Abstract
Background
Malaria intervention in Ethiopia has been strengthened significantly in the past decade. The Ethiopian government recently stratified the country based upon annual parasite incidence into malaria free, low, moderate and high transmission strata. Districts with low transmission were targeted for indigenous transmission elimination. Surveillance on malaria disease incidence is needed for planning control and elimination efforts.
Methods
Clinical malaria was monitored prospectively in health facilities in Jimma town, Oromia Region, southwestern Ethiopia from July 2014 to June 2015. Seasonal cross-sectional parasite prevalence surveys in local communities were conducted in 2014 and 2015 in eight kebeles. Case report forms were administered to obtain sociodemographic and epidemiological information from patients.
Results
A total of 1434 suspected malaria cases were examined from the health facilities and 428 confirmed malaria cases were found. Among them, 327 (76.4 %) cases were Plasmodium vivax, 97 (22.7 %) were Plasmodium falciparum, and 4 (0.9 %) were mixed infection of P. vivax and P. falciparum. The annual malaria incidence rate was 1.7 cases per 1000 people at risk. Parasite prevalence in the community was less than 3 %. Household ownership of insecticide-treated nets (ITNs) was 47.3 % (1173/2479) and ITN usage was 37.9 %. All ITNs were long-lasting insecticidal nets, and repellent use was not found in the study area. Being male and traveling were the significant risk factors for P. falciparum malaria. For P. vivax malaria, risk factors included occupation and history of malaria illness during the preceding 30 days.
Conclusion
Epidemiological evidence suggested low clinical malaria incidence and prevalence in Jimma town. More aggressive measures may be needed to further suppress vivax transmission. Strategies should be planned targeting sustained control and elimination.
Similar content being viewed by others
Background
Ethiopia is one of the few countries in Africa where Plasmodium vivax and Plasmodium falciparum coexist [1, 2]. It is also one of the few African countries with a policy of providing malaria prevention and control services including malaria diagnosis and treatment, insecticide-treated bed nets (ITNs), and indoor residual spray (IRS) free of charge [3–6]. This policy ensures that malaria interventions reach the poor, and in turn enables them to increase economic productivity [4]. In Ethiopia, major malaria intervention scale-up efforts began in 2004/2005 with the introduction of artemisinin-based combination therapy (ACT) as the first-line treatment of P. falciparum malaria. ACT has been free of charge for all ages in the public sector since then [3–5]. IRS campaigns have targeted epidemic-prone areas, and IRS coverage has reached to 37 % of the at-risk population in 2013 [4–6]. Long-lasting insecticidal nets (LLINs) have been distributed free of charge to the entire population through mass campaigns to step up vector control and prevention since 2006 [3, 4]. In 2008, the Ethiopian government distributed 20.4 million LLINs, and 11.2 million were replaced in 2010 and 7 million in 2011 [3, 4]. The combined improvement in coverage of essential interventions has coincided with a considerable reduction in malaria morbidity and mortality as well as epidemic episodes and affected areas. While no epidemics have been reported in the country since 2004 [7], a comprehensive longitudinal study showed that malaria remains a major public health problem in Ethiopia [8, 9].
The latest National Malaria Strategic Plan of Ethiopia stratified districts into malaria free, low, moderate and high transmission areas based upon annual parasite incidence [4, 10]. An ambitious goal was set to eliminate indigenous transmission in low transmission areas [4, 10]. In some districts where malaria control is shifted to elimination, changes have been made in the malaria treatment guideline. For example, a single low-dose primaquine is added to the artemether-lumefantrine (Coartem®) treatment regimen for P. falciparum to kill gametocytes and reduce malaria transmission. A 14-day course of primaquine is added to the chloroquine treatment regimen for radical cure of P. vivax malaria in low transmission area [10]. In the past 10 years, IRS has been performed on an annual basis and LLINs have been distributed to all residents in Jimma town free of charge [4]. However, data on the current coverage of interventions (e.g., LLIN ownership and population coverage), epidemiological characteristics (e.g., age and gender distribution) and clinical malaria risk factors broadly in Ethiopia as well as in local areas are still lacking.
