Abstract
Background
Scale-up of malaria interventions seems to have contributed to a decline in the disease but other factors may also have had some role. Understanding changes in transmission and determinant factors will help to adapt control strategies accordingly.
Methods
Four sites in Ethiopia and Uganda were set up to monitor epidemiological changes and effectiveness of interventions over time. Here, results of a survey during the peak transmission season of 2012 are reported, which will be used as baseline for subsequent surveys and may support adaptation of control strategies. Data on malariometric and entomological variables, socio-economic status (SES) and control coverage were collected.
Results
Malaria prevalence varied from 1.4 % in Guba (Ethiopia) to 9.9 % in Butemba (Uganda). The most dominant species was Plasmodium vivax in Ethiopia and Plasmodium falciparum in Uganda. The majority of human-vector contact occurred indoors in Uganda, ranging from 83 % (Anopheles funestus sensu lato) to 93 % (Anopheles gambiae s.l.), which is an important factor for the effectiveness of insecticide-treated nets (ITNs) or indoor residual spraying (IRS). High kdr-L1014S (resistance genotype) frequency was observed in A. gambiae sensu stricto in Uganda. Too few mosquitoes were collected in Ethiopia, so it was not possible to assess vector habits and insecticide resistance levels. ITN ownership did not vary by SES and 56–98 % and 68–78 % of households owned at least one ITN in Ethiopia and Uganda, respectively. In Uganda, 7 % of nets were purchased by households, but the nets were untreated. In three of the four sites, 69–76 % of people with access to ITNs used them. IRS coverage ranged from 84 to 96 % in the three sprayed sites. Half of febrile children in Uganda and three-quarters in Ethiopia for whom treatment was sought received diagnostic tests. High levels of child undernutrition were detected in both countries carrying important implications on child development. In Uganda, 7–8 % of pregnant women took the recommended minimum three doses of intermittent preventive treatment.
Conclusion
Malaria epidemiology seems to be changing compared to earlier published data, and it is essential to have more data to understand how much of the changes are attributable to interventions and other factors. Regular monitoring will help to better interpret changes, identify determinants, modify strategies and improve targeting to address transmission heterogeneity.
Similar content being viewed by others
Background
The malaria burden has declined worldwide in the past decade. Malaria mortality rates decreased by 47 % globally and by 54 % in the WHO African Region between 2000 and 2013 [1]. During the same period, prevalence of infection in children aged 2–10 years decreased from 26 to 14 %—a 48 % decline [1]. Factors that are believed to have had a significant on this trend impact include the scale-up of key vector control interventions, availability of rapid diagnostic tests (RDTs) and effective treatment with artemisinin-based combination therapy (ACT) [2, 3]. However, the trend has not been uniform. In some areas with high baseline transmission and/or where high coverage levels have not been achieved, the malaria burden has not declined [4–7].
The changing epidemiology of malaria requires adaptation of interventions to address shifts in geographical, behavioural and demographic risk characteristics, especially as transmission declines and becomes more clustered [8]. A deeper understanding of possible determinants of change is critically important. Local knowledge of the burden and features of the disease will be important to adapt interventions and maintain cost-effectiveness and equity. Features that need to be monitored include: changes in vector habits and insecticide resistance, parasite infection patterns and drug resistance, climatic, socio-economic and demographic changes, gaps or issues in implemented interventions, and effectiveness and relevance of some control strategies.
Furthermore, a good surveillance system is essential to identify most at-risk populations and geographical areas and to assess trends and impact of interventions [9]. In addition to surveillance and monitoring of empirical data, appropriate mathematical models are useful to understand the malaria transmission dynamics. Examining various scenarios, including the extent to which a set of interventions can reduce malaria to low levels, could help to use resources optimally.
Detailed epidemiological studies have been carried out with the aim of understanding the likelihood of interruption of malaria transmission in Africa. One of the best examples of such studies was the epidemiological research undertaken in the Garki Project during 1969–1976 in a lowland rural Sudan savanna in northern Nigeria [10]. The goal was to test the effects of indoor residual spraying (IRS) and mass drug administration and to develop and test a mathematical model of transmission. Although potent interventions were applied, interruption of transmission was not achieved. Part of this failure was attributed to non-uniform exposure to sprayed surfaces due to at least partial genetically determined outdoor resting populations of Anopheles gambiae s.l. However, the model constructed by the project proved useful for planning malaria control interventions. More recently, a malaria model was developed, which has been proposed to be used in elimination scenario planning [11, 12]. Models could be used to extrapolate realistic predictions in larger geographical areas for selective control planning and evaluation of effectiveness of interventions in bringing down transmission to a low level. Data from the present project could be used to validate such models and to stratify areas for optimum impact within available resources. The project which is the subject of this paper was named ‘Beyond Garki’ to recognize the contribution of the Garki Project to the understanding of malaria epidemiology in Africa.
The project is led by Malaria Consortium and implemented in collaboration with the Ethiopian Public Health Institute and Ministries of Health in Ethiopia and Uganda, alongside Regional/District Health Offices in the study sites. Here, the project is described and data on several variables presented, including malaria epidemiology, vector behaviour and insecticide resistance, demographic and socio-economic factors, treatment-seeking behaviour and coverage of interventions in the study sites from a baseline survey carried out in October and November of 2012. Three more rounds of surveys have been carried out up to November 2014. The detailed results of these surveys and other data in comparison with the baseline survey will be published elsewhere.
Methods
Study sites
A ‘study site’ in the context of the project is defined as a ‘health centre and the catchment population in selected villages around it’. Two study sites were selected per country in Ethiopia and Uganda, representing different epidemiological settings in rural environments (Table 1).
The selection of the study sites was based on the need to represent different epidemiological (transmission) settings, geographical location and accessibility, and availability of adequate baseline morbidity data. The four study sites represented settings ranging from low seasonal transmission in the Ethiopia sites to high perennial transmission in the Uganda sites. Only villages in close proximity to the health centres were selected, covering a radius of approximately 2–6 kms to reduce potential bias in the analysis of treatment-seeking and use of services by the study population.
Ethiopia
Most areas below 2000 m above sea level are considered malarious in Ethiopia. An estimated 60 % of the population live in areas at risk of malaria transmission [13]. Both Plasmodium falciparum and Plasmodium vivax are common. The Malaria Indicator Survey (MIS) during October-December 2011 showed that nationally the prevalence of malaria was 1.3 % in areas below 2000 m; 77 % of the positive slides were P. falciparum infections [14]. There is marked seasonality in transmission and geographic variation in intensity. Many areas are epidemic-prone. Anopheles arabiensis is the main vector species [15]. Anopheles pharoensis, Anopheles funestus and Anopheles nili are considered secondary vectors. Resistance of the main vector against DDT and pyrethroids is widespread in the country [16].
Ethiopia’s organized malaria control began in 1959 when the Malaria Eradication Service was established a year after a major epidemic claimed an estimated 150,000 lives [17]. A blanket DDT spraying campaign was used until the early 1970s, when the eradication strategy was abandoned and replaced with a control programme [18]. The programme, based on selective spraying and treatment of cases, continued until the mid-1990s after which the specialized service was gradually integrated into the general health services. There has been a substantial increase in coverage of key interventions in the country. More than 64 million long-lasting insecticidal nets (LLINs) were distributed through mass campaigns between 2005 and 2014 [13]. IRS is also implemented in many areas. Through the expansion of basic health services, mainly health posts, diagnostic and treatment services have increased over the years.
Uganda
Malaria is highly endemic in approximately 95 % of the country where 90 % of the population live. The MIS in November and December 2009 reported that 42 % of children under the age of five tested positive for malaria with microscopic diagnosis [19]. Plasmodium falciparum is responsible for 99 % of malaria cases. The disease accounts for 25–40 % outpatient visits and nearly half of inpatient paediatric deaths [20]. The main malaria vectors are Anopheles gambiae s.s., A. arabiensis and A. funestus [19, 20].
Although IRS was implemented in limited sites as part of the WHO pilot programme between 1959 and 1963, the operation was not scaled up [21]. Treatment of cases remained the only malaria control measure for many years. The Malaria Control Unit was established in 1995 and grew into the National Malaria Control Programme. The main preventive interventions in Uganda are LLINs, IRS in selected districts, and intermittent preventive treatment in pregnancy (IPTp). Uganda has scaled up effective case management and in some regions village health teams (VHTs) were trained to test and treat common childhood illnesses including malaria through Integrated Community Case Management (ICCM). In 2009, 47 % of households owned at least one insecticide-treated net (ITN) compared to 16 % in 2006 [19, 22]; this increased to 60 % in 2011 [23]. These combined efforts are believed to have resulted in reduced transmission in many areas [24].
Up to 10 districts in northern Uganda have been sprayed in the past 6–7 years within the IRS programme supported by the US Government’s Presidential Malaria Initiative (PMI) [20]. Starting from 2014, more northern and eastern districts were added while the operation ended in others (including Apac, the district containing the study site Aduku) due to a decline in transmission. A large reduction in malaria prevalence was observed in children living in sprayed areas compared to those living in unsprayed areas [25]. Meanwhile, more than 21 million LLINs were distributed in a nation-wide mass campaign during 2012–2014.
Study components
Repeat cross-sectional surveys were conducted in the selected sites (of which only results from the first study are presented here as the baseline data). The study also included longitudinal collection of meteorological and morbidity data at health facilities. The main components include: household surveys, malariometric and serological surveys, entomological surveys, health facility-based morbidity studies, and climatic studies. For the household surveys, the required sample size was estimated for each site by assuming 5 and 50 % baseline prevalence in children below 10 years in Ethiopia and Uganda, respectively.
Household surveys
All households in villages around each health centre (within radius of 2–6 kms) were enumerated and included in the sampling frame. A simple random sample of 571 and 234 households were selected in each site in Ethiopia and Uganda, respectively. The sample sizes were determined separately for the two countries based on expected malaria prevalence rates, household sizes and a 10 % non-response rate using appropriate statistical procedures, and sample sizes for each site were calculated independently assuming simple random sampling. The household surveys included interviews with household heads and women aged 15–49 years of age using handheld devices (smartphones with Pendragon Forms 5.1 or tablets with Open Data Kit). Data were collected on variables indicating socio-economic status (SES), prevention methods, knowledge about malaria, ITN ownership and use, as well as number of children born to interviewed women who were alive and dead, febrile illness in children, treatment sought and protection against malaria during pregnancy.
Malariometric surveys
Each member of the sampled households (except infants under 6 months) was given a subject card and asked to visit a malariometric testing site within the village to obtain anthropometric measurements and collect blood samples. Bodyweight, temperature, height and mid-upper arm circumference were measured for children under five. Thick and thin blood films for microscopy, dry blood spots for serology and blood samples for haemoglobin measurement using the HemoCue machine (Hb 301, Ängelholm, Sweden) were obtained for all subjects. RDTs (CareStart™ pf-HRP2/pan-pLDH by Access Bio USA in Ethiopia and SD Bioline in Uganda) were used to test subjects with body temperature 37.5 °C and above or history of fever in the previous 48 h. Individuals with fever or history of fever were tested by RDT for the purpose of providing anti-malarial treatment. Treatment was provided at the field site according to national guidelines for mild and moderate anaemia (using ferrous sulphate) and uncomplicated malaria cases (using artemether–lumefantrine for P. falciparum and chloroquine for P. vivax), while severe cases were referred to the site’s health centre.
Slides were stained with Giemsa and examined by two independent microscopists for presence/absence of asexual parasites and gametocytes and species identification. In the case of discrepant results, a third microscopist examined the slides for a final verification.
Serological analysis of dry blood spots from the Uganda sites was carried out to determine antibody responses to assess malaria transmission intensity over an extended period of time. The antibody response of individuals against merozoite surface protein-119 (MSP-119) was determined using an enzyme-linked immunosorbent assay (ELISA). Serum obtained from the dried blood spots on filter papers was analysed at the Medical Research Council (MRC) Laboratory in Uganda for total IgG antibodies using P. falciparum antigen MSP-119 (CTK Biotech, USA, cat. No. A3003) following previously described methods [26, 27].
Entomological surveys
Anopheles mosquitoes were sampled to determine species composition, densities, behaviour and insecticide resistance using light trap collection, exit trap collection, room search, pyrethrum spray catch and human landing catch (HLC) in 12 houses per site selected using simple random sampling from the sampling frame for the household survey. Mosquitoes were identified using morphological features and individually packed in microtubes for molecular analysis, which were carried out at Rothamsted Research in the UK [28]. Genomic DNA was extracted using the Livak method. A. gambiae s.l. samples were analysed to determine whether they were A. gambiae s.s. or A. arabiensis [29, 30]. Anopheles gambiae s.l. samples were analysed mainly for knock down resistance (kdr) mutations (but also for mutation in the ace-1 gene which encodes the acetylcholinesterase enzyme although not reported here) [31].
Other study components
Other components of the study not reported in the present paper include: use of automatic weather stations (BWS200 automatic weather station, Campbell Scientific, Stellenbosch, South Africa) installed in all sites to record hourly meteorological data, compilation of outpatient morbidity data for every suspected or confirmed malaria patient seen at the health facility in each site, and mathematical modelling of transmission.
Ethical considerations
Ethical clearance was obtained from the appropriate review boards (Uganda: UNCST 1348; Ethiopia: 3-10/819/05). In addition, written consent was obtained from respondents for interviews, for all subjects that participated in malariometric surveys, and from household heads for entomology sentinel houses.
Data entry and analysis
EpiData v3.1 (The EpiData Association, Odense, Denmark) was used for data entry where necessary. Stata versions 12 and 13 (StataCorp LP, College Station, TX, USA) and Microsoft Excel (Microsoft Corporation) were used for data analysis.
Household survey data
Chi squared tests were used where appropriate to assess significant differences between groups of interest, taking into account clustering at household level. Principal components analysis (PCA) was used to calculate wealth index for each household, computed separately for each country.
Malaria infection rates
Infection prevalence data were analysed in relation to potential household or individual risk factors such as coverage and use of prevention methods, housing conditions, demographic factors and socio-economic status.
Undernutrition
Undernutrition was studied using the anthropometric data for children under five. A Stata program file ZSCORE06 developed by Jef Leroy (Boston College Department of Economics) was used to calculate anthropometric z-scores using the 2006 WHO Child Growth Standards [32].
Anaemia
Anaemia was classified as mild, moderate or severe based on the concentrations of haemoglobin (Hb) as follows; (a) mild anaemia: for non-pregnant women, Hb 10.0–11.9 g/dl; for pregnant women and children under 5, Hb 10.0–10.9 g/dl; for men: Hb 10.0–12.9 g/dl; (b) moderate anaemia: Hb 7.0–9.9 g/dl; c) severe anaemia: Hb <7.0 g/dl [33].
Serological studies
Optical density (OD) values were analysed in Microsoft Excel using a macro file provided by C. Drakeley, London School of Hygiene and Tropical Medicine (LSHTM). Normalized OD values were used for data analysis using a Stata procedure provided by C. Drakeley (LSHTM). A cut-off value of 0.177 was used to determine seropositive samples. Age seroprevelance curves were generated using methods described by Corran et al. [34]. Data for children below 2 years was excluded to avoid potential bias caused by maternal antibodies [35].
Under-5 mortality rates
A variant of the Brass indirect method [36] was used to calculate under-five mortality rate (U5MR) using the summary birth history dataset provided by women of child-bearing age which included age of mother, total number of live births and total number of deaths [37]. Mortality rates were not calculated for the 3 years period before the survey date (2009–2012), namely data related to women aged 15–19, because of the selection effect where women from lower socioeconomic classes tend to start childbearing early and their children face above average mortality risks [38]; and because random errors are larger for estimates based on the reports of young women, since they have fewer children ever born.
Entomological data
Various entomological parameters were estimated including species compositions, and indoor resting and biting habits. Human biting rates (i.e., the number of bites per person per night) were calculated taking into account the number of collectors working simultaneously, the number of collection nights, and the assumed night-time behaviour of the local human populations. It was assumed that an average villager in each of the sites spends 1 h on average outdoors between 18:00 h and 22:00 h, and all villagers are indoors after 22:00 h.
Results
A total of 1521 households with complete records were included in the analysis of this first survey from the four sites in Ethiopia (average 540 per site) and Uganda (average 221 per site). Household response rates were 94.6 and 94.4 % in Ethiopia and Uganda, respectively. In total, 8079 people were registered. Nearly all the registered household members were usual residents, ranging from 97.2 % in Aduku (Apac district, Uganda) to 100 % in Guba (Halaba Special district, Ethiopia). Females constituted 51.6–53.2 % of the populations in the study sites.
Blood samples for microscopy and serology and body temperature measurements were obtained for 66 % of registered household members for whom subject cards were issued (the rest did not visit the testing site or did not provide consent). Blood samples for haemoglobin measurements were obtained from 62 % of registered subjects (some tests were missed due to shortage of equipment).
Malaria prevalence
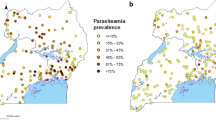
Prevalence of malaria infection varied between the four sites, ranging from 1.4 % in Guba to 9.9 % in Butemba (Fig. 1). The predominant species in Ethiopia was Plasmodium vivax which accounted for 52–67 % of all infections. In Uganda, P. falciparum accounted for 95–100 %. Malaria prevalence varied among age groups in most sites (Fig. 2). The risk of infection tended to be high in older subjects in Aduku. The age group pattern in both sites of Ethiopia was typical of low endemicity where all age groups are more-or-less equally affected, whereas the pattern in Butemba (Uganda) reflected moderate endemicity where malaria prevalence rates in older children and adults are less than rates in younger children as a result of acquisition of partial immunity due to repeated infections over time [39]. Aduku exhibited a pattern somewhat between low and moderate endemicity. The seemingly high prevalence in the under-one age group in the Hembecho site was due to a small sample size (one infection out of seven infants).
Subjects with fever had higher infection prevalence than those without fever in Butemba (p = 0.016) and Guba (p < 0.001). History of fever, however, had no association with malaria infection risk in all sites except in Guba where subjects with history of fever were more likely to be positive (X2 = 30.86, p < 0.001).
The positive and negative predictive values of RDTs for these cases were calculated for each site using the slide positivity rates determined by microscopy. The negative predictive value (NPV) of RDT for P. falciparum was high (≥99 %) in all three sites with sufficient sample sizes for this analysis (Aduku, Butemba and Hembecho). The positive predictive value (PPV) of RDT was low in all three sites (≤44 %). RDT results for P. vivax infection exhibited a similar pattern of high NPV (≥98 %) and low PPV (≤40 %) for the two sites in Ethiopia.
Anaemia
There was a uniformly high prevalence of mild anaemia in all sites, ranging from 53 % in Aduku to 65 % in Hembecho. However, substantial variation was observed in prevalence of moderate and severe anaemia, ranging from 2.4 % in Aduku to 6.0 % in Butemba.
Age-specific seroprevalence
A total of 1123 samples were analysed for the antigen MSP-119, 602 for Aduku and 521 for Butemba. Overall seropositivity (i.e., the proportion of individuals with a positive antibody response to MSP-119) was higher in Butemba (45.7 %) than Aduku (22.9 %), indicating higher overall exposure to malaria. The seroconversion rate (λ) was higher in Butemba compared to Aduku, indicative of a higher transmission intensity (Fig. 3).
Age-specific seroprevalence for P. falciparum anti-MSP-119 antibodies. Dots, continuous lines and broken lines represent data, fitted estimates and 95 % confidence intervals, respectively. λ is the seroconversion rate. The 0–2 years age group was omitted because of distortions caused by presence of maternal antibody in high endemicity settings [35]
Under-five mortality rates
Summary birth histories of 1503 women were available: 1041 women in Ethiopia and 462 in Uganda. Of these 88 % had complete data and were retained in the analysis. In total 3317 live births were reported in Ethiopia and 1101 in Uganda and the average number of live births to women aged 15–49 years was approximately 3.5 and 2.9 in Ethiopia and Uganda, respectively. As expected, the number of live births increased in older age groups. Mortality rates among live births were 4.1 % in Ethiopia and 6.2 % in Uganda. The trend observed for Ethiopia is a reduction of almost 60 % in the U5MR from approximately 60 deaths per 1000 live births for the years prior to 2000 to approximately 23 deaths per 1000 live births post-2000 (Fig. 4). A similar reduction was observed in Uganda. Pre-2000, the U5MR was approximately 113 per 1000 live births, whereas post-2000, the rates fluctuated between 34 and 67.
Ownership and use of ITNs
ITN coverage varied between countries and sites. Hembecho had the highest coverage whereas Guba had the lowest (Table 2). Net ownership did not vary with SES. Purchasing of nets was evident in Uganda but not in Ethiopia. In Uganda, 42/606 (7 %) of the mosquito nets were purchased by the households themselves. None of these nets were identified as ITNs (15 were not ITNs and 27 were of unknown type). Thirty-one were bought from open market, 10 from shops and 1 from a pharmacy. The proportion of nets purchased was not higher in the upper (richer) socioeconomic groups.
Access of household members to ITNs was calculated for each study site. To assess access of nets within the household, first the potential number of people who could use the available nets was computed assuming one net for two people. When the potential number of people who could use the available nets exceeded the total number of people who spent the previous night in the household, the two figures were assumed to be equal. The sum of all potential users in the sample was then divided by the total number of people who spent the previous night in surveyed households to estimate the access rates or the proportion of population with access to an ITN within the household.
The ITN use rates among those who have access were then estimated for each site. The data show that there was variation between and within countries in terms of access to nets. Hembecho had the highest access rate while Guba had the lowest.
Among people with access to ITNs, use rates were high in three of the four sites (ranging from 69 to 76 %), with the exception of Guba in Ethiopia, where only a third of those with access used a ITN the previous night. There was no inequity between the sexes in ITN use for any site, except for adults in Aduku, where significantly more females used an ITN (OR = 1.6; 95 % CI 1.1–2.2).
Indoor residual spraying coverage
No IRS was carried out in Butemba. The remaining three study areas contained households that had been sprayed in the last 12 months, with or without concurrent ITN use. The coverage of IRS was consistently high across the villages within each study site. On average, 84 % of households in Aduku were sprayed within the last 12 months, 96 % in Hembecho and 85 % in Guba.
Prevalence of infection by use of preventive measures
Individuals who used an ITN the night before the survey had significantly lower malaria infection prevalence in Aduku (2.3 % versus 5.7 %; p = 0.020). No statistically significant difference was observed in the other sites. There was no statistically significant difference in malaria infection prevalence between houses with open/partially open or closed eaves in all sites. However, in Guba (the site with the lowest ITN ownership and use rates), houses with open eaves had slightly higher risk of infection with P. falciparum although this was not statistically significant (p = 0.087).
Out of the three sites where IRS was implemented, prevalence of infection was significantly lower in individuals living in sprayed houses compared to those in unsprayed houses only in Guba (p = 0.042). Among individuals who slept in sprayed houses, the prevalence of infection was significantly lower in those who used ITNs in Aduku (p = 0.026), indicating that ITNs are more protective in this site than they are in the other sites. No such association was observed in the other two sprayed sites.
Febrile illness in children and treatment-seeking behaviour
A higher proportion of children under 10 years of age had fever or history of fever in the two Ugandan sites compared to the Ethiopian sites (Table 3). In Uganda, treatment was sought for more than 90 % of all children with fever, whereas in Ethiopia, this ranged between 62 and 80 %. In Aduku, first treatment was most commonly sought from privately owned clinics, pharmacies or drug shops, while in Butemba similar proportions were treated in the public and private sectors. In both Ethiopian sites, first treatment was primarily sought in the public sector. Village Health Teams (VHTs) in Uganda and Health Extension Workers (HEWs) in Ethiopia played important roles in providing early diagnosis and treatment of uncomplicated malaria within their communities.
Anti-malarials were given to the majority of children with fevers in all sites with the exception of Hembecho, where only 36 % of children received an anti-malarial. ACT use varied across the sites (In Ethiopia, chloroquine was used as well as ACT as it was first-line treatment for vivax malaria). For approximately half of the children that were given an anti-malarial in Uganda, treatment was started within 24 h following onset of fever. In Ethiopia, only 2–7 % of children who received an anti-malarial started the treatment within 24 h of onset of fever. Approximately 52–60 % and 77–92 % of children with fever who sought treatment received a malaria diagnostic test (either RDT or microscopy) in Uganda and Ethiopia, respectively.
Prevention of malaria in pregnancy
Overall, IPTp use was more common in Butemba compared to Aduku (X2 = 15.1, p = 0.004), and 36.9 % of the women who gave birth within 2 years preceding the survey took two doses of IPTp, compared to 20.5 % in Aduku (Table 4). The proportion of women that took three or more doses was around 7–8 % in both sites (IPTp is not implemented in Ethiopia).
Knowledge about malaria
A large majority of respondents in Uganda identified mosquito bites as the cause of malaria (93 % in Aduku and 77 % in Butemba), whereas in Ethiopia the percentages were relatively lower (65 % in Hembecho and 39 % in Guba). In Uganda, 89 and 79 % of respondents had heard information about malaria in Aduku and Butemba, respectively, whereas messages about malaria were less frequently heard in Ethiopia (X2 = 158.6, p < 0.001) (61 % in Hembecho and 36 % in Guba). The main sources of information in the Uganda sites were radio and health workers, while in Ethiopia, community leaders and health workers were mentioned most often.
Mosquito density and species composition
In Ethiopia, only 22 A. gambiae s.l. females were collected from both sites, which were probably A. arabiensis. In Uganda, a total of 1670 and 315 anopheline females were collected using the various trapping techniques in the Aduku and Butemba sites, respectively. In Aduku, the most common vector species was A. funestus s.l. (53 % of the collections), while A. gambiae s.l. made up only 18 % of the collections. The majority of other anophelines considered as non-vectors belonged to the Anopheles coustani complex. In Aduku, 94 % of the A. gambiae s.l. were identified as A. arabiensis and 6 % as A. gambiae s.s. (molecular identification of A. funestus s.l. is yet to be completed).
In Butemba, A. gambiae s.l. was the dominant species (89 % of all collections) and no A. funestus s.l. was collected. Molecular analysis showed that 93 % of all A. gambiae s.l. mosquitoes collected in Butemba were A. gambiae s.s. while 7 % were A. arabiensis.
Vector biting habits
In Butemba, the only vector species caught by HLC was A. gambiae s.l. However, only seven mosquitoes were caught indoors which was not sufficient to deduce any feeding patterns. In Aduku, the A. gambiae s.l. identified were 17 A. arabiensis and one A. gambiae s.s. These were observed to feed primarily after midnight indoors (Fig. 5). The number collected was too few to determine any behavioural differences between the sibling species. A. funestus s.l. was observed to feed much earlier in the evening both indoors and outdoors. This result, however, requires confirmation and as the molecular analysis of A. funestus s.l. has not been completed to confirm if the species under question is an important vector, this result should be interpreted with caution. Additionally, A. coustani s.l. showed a similar feeding pattern to A. funestus s.l.
Human biting rates (HBR) for all anopheline species combined in Aduku was 7.3 anopheline bites per person night (Table 5). Anopheles funestus s.l. was the largest contributor to the HBR followed by A. coustani s.l. and A. gambiae s.l. In Butemba, the HBR was 0.5 bites per person night. If given equal opportunity (or if human baits are present both indoors and outdoors) during 1800–0000 h, A. gambiae s.l. showed a clear preference for biting outdoors (75 %) in Aduku. In contrast, A. coustani s.l. clearly preferred biting indoors (only 24 % preferred biting outdoors), whereas A. funestus s.l. readily fed both indoors and outdoors.
Nevertheless, assuming that an average villager spends one hour outside prior to 2200 h and is indoors after 2200 h, the majority of human-vector contact for all three species occurred indoors, ranging from 83 % for A. funestus s.l. to 93 % for A. gambiae s.l. Assuming that by 2200 h all residents would go to bed, the proportion of contact before this time could be used as a proxy for the potential risk of malaria exposure. The largest proportion of human-vector contact for A. funestus s.l. (65 %) and A. coustani s.l. (76 %) took place before 2200 h. For A. gambiae s.l. only 13 % of the human-vector contact occurred before 2200 h. As mentioned above the results for A. funestus s.l. should be interpreted fully only when species data is available.
Kdr-L1014S genotype frequencies
In Butemba, kdr-L1014S (kdr-east) frequencies were 94.7 % (n = 113) in A. gambiae s.s.; no susceptible individuals were observed. The few A. arabiensis analysed (n = 7) were all susceptible. In Aduku, A. gambiae s.s. showed a high L1014S frequency of 81 % (n = 13), while for A. arabiensis frequency was 3 % (n = 252).
Undernutrition
Undernutrition was an important problem in both countries. However, a far greater proportion of children under five in the Ethiopian sites (29-32 %) were underweight compared with the Ugandan sites (6–7 %). The Ethiopia sites had the highest percentage of children affected by undernutrition. Approximately two-thirds of children in the Ethiopian sites were stunted (Fig. 6). Wasting, which is an indicator of acute undernutrition, was most prevalent in the Guba site (16.8 %) and affected a much lower proportion of children in the Hembecho site (3.4 %). In the Butemba site of Uganda, more than a quarter of children under five were stunted (no data was collected on stunting and wasting in Aduku). In Butemba, 8.1 % of children were affected.
Discussion
A comprehensive survey was carried out in four sites in Ethiopia and Uganda to gather baseline data for future surveys aimed at monitoring changes in malaria epidemiology and effectiveness of interventions over time. Malaria transmission during October–November 2012 in all locations was low or moderate including in previously highly endemic areas although the surveys were conducted during months normally considered as falling in the peak transmission season.
Malaria prevalence in the Ugandan sites was much lower than historically recorded, particularly in the district of Apac [40]. However, as the studies are only in few sites, they may not reflect national trends. It is likely that the combined use of both IRS and LLINs led to the observed decline. Apac has been under the IRS programme since 2007. As baseline transmission levels vary across sites, it is inappropriate to compare the four study sites; however, it is worth noting that the highest malaria prevalence was observed in the site that was not under IRS in Uganda. The point prevalence data may not represent the actual average situation in a site. There is considerable seasonality of transmission in both countries as well as inter-annual variations. Especially in Ethiopia, the variations are likely to be governed by meteorological conditions [41, 42]. It is not always possible to accurately determine the peak of the transmission seasons correctly. In Ethiopia, prevalence rates were generally comparable to rates reported in the 2011 MIS survey [14]. However, the MIS survey reported dominance of P. falciparum contrary to the finding of the present study the previous year. The dominance of P. vivax in the present study could be due to reduced transmission conditions either as the result of transmission factors being relatively less favourable for P. falciparum or overall reduction of transmission due to the effects of interventions. In Uganda, however, prevalence rates were much lower than rates reported in the 2009 MIS survey during the same season [19].
Serology data can indicate systematic changes in transmission intensity by looking at the age-seroprevalence distribution [35]. Serological data confirm a higher level of transmission in Butemba compared to Aduku in Uganda. The age group pattern of infection prevalence also indicates a relatively high transmission situation in Butemba. The model does not fit some of the younger age groups well in Aduku, which could reflect transmission changes due to the ongoing IRS programme using the carbamate insecticide bendiocarb. Similarly, in Butemba, the youngest age group shows a lower than expected seroprevalence rate, which could be due to the LLIN mass distribution campaign in 2010. However, small sample sizes in younger age groups could also account for these findings, and additional serology data from subsequent rounds and additional analyses of samples from Ethiopia, as well as use of other antigens, can provide more robust datasets to analyse the interventions in terms of transmission changes.
Entomological results yielded important information on the situation of vectors. A. gambiae s.s. dominated in Butemba, while in Aduku, A. funestus s.l. and A. arabiensis were the main vectors. Prior to the scale up of interventions, A. funestus s.l. was the main vector in Apac [40], suggesting more success of the vector control efforts against this vector. Most human-vector contact with A. gambiae s.l. occurs indoors in Aduku, Uganda, which shows that the use of interventions against indoor-biting vectors should be considered effective. However, the early feeding observed for A. funestus s.l. requires confirmation with data from subsequent surveys. A substantial number of A. coustani s.l. were collected by human landing catch suggesting attraction to humans. Its status as a malaria vector is unknown in Uganda. A study in Zambia found a high degree of anthropophily in A. coustani s.l. [43] and it was reported to be contributing to transmission in Kenya [44]. Forthcoming analyses will determine infectivity rates of vectors to calculate entomological inoculation rates.
Insecticide resistance is increasing in many areas of Uganda [45, 46]. High kdr-L1014S frequencies were observed in A. gambiae s.s. and low frequencies in A. arabiensis, a finding which was in line with another study from Eastern Uganda [47]. Further studies will be needed to confirm the magnitude and impact of insecticide resistance in both countries. No tests were done in Ethiopia due to small sample size but widespread resistance to DDT and pyrethroids has been reported [16, 48]. Resistance against pyrethroids and DDT has reached a high level in both countries, probably due to the substantial increase in the distribution and use of LLINs in recent years as these insecticides share a similar mode of action in terms of knock-down resistance.
Regarding diagnosis and treatment, there are variations observed in both countries, especially around use of services. In Uganda, seeking treatment in the private sector was common. These findings are in line with the MIS 2009 survey, which observed that treatment was sought for 82 % of fevers of children under five treatment and 56 % of children with a fever were taken to the private sector [19, 49]. In Ethiopia, a great majority of febrile children were taken to public health facilities for treatment.
As part of the malariometric survey, febrile subjects were tested with RDTs which provided an opportunity to compare with subsequent microscopy results. The RDTs used had high negative predictive value, but their positive predictive value was uniformly low in all sites. This shows that negative results with the RDTs for febrile patients can be a good indicator of absence of infection but a positive result may not always be a reliable indicator of presence of infection. These results may be explained by the nature of the RDTs that test for the presence of parasite antigens which circulate in the blood for several weeks post-infection [50, 51].
Prevention of malaria in pregnancy was one of the interventions implemented in Uganda. The survey results indicated that IPTp uptake was low in Uganda and needs to be strengthened if the current recommendation of at least three doses is to be reached [52]. The rather low coverage or use levels are in line with findings from the DHS survey in 2011 which found that 25 % of women reported to have taken at least two doses of IPTp [23]. While data for Aduku was similar to the national average in 2011, in Butemba a higher proportion of women reported using IPTp, likely due to activities of various projects in this region working to improve its uptake.
Knowledge of malaria varied among the sites. Households in the Ugandan sites had overall better knowledge about malaria compared to the Ethiopian sites. However, in Butemba, other causes of malaria were frequently cited, unlike in Aduku where most respondents correctly identified mosquitoes as the only cause of malaria, likely because of behavioural change communication (BCC) campaigns that accompanied IRS implementation. In Guba, knowledge about the cause of malaria needs to be improved through appropriate community education. In connection with this, the low ITN ownership and use rates in Guba and possibly surrounding areas in Ethiopia will require close attention by the health services.
Universal coverage is defined differently in the two countries: two ITNs per household in Ethiopia and one ITN for two people in Uganda. In one of the Ethiopian sites (Hembecho), the goal was nearly achieved with 1.9 ITNs per household whereas ownership in the second site (Guba) was the lowest of all sites (0.7 ITNs). The cause of the low ownership rate in Guba requires more investigation. In Uganda, although the percentages of sleeping places that can potentially be covered with ITNs available in the households were 44 and 71 % in Aduku and Butemba, respectively, the percentages of households with one ITN for two people were 24 and 30 %, respectively. The ITN coverage is expected to increase substantially following a recent nationwide campaign in Uganda.
Uniformly high IRS coverage was observed in the sprayed sites. In one of the four sites (Aduku), individuals who slept under an ITN the night before the survey had lower risk of infection than those who did not. This could be due to low level of pyrethroid resistance in that site [53]. The lack of association in ITN use and malaria in the other sites does not necessarily translate to lack of effectiveness. It may be partly explained by ‘mass’ effects of ITNs or IRS (in the sprayed sites) which could confound the result, as people who did not use nets might have been ‘protected’ by either all other nets in the villages or the IRS due to mortality effects on mosquito vectors or a combination of these.
It is not clear whether ITN and IRS have an additive or synergistic action when used in combination. The current WHO recommendation is that where LLIN coverage is high and they remain effective, IRS may have limited utility in reducing malaria morbidity and mortality, unless the combined use is for resistance management [54]. In Guba where both ITN ownership and use rates were low, malaria infection risk was higher in individuals living in unsprayed houses than in sprayed ones, indicating the potential benefit of the combined use of both interventions in such situations.
One of the main observations in the present survey was the high level of undernutrition in the study sites. Undernutrition during the critical first 1000 days of a child’s life could have devastating consequences by increasing morbidity and mortality and development of the child. Recently, stunting (or low height for age) has been chosen as a key indicator to measure global and national progress towards reduction of undernutrition [55]. Undernutrition is particularly a severe problem in the Ethiopia sites where two-thirds of the children were classified as stunted. Critical nutrition interventions should be strengthened addressing both maternal and child undernutrition. These include, among others, promoting optimal breastfeeding practices, micronutrient supplementation, reducing incidence of low birth weight and prevention of disease.
Conclusion
Low malaria prevalence was observed in some sites that previously had high endemicity but there was substantial variation between sites. Intensified vector control and effective treatment seem to have played key roles in bringing endemicity down over recent years, as reflected in the results of serological analyses. Malaria control efforts should be sustained to reduce transmission further, maintain the gains and prevent resurgence. Control strategies should be adapted to the changing patterns and heterogeneity of transmission which may require a thorough epidemiological stratification and selective targeting of interventions. A tendency of early biting in A. funestus s.l. in Aduku requires further investigation. The impact of pyrethroid resistance in A. gambiae s.s. in Uganda on effectiveness of LLINs should be studied further. Pre-emptive rotation of insecticides should be considered by IRS programmes. Non-pyrethroid IRS may be considered when feasible and where other measures have inadequate impact, or in areas where there are major obstacles to achieving high ownership and use of LLINs. Robust continuous distribution systems are needed to replace LLINs and maintain high coverage. Whether low LLIN coverage in Guba is indicating a similar problem elsewhere should be investigated and more efforts are needed to create a culture of net use. Health services should create conditions to make LLINs available in the commercial market at affordable prices and educate communities on the benefits of LLINs over untreated nets.
The findings will serve as a baseline against which results of future surveys or other studies could be compared. Although results from a small number of study sites may not be nationally representative, the approach provides more comprehensive information on a range of potential determinants of malaria rates than more geographically extensive surveys and surveillance, and they will provide a basis for and may prompt further investigations of some of the observations. It is necessary to continue monitoring the epidemiological changes and more studies through in-depth analysis and modelling will reveal the impact of the various interventions on malaria transmission.
Change history
25 November 2019
Please be advised that one of the author names is incorrectly spelled in the published article: ‘Irene Kyomuhagi’ should be ‘Irene Kyomuhangi’.
References
WHO. World malaria report 2014. Geneva: World Health Organization; 2014.
Aregawi M, Lynch M, Bekele W, Kebede H, Jima D, Taffese HS, et al. Time series analysis of trends in malaria cases and deaths at hospitals and the effect of antimalarial interventions, 2001–2011, Ethiopia. PLoS One. 2014;9:e106359.
Karema C, Aregawi MW, Rukundo A, Kabayiza A, Mulindahabi M, Fall IS, et al. Trends in malaria cases, hospital admissions and deaths following scale-up of anti-malarial interventions, 2000–2010, Rwanda. Malar J. 2012;11:236.
Bennett A, Kazembe L, Mathanga DP, Kinyoki D, Ali D, Snow RW, Noor AM. Mapping malaria transmission intensity in Malawi, 2000–2010. Am J Trop Med Hyg. 2013;89:840–9.
Kamuliwo M, Chanda E, Haque U, Mwanza-Ingwe M, Sikaala C, Katebe-Sakala C, et al. The changing burden of malaria and association with vector control interventions in Zambia using district-level surveillance data, 2006–2011. Malar J. 2013;12:437.
Mukonka VM, Chanda E, Haque U, Kamuliwo M, Mushinge G, Chileshe J, et al. High burden of malaria following scale-up of control interventions in Nchelenge District, Luapula Province, Zambia. Malar J. 2014;13:153.
Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, et al. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop. 2012;121:184–95.
Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11.
WHO. Disease surveillance for malaria control: an operational manual. Geneva: World Health Organization; 2012.
Molineaux L, Gramiccia G. The Garki Project: research on the epidemiology and control of malaria in the Sudan Savanna of West Africa. Geneva: World Health Organization; 1980.
Griffin JT, Hollingsworth TD, Okell LC, Churcher TS, White M, Hinsley W, et al. Reducing Plasmodium falciparum malaria transmission in Africa: a model-based evaluation of intervention strategies. PLoS Med. 2010;7:e1000324.
WHO. From malaria control to malaria elimination: a manual for elimination scenario planning. Geneva: World Health Organization; 2014.
PMI. Malaria Operational Plan FY 2015—Ethiopia. President’s Malaria Initiative, USAID; 2014.
EHNRI, Partners. Ethiopia National Malaria Indicator Survey 2011. Addis Ababa: Ministry of Health; 2012.
Abose T, Yeebiyo Y, Olana D, Alamirew D, Beyene Y, Regassa L, Mengesha A. Re-orientation and definition of the role of malaria vector control in Ethiopia. WHO/MAL/98.1085. Geneva: World Health Organization; 1998.
Balkew M, Ibrahim M, Koekemoer LL, Brooke BD, Engers H, Aseffa A, et al. Insecticide resistance in Anopheles arabiensis (Diptera: Culicidae) from villages in central, northern and south west Ethiopia and detection of kdr mutation. Parasit Vectors. 2010;3:40.
Fontaine RE, Najjar AE, Prince JS. The 1958 malaria epidemic in Ethiopia. Am J Trop Med Hyg. 1961;10:795–803.
Gish O. Malaria eradication and the selective approach to health care: some lessons from Ethiopia. Int J Health Serv Plan Adm Eval. 1992;22:179–92.
UBOS, ICF: Uganda Malaria Indicator Survey 2009. Kampala and Claverton: Uganda Bureau of Statistics and ICF Macro; 2010.
PMI. Malaria operational plan FY 2015—Uganda. President’s Malaria Initiative, USAID; 2014.
MCP. Uganda malaria control strategic plan 2005/06–2009/10. Kampala: Ministry of Health.
UBOS, MII. Uganda demographic and Health survey. Calverton: UBOS and Macro International Inc.; 2007.
UBOS, ICF. Uganda demographic and health survey 2011. Kampala and Claverton: Uganda Bureau of Statistics and ICF International Inc.; 2012.
Kigozi R, Baxi SM, Gasasira A, Sserwanga A, Kakeeto S, Nasr S, et al. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS One. 2012;7:e42857.
Steinhardt LC, Yeka A, Nasr S, Wiegand RE, Rubahika D, Sserwanga A, et al. The effect of indoor residual spraying on malaria and anemia in a high-transmission area of northern Uganda. Am J Trop Med Hyg. 2013;88:855–61.
Corran PH, Cook J, Lynch C, Leendertse H, Manjurano A, Griffin J, et al. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195.
Egan AF, Chappel JA, Burghaus PA, Morris JS, McBride JS, Holder AA, Kaslow DC, Riley EM. Serum antibodies from malaria-exposed people recognize conserved epitopes formed by the two epidermal growth factor motifs of MSP1 (19), the carboxy-terminal fragment of the major merozoite surface protein of Plasmodium falciparum. Infect Immun. 1995;63:456–66.
Bass C, Nikou D, Vontas J, Donnelly MJ, Williamson MS, Field LM. The Vector Population Monitoring Tool (VPMT): high-throughput DNA-based diagnostics for the monitoring of mosquito vector populations. Malar Res Treat. 2010;2010:190434.
Bass C, Williamson MS, Field LM. Development of a multiplex real-time PCR assay for identification of members of the Anopheles gambiