasmodium falciparum malaria compared to microscopy at rural health centres? Malar J. 2010;9:349.
Thurber MI, Ghai RR, Hyeroba D, Weny G, Tumukunde A, Chapman CA, et al. Co-infection and cross-species transmission of divergent Hepatocystis lineages in a wild African primate community. Int J Parasitol. 2013;43:613–9.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Humar A, Ohrt C, Harrington MA, Pillai D, Kain KC. ParaSight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg. 1997;56:44–8.
Ohrt C, Purnomo Sutamihardja MA, Tang D, Kain KC. Impact of microscopy error on estimates of protective efficacy in malaria-prevention trials. J Infect Dis. 2002;186:540–6.
Farcas GA, Zhong KJY, Lovegrove FE, Graham CM, Kain KC. Evaluation of the Binax Now ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am J Trop Med Hyg. 2003;69:589–92.
Hayakawa T, Culleton R, Otani H, Horii T, Tanabe K. Big bang in the evolution of extant malaria parasites. Mol Biol Evol. 2008;25:2233–9.
Tyagi S, Pande V, Das A. New insights into the evolutionary history of Plasmodium falciparum from mitochondrial genome sequence analyses of Indian isolates. Mol Ecol. 2014;23:2975–87.
Nankabirwa J, Zurovac D, Njogu JN, Rwakimari JB, Counihan H, Snow RW, et al. Malaria misdiagnosis in Uganda—implications for policy change. Malar J. 2009;8:66.
Ochola LB, Vounatsou P, Smith T, Mabaso MLH, Newton C. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis. 2006;6:582–8.
Nankabirwa JI, Yeka A, Arinaitwe E, Kigozi R, Drakeley C, Kamya MR, et al. Estimating malaria parasite prevalence from community surveys in Uganda: a comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar J. 2015;14:528.
Hopkins H, Bebell L, Kambale W, Dokomajilar C, Rosenthal PJ, Dorsey G. Rapid diagnostic tests for malaria at sites of varying transmission intensity in Uganda. J Infect Dis. 2008;197:510–8.
Uganda Ministry of Health. The Uganda Malaria reduction strategic plan, 2014–2020. Kampala, Uganda; 2014. p. 83.
D’Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M, Genton B. Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Med. 2009;6:e252.
Batwala V, Magnussen P, Hansen KS, Nuwaha F. Cost-effectiveness of malaria microscopy and rapid diagnostic tests versus presumptive diagnosis: implications for malaria control in Uganda. Malar J. 2011;10:372.
Authors’ contributions
RRG was involved in all stages of the study. MIT was involved in sample collection and design of PCR. AB confirmed microscopic diagnosis. CAC and TLG participated in the design of the study. TLG oversaw molecular analyses. TLG and AB were involved with the interpretation of results. All authors contributed to the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the patients that participated in this study, and the healthcare professionals at our study clinics who facilitated this research—especially nurses Lucy Kanjanse and Dennis Natamba. Finally, we thank Drs. Patrick Omeja and Geoffrey Weny for their valuable contributions to this project, and Alex Tumukunde for methodological assistance.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Blood samples were collected with informed consent in accordance with World Health Organisation guidelines. Research approval was granted by the Uganda National Council for Science and Technology. Permission to conduct this research was granted by the Institutional Review Committees associated with Makerere University, McGill University, and the University of Wisconsin-Madison. Samples were shipped internationally following IATA guidelines and regulations.
Funding
This research was supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant (CAC) and the Fonds de Recherche du Quebec Nature et Technologies International Internship award (RRG), as well as by NIH Grant TW009237 as part of the joint NIH-NSF Ecology of Infectious Disease program and the UK Economic and Social Research Council. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ghai, R.R., Thurber, M.I., El Bakry, A. et al. Multi-method assessment of patients with febrile illness reveals over-diagnosis of malaria in rural Uganda. Malar J 15, 460 (2016). https://doi.org/10.1186/s12936-016-1502-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-016-1502-4