The present study determined the current epidemiological characteristics of malaria in urban and suburban sites of southwestern Ethiopia. Prospective passive case detection was conducted in health centers and district hospitals to determine clinical malaria incidence in parallel with cross-sectional surveys to determine parasite prevalence in the community. This information on malaria epidemiology can inform malaria control and elimination strategies in Ethiopia.
Methods
Study area
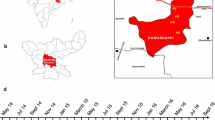
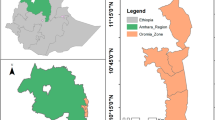
The study was conducted in Jimma town (7°41′ N, 36°50′ E, 1710–1800 m above sea level) in a highland low-transmission epidemic-prone area in Oromia Region, southwestern Ethiopia (Fig. 1). The population of Jimma town was about 176,500 in 2015. Jimma town has a warm climate with a mean annual temperature of 23 °C, a mean annual maximum temperature of 27 °C, and a mean annual minimum temperature of 19 °C. The mean annual precipitation is about 1500 mm, with one rainy season from May to September. December and January are the driest months.
Passive malaria case detection (PCD)
PCD was conducted prospectively in all public health centres and hospitals in Jimma town including Becho Bore Health Centre, Jimma Health Centre, Jimma Higher Two Health Centre, Mendera Kochi Health Centre, and Shenen Gibe Hospital, except Jimma University Special Hospital (Fig. 1). Jimma University Special Hospital usually accepts referred severe malaria cases, to avoid double count, malaria cases from this hospital were not included in data analysis. These are all the government-run malaria treatment centres and hospitals. A clinical malaria case is defined as an individual with malaria-related symptoms (fever, i.e., axillary temperature ≥37.5 °C, chills, severe malaise, headache or vomiting) at the time of examination or 1–2 days prior to the examination, with a Plasmodium positive blood smear [11, 12]. All febrile patients with malaria-like symptoms attending these health facilities were enrolled into this study upon the signing of consent or assent forms for minors. Standard case report forms were used to collect the following information from each patient: village of residency, demographic characteristics, occupation, education level, clinical symptoms, blood hemoglobin level, history of malaria in the preceding 30 days, history of travel in the 2 weeks preceding the clinic visit, history of fever, treatment drug prescribed, and ownership and use of malaria prevention measures. If ITN was used, the types and usage (used or not during the preceding night) of ITN were recorded. Thick and thin blood smears were prepared for each patient. Slides were stained with 10 % Giemsa and examined microscopically by experienced laboratory technologists at the Medical Parasitology Laboratory of Jimma University. Species were identified and parasites were counted against 200 leukocytes. All positive slides and 10 % of negative slides were reexamined blindly by another senior laboratory technologist. PCD was conducted from July 2014 to June 2015 (Fig. 2).
Cross-sectional parasitological survey
Cross-sectional parasite prevalence surveys were conducted three times in October 2014 (high-transmission season), March 2015 (end of high-transmission season), and August 2015 (end of low-transmission season), in eight kebeles (the smallest administrative unit in Ethiopia, equivalent to a district in a city) representing both urban and suburban settings (Figs. 1, 2). Households were randomly selected from each kebele to maximize coverage so that the surveyed population would represent the eight kebeles. To avoid sampling bias, one volunteer was arbitrarily selected from each selected household for participation of this study. Peripheral blood was collected by the standard finger-prick method using disposable lancets in October 2014, March and August 2015, respectively. Thick and thin smears were prepared. Slides were stained and examined at the Jimma University Specialized Hospital laboratory according to standard World Health Organization procedures. Smears were stained with Giemsa solution, and examined microscopically at a magnification of 100× by experienced laboratory technologists. Parasitaemia was determined, and quality control for slide reading was implemented as described above. Each study participant found positive for malaria was referred to a nearby health facility for treatment, according to the national malaria diagnosis and treatment guidelines.
A subset of the blood samples was subjected to further PCR analysis to confirm parasite infection and to identify the parasite species [13–15]. The initially plan was to do PCR on all blood samples, due to the zero prevalence rate detected by microscopy, PCR was only done on randomly selected samples after the second round survey.
Sample size was estimated based on the binomial model. Because the prevalence of malaria was low in the area, an estimate of 10 % prevalence was used for the peak-transmission season and 5 % for the low-transmission season in sample size determination [13, 16]. The resulting sample sizes were 559 persons for the peak-transmission season and 386 for the low-transmission season for prevalence estimation at precision level of 5 %. For the PCR method, assuming ≤5 % prevalence based on microscopic results, with a Type I error of 5 % and population (blood samples) size of 850, 218 samples could detect a marginal error of 2.5 %. To allow for 10 % PCR failure rate, 240 samples is enough.
Demographic survey
The demographic survey was conducted in the same eight kebeles as the parasitological surveys (Figs. 1, 2). Households were randomly selected from each kebele to maximize coverage so that the surveyed population would represent the eight kebeles. All individuals living in the selected households were included in the study. For all consenting individuals, demographic characteristics (age, sex, occupation, and education level) and information on preventive measures (ITN, IRS, repellent, and others) were collected through questionnaires. The survey was conducted from May to June 2014. Information collected on ITNs included ITN ownership, type, and usage. The total population at each kebeles in Jimma was obtained from the Central Statistical Agency of Ethiopia based on 2015 demographic projections. Household ITN ownership was calculated as percentage of households that owns at least one ITN based on demographic survey.
Data analysis
The density of parasitaemia was expressed as the number of asexual Plasmodium per microlitre of blood, assuming a leukocyte count of 8000 per microlitre. Annual malaria incidence rate was calculated as the number of cases per 1000 population at risk. Parasite prevalence rate was calculated as the number of infected individuals per 100 people. The incidence rate was stratified into three age groups: age < 5, 5 ≤ age < 15, and ≥15 years. Differences in sex-, age-, and occupation-specific incidence rates were analysed using χ2 test and odds ratios (ORs) were calculated for different categories separately against their respective reference group. Risk factors of clinical malaria were analysed using multivariate nominal logistic regression analysis. Risk factors included age, sex, occupation, history of malaria in the preceding 30 days, history of travel in the preceding 2 weeks, and preventive measure usage. Risk factor analysis was done separately for P. falciparum and P. vivax. SPSS (IBM Corporation, Armonk, New York, USA) was used for data analysis.
Ethical statement
Ethical clearance was obtained from the Institutional Review Boards (IRBs) of Jimma University, Ethiopia, and the University of California at Irvine, USA. Written consent (assent for children under 18 years) was obtained from heads of households and study participants. All cases with a history of fever in the preceding 3 days, as well as those with fever on examination and a positive test for malaria parasite in the blood film examination, were offered anti-malarial treatment by physicians from Jimma University as per national malaria diagnosis and treatment guidelines. Infants younger than 6 months and individuals who were unwilling to participate were excluded from the study.
Results
Epidemiological characteristics of clinical malaria
A total of 1434 suspected cases were examined and clinical malaria was confirmed in 428 cases. Among the confirmed cases, 327 (76.4 %) were P. vivax, 97 (22.7 %) were P. falciparum, and four (0.9 %) were mixed infections of P. vivax and P. falciparum. No Plasmodium malariae or Plasmodium ovale was detected. Demographic information was obtained from 10,119 individuals comprising 5278 (52.2 %) females and 4841 (47.8 %) males, with an average age of 20.7 years (Additional file 1). Among the confirmed malaria cases, 299 resided within the eight villages where demographic information was collected and available. Among these cases, 224 (74.9 %) were P. vivax, 71 (23.7 %) were P. falciparum, and 4 (1.3 %) were mixed infections of P. vivax and P. falciparum. The annual malaria incidence rate was 1.7 cases per 1000 people at risk (Table 1).
Total number of case report forms and confirmed malaria cases decreased monotonously from July 2014 to June 2015 (Fig. 3). Plasmodium falciparum malaria was seasonal and was mainly observed from July to October 2014, but the seasonality in clinical P. vivax malaria was not obvious (Fig. 3). While males had significantly higher P. falciparum malaria incidence rates (OR = 1.68, 95 % CI [1.04, 2.71], P < 0.05) (Table 1) than females, there was no gender difference in P. vivax malaria. Incidence rates did not vary by age for either malaria species (Table 1). There was a significant difference in P. vivax incidence rates among different occupation groups (Table 1). Trader and travellers had the lowest P. vivax incidence rate (0.53 cases per 1000 people year), whereas office workers and teachers had the highest incidence rate compared to the other groups (3.92 cases per 1000 people year; OR = 3.39, 95 % CI [2.08, 5.50]). Although incidence rates of P. falciparum malaria varied substantially among occupation groups, the variation was not statistically significant due to small number of falciparum cases detected (Table 1).
Five categories of malaria preventive measures were included in the questionnaire: bed nets, indoor residual spraying (IRS), repellent, others, and none. The household ITN ownership rate (percentage of households that owns at least one ITN) was 47.3 % (1173/2479) and ITN usage rate (i.e., proportion of individuals who reported to use the net the night before the survey) was 37.5 %. All ITNs were LLINs, and no repellent use was found in the questionnaire survey. The use of LLIN was not significantly associated with clinical malaria risk (Table 2). Travel during the preceding 14 days did not affect P. vivax malaria incidence, but it increased the likelihood of P. falciparum malaria by fourfolds (OR = 4.14, 95 % CI [2.11, 8.14], P < 0.001) (Table 2). On the other hand, having malaria during the preceding 30 days increased the likelihood of P. vivax malaria by nearly threefolds (OR = 2.63, 95 % CI [1.51, 4.57], P < 0.001) (Table 2).
Multiple logistic regression analysis indicated that male had marginally higher risk than females, and that people who travelled during the preceding 14 days had a significantly higher risk of having P. falciparum malaria after adjusting for other risk factors (Additional file 2). For P. vivax malaria, being an office worker or teacher, or had malaria during the preceding 30 days were the significant risk factors (Additional file 3).
Parasite prevalence in cross-sectional survey
In the cross-sectional survey low parasite prevalence was found consistently among the three sampling time points (Table 3). Overall, parasite prevalence was 0.8 % by microscope and 2.4 % by PCR. Microscopically identified parasite prevalence was about 2.7 % (0.8 % P. falciparum and 1.9 % P. vivax) during the high-transmission season in October 2014. Unfortunately, PCR was not done for this survey due to the loss of blood samples during sample transportation. Microscopy did not identify any infection for the surveys conducted in March and August 2015, but PCR analysis detected a prevalence of 2.6 and 1.7 %, respectively (Table 3). Overall, there were 23 (56.1 %) P. vivax positive samples and 18 (43.9 %) P. falciparum positive samples in the three surveys. No P. malariae, P. ovale, or mixed infections were identified by either microscopy or PCR.
Discussion
With the support of the Global Fund to Fight AIDS, Tuberculosis and Malaria, the President’s Malaria Initiative and from the African national governments, there has been a massive scale-up of antimalarial interventions in the past decade. These interventions have led to wide-scale reductions in malaria morbidity and mortality and have changed the landscape of malaria epidemiology in Africa. For example, many studies have shown that household ITN ownership has reached more than 80 % in Eastern Africa [17–19] and that the scaling-up of malaria control has greatly reduced malaria-related burdens [20–26]. Despite high coverage of ITNs and IRS, some areas, however, showed a resurgence of malaria in recent years [27–32]. This phenomenon could be linked to strong insecticide resistance in Anopheles mosquitoes [33–35]. Compare to findings from a previous study, parasite prevalence rate in Jimma has been reduced by more than 50 % in the last 5 years (5.2 % in 2010 vs. 2.5 % in 2015). While it is a significant reduction in malaria prevalence, parasite species compositions are comparable between the two studies [8].
In this study, household ITN ownership in Jimma town was 47 % and usage was 38 %. These percentages were close to the national average [5] but far below the government target of universal coverage of at-risk populations by 2015 [4]. ITN users in the study area had a slightly lower but insignificant risk of clinical malaria compared to those who did not use ITNs. This coincided with earlier reports that suggested ITN use did not reduce P. falciparum prevalence [16, 32, 36]. The ineffectiveness of ITNs may be attributed to factors such as insecticide resistance [37–39], early biting and outdoor transmission [16, 29, 36]. In Jimma, Anopheles arabiensis is the major malaria vector [16, 37, 40, 41]. Previous blood-meal analysis in An. arabiensis in Ethiopia found that the human blood index (HBI) and bovine blood index (BBI) were similar; i.e., the vector fed almost equally on human and animals [37, 40]. This implies that even if ITN usage by humans is 100 %, indoor prevention measures such as ITNs and IRS do not protect people who rest and work outdoor [37, 39, 40, 42].
Changes in age and gender distribution represent a common epidemiological shift in malaria-eliminating countries, where increasing proportions of adults and males were observed among all malaria cases regardless of malaria parasite species [43, 44]. In this study, males in the study area had higher clinical P. falciparum malaria incidence rates than females, consistent with previous studies [8, 45]. The reason for this is unclear, but it is possible that males may spend longer time outdoor during evening and thus expose more frequent to mosquito biting. Age is a malaria risk factor especially in areas of high transmission intensity [37, 43, 45–47]. In high-transmission settings, young children typically have the highest parasite prevalence and clinical incidence [12, 48–50], whereas in areas of very low transmission both children and adults are vulnerable groups [43, 44]. The finding that age was not a significant risk factor of malaria prevalence is consistent with the epidemiological situation in low transmission areas.
It is not surprising that a history of malaria illness in the preceding 30 days posed a risk for P. vivax malaria but not P. falciparum malaria. This was likely due to the relapse of vivax parasite arising from persistent liver stages of hypnozoites [51]. In the tropics, P. vivax relapse is a common phenomenon. This finding has critical implications in guiding malaria control and elimination in Ethiopia. For P. falciparum malaria, it seems that the current front-line treatment policy, e.g., first-line treatment with ACT, is possibly effective and sufficient for the elimination of falciparum malaria, given the continuous decline in clinical falciparum malaria incidence [1]. By contrast, clinical P. vivax malaria remains unchanged in the study area. More aggressive treatment such as radical cure with primaquine may be needed to avoid relapse of vivax malaria. However, it is noteworthy that extreme caution must be taken when implement primaquine for radical cure of vivax malaria because primaquine may cause acute hemolysis in individuals with Glucose-6-phosphate dehydrogenase (G6PD) deficiency, although Such effect may be less severe or absent if dosage was low [52].
It is interesting that occupation was found to be a risk factor for P. vivax malaria but not for P. falciparum malaria in the present study. Indoor workers such as teachers and office workers showed a four-fold higher risk of getting vivax malaria than outdoor workers. A possible explanation could be differential treatment-seeking behavior as the office workers and teachers are financially capable of seeking health care at the health centres [9]. Although anti-malarial treatment is free in Ethiopia, all patients are required to pay clinical registration (~US $1.00) and laboratory test (~US $0.25 per test) fees. On the other hand, individuals who travelled in the preceding 14 days showed a high risk of getting falciparum malaria. This likely resulted from being exposed to P. falciparum in surrounding rural areas with high transmission intensity [43, 44].
In this study, P. falciparum malaria was seasonal, however, seasonality in P. vivax malaria was not clear. The high number of clinical vivax cases from July to September in 2014 was inconsistent with entomological observations and rainfall patterns in the area, where the peak transmission season was expected to be from October to December [16]. Therefore, the declining trend in clinical malaria from July 2014 to June 2015 may be an overall declining in malaria transmission in the area, but yet needs to be confirmed with long-term observations.
The limitation of this study is that malaria cases were collected only from government-run malaria treatment centres and hospitals of the study areas. By the government policy, sales of anti-malarial drugs by private shops are prohibited, so self-treatment of malaria in Ethiopia is very unlikely. Malaria cases could have been underestimated because patients may seek treatments in other small private clinics that were not included here. Also, another major hospital, Jimma University Special Hospital that is specialized in treating severe malaria was not included. Although the number of malaria cases is known to be small and some case may have been reported from elsewhere, this may slightly influence the estimations of this study.
Conclusion
The urbanization process results in profound socioeconomic and landscape changes that generally reduce malaria transmission [53, 54]. However, population movement between urban areas and surrounding malarious rural areas presents an important risk to residents in urban areas. In Ethiopia, where P. falciparum and P. vivax coexist, the effects of urbanization and human movement on the clinical disease incidence of these two species were not the same, and should be investigated further. Clinical malaria disease incidence and parasite prevalence data in Jimma town suggest very low transmission that could be targeted for elimination. Epidemiological studies and risk factor analysis suggest malaria control and elimination in urban settings where P. falciparum and P. vivax coexist require new strategies.
References
WHO. World malaria report 2015. Geneva: World Health Organization; 2015.
Federal Ministry of Health of Ethiopia. National malaria guidelines. Addis Ababa: Third Edition; 2012.
Federal Ministry of Health of Ethiopia. Ethiopia National Malaria Indicator Survey 2011 Technical Summary. Addis Ababa; 2012.
Federal Ministry of Health of Ethiopia. National strategic plan for malaria prevention, control and elimination in Ethiopia, 2011–2015 (2003/2004 –2007/2008 E.C.). Addis Ababa; 2010.
WHO. World malaria report 2014. Geneva: World Health Organization; 2014.
Aregawi M, Lynch M, Bekele W, Kebede H, Jima D, Taffese HS, et al. Time series analysis of trends in malaria cases and deaths at hospitals and the effect of antimalarial interventions, 2001-2011, Ethiopia. PLoS One. 2014;9:e106359.
President’s Malaria Initiative. Ethiopia: Malaria Operational Plan FY 2015. http://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy-15/fy-2015-ethiopia-malaria-operational-plan.pdf?sfvrsn=3 Accessed 20 Jan 2016.
Alemu A, Tsegaye W, Golassa L, Abebe G. Urban malaria and associated risk factors in Jimma town, south-west Ethiopia. Malar J. 2011;10:173.
Olana D, Chibsa S, Teshome D, Mekasha A, Graves PM, Reithinger R. Malaria, Oromia Regional State, Ethiopia, 2001–2006. Emerg Infect Dis. 2011;17:1336–7.
Federal Ministry of Health of Ethiopia. National strategic plan for malaria prevention, control and elimination in Ethiopia 2014–2020. Addis Ababa; 2014.
Afrane YA, Zhou G, Githeko AK, Yan G. Utility of Health Facility-based Malaria Data for Malaria Surveillance. PLoS One. 2013;8:e54305.
Zhou G, Afrane YA, Malla S, Githeko AK, Yan G. Active case surveillance, passive case surveillance and asymptomatic malaria parasite screening illustrate different age distribution, spatial clustering and seasonality in western Kenya. Malar J. 2015;14:41.
Tadesse FG, Pett H, Baidjoe A, Lanke K, Grignard L, Sutherland C, et al. Submicroscopic carriage of Plasmodium falciparum and Plasmodium vivax in a low endemic area in Ethiopia where no parasitaemia was detected by microscopy or rapid diagnostic test. Malar J. 2015;14:303.
Lo E, Yewhalaw D, Zhong D, Zemene E, Degefa T, Tushune K, et al. Molecular epidemiology of Plasmodium vivax and Plasmodium falciparum malaria among duffy-positive and duffy-negative populations in Ethiopia. Malar J. 2015;14:84.
Lo E, Zhou G, Oo W, Afrane Y, Githeko A, Yan G. Low parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of Western Kenya. PLoS One. 2015;10:e0121763.
Degefa T, Zeynudin A, Godesso A, Michael YH, Eba K, Zemene E, et al. Malaria incidence and assessment of entomological indices among resettled communities in Ethiopia: a longitudinal study. Malar J. 2015;14:24.
Zhou G, Li JS, Ototo EN, Atieli HE, Githeko AK, Yan G. Evaluation of universal coverage of insecticide-treated nets in western Kenya: field surveys. Malar J. 2014;13:351.
Berhane A, Mihreteab S, Ahmed H, Zehaie A, Abdulmumini U, Chanda E. Gains attained in malaria control coverage within settings earmarked for pre-elimination: malaria indicator and prevalence surveys 2012, Eritrea. Malar J. 2015;14:467.
Kateera F, Ingabire CM, Hakizimana E, Rulisa A, Karinda P, Grobusch MP, et al. Long-lasting insecticidal net source, ownership and use in the context of universal coverage: a household survey in eastern Rwanda. Malar J. 2015;14:390.
Aregawi MW, Ali AS, Al-mafazy AW, Molteni F, Katikiti S, Warsame M, et al. Reductions in malaria and anaemia case and death burden at hospitals following scale-up of malaria control in Zanzibar, 1999–2008. Malar J. 2011;10:46.
Karema C, Aregawi MW, Rukundo A, Kabayiza A, Mulindahabi M, Fall IS, et al. Trends in malaria cases, hospital admissions and deaths following scale-up of anti-malarial interventions, 2000–2010, Rwanda. Malar J. 2012;11:236.
Okiro EA, Hay SI, Gikandi PW, Sharif SK, Noor AM, Peshu A, et al. The decline in paediatric malaria admissions on the coast of Kenya. Malar J. 2007;6:151.
Lim SS, Fullman N, Stokes A, Ravishankar N, Masiye F, Murray CJ, et al. Net benefits: a multicountry analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes. PLoS Med. 2011;8:e1001091.
Noor AM, Kinyoki DK, Mundia CW, Kabaria CW, Mutua JW, Alegana VA, et al. The changing risk of Plasmodium falciparum malaria infection in Africa 2000–10: a spatial and temporal analysis of transmission intensity. Lancet. 2014;383:1739–47.
Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, et al. The rise and fall of malaria in a west African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14:476–88.
Gimnig JE, Otieno P, Were V, Marwanga D, Abong’o D, Wiegand R, et al. The effect of indoor residual spraying on the prevalence of malaria parasite infection, clinical malaria and anemia in an area of perennial transmission and moderate coverage of insecticide treated nets in western Kenya. PLoS One. 2016;11:e0145282.
Kibret S, Wilson GG, Tekie H, Petros B. Increased malaria transmission around irrigation schemes in Ethiopia and the potential of canal water management for malaria vector control. Malar J. 2014;13:360.
Coulibaly D, Travassos MA, Kone AK, Tolo Y, Laurens MB, Traore K, et al. Stable malaria incidence despite scaling up control strategies in a malaria vaccine-testing site in Mali. Malar J. 2014;13:374.
Jagannathan P, Muhindo MK, Kakuru A, Arinaitwe E, Greenhouse B, Tappero J, et al. Increasing incidence of malaria in children despite insecticide-treated bed nets and prompt anti-malarial therapy in Tororo, Uganda. Malar J. 2012;11:435.
Okiro EA, Bitira D, Mbabazi G, Mpimbaza A, Alegana VA, Talisuna AO, et al. Increasing malaria hospital admissions in Uganda between 1999 and 2009. BMC Med. 2011;9:37.
Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P, et al. Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PLoS One. 2011;6:e20318.
Kamya MR, Arinaitwe E, Wanzira H, Katureebe A, Barusya C, Kigozi SP, et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg. 2015;5:903–12.
Ngufor C, Chouaïbou M, Tchicaya E, Loukou B, Kesse N, N’Guessan R, et al. Combining organophosphate-treated wall linings and long-lasting insecticidal nets fails to provide additional control over long-lasting insecticidal nets alone against multiple insecticide-resistant Anopheles gambiae in Côte d’Ivoire: an experimental hut trial. Malar J. 2014;13:396.
Wanjala CL, Mbugi JP, Ototo E, Gesuge M, Afrane YA, Atieli HE, et al. Pyrethroid and DDT resistance and organophosphate susceptibility among Anopheles spp. mosquitoes, western Kenya. Emerg Infect Dis. 2015;21:2178–81.
Quiñones ML, Norris DE, Conn JE, Moreno M, Burkot TR, Bugoro H, et al. Insecticide resistance in areas under investigation by the International Centers of Excellence for Malaria Research: a challenge for malaria control and elimination. Am J Trop Med Hyg. 2015;93(3 Suppl):69–78.
West PA, Protopopoff N, Rowland M, Cumming E, Rand A, Drakeley C, et al. Malaria risk factors in north west Tanzania: the effect of spraying, nets and wealth. PLoS One. 2013;8:e65787.
Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in South-West Ethiopia. Parasit Vectors. 2013;6:44.
Yewhalaw D, Wassie F, Steurbaut W, Spanoghe P, van Bortel W, Denis L, et al. Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS One. 2011;6:e16066.
Taye B, Lelisa K, Emana D, Asale A, Yewhalaw D. Seasonal dynamics, longevity, and biting activity of anopheline mosquitoes in southwestern Ethiopia. J Insect Sci. 2016;16:6.
Animut A, Balkew M, Gebre-Michael T, Lindtjørn B. Blood meal sources and entomological inoculation rates of anophelines along a highland altitudinal transect in south-central Ethiopia. Malar J. 2013;12:76.
Kibret S, Lautze J, Boelee E, McCartney M. How does an Ethiopian dam increase malaria? Entomological determinants around the Koka reservoir. Trop Med Int Health. 2012;17:1320–8.
Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14:244.
Zhou G, Sun L, Xia R, Duan Y, Xu J, Yang H, et al. Clinical malaria along the China–Myanmar border, Yunnan Province, China, January 2011–August 2012. Emerg Infect Dis. 2014;20:675–8.
Cotter C, Sturrock HJW, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11.
Bisanzio D, Mutuku F, LaBeaud AD, Mungai PL, Muinde J, Busaidy H, et al. Use of prospective hospital surveillance data to define spatiotemporal heterogeneity of malaria risk in coastal Kenya. Malar J. 2015;14:482.
Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, et al. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China–Myanmar border. Malar J. 2013;12:361.
Parker DM, Carrara VI, Pukrittayakamee S, McGready R, Nosten FH. Malaria ecology along the Thailand–Myanmar border. Malar J. 2015;14:388.
Munyekenye OG, Githeko AK, Zhou G, Mushinzimana E, Minakawa N, Yan G. Plasmodium falciparum spatial analysis, western Kenya highlands. Emerg Infect Dis. 2005;11:1571–7.
Hetzel MW, Morris H, Tarongka N, Barnadas C, Pulford J, Makita L, et al. Prevalence of malaria across Papua New Guinea after initial roll-out of insecticide-treated mosquito nets. Trop Med Int Health. 2015;20:1745–55.
Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, et al. High rates of asymptomatic, sub-microscopic Plasmodium vivax infection and disappearing Plasmodium falciparum malaria in an area of low transmission in Solomon Islands. PLoS Negl Trop Dis. 2015;9:e0003758.
White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297.
Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis. 2004;39:1336–45.
Wilson ML, Krogstad DJ, Arinaitwe E, Arevalo-Herrera M, Chery L, Ferreira MU, et al. Urban malaria: understanding its epidemiology, ecology, and transmission across seven diverse ICEMR network sites. Am J Trop Med Hyg. 2015;93(3 Suppl):110–23.
Tatem AJ, Gething PW, Smith DL, Hay SI. Urbanization and the global malaria recession. Malar J. 2013;12:133.
Authors’ contributions
GZ, DY, and GY designed the research. TD, EZ, ML, DZ, EK, EL and KT participated in the field work, collected the data and samples. EL, DZ and XW performed the laboratory work. GZ did the data analysis. GZ drafted the manuscript, GY, EL and DY edited the manuscript. All authors were involved in the interpretation and discussion of the results, and provided comments and approved the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We are greatly indebted to technicians and staffs from the Tropical and Infectious Diseases Research Centre of Jimma University for sample collection. We are grateful to the communities and hospitals for support and willingness to participate in this research. The work was supported by Grants from the National Institutes of Health (R21 AI101802 and D43 TW001505).
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional files
12936_2016_1298_MOESM2_ESM.docx
Additional file 2. Results of multiple regression. Dependent variable was Plasmodium falciparum (confirmed positive or negative).
12936_2016_1298_MOESM3_ESM.docx
Additional file 3. Results of multiple regression. Dependent variable was Plasmodium vivax (confirmed positive or negative).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, G., Yewhalaw, D., Lo, E. et al. Analysis of asymptomatic and clinical malaria in urban and suburban settings of southwestern Ethiopia in the context of sustaining malaria control and approaching elimination. Malar J 15, 250 (2016). https://doi.org/10.1186/s12936-016-1298-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-016-1298-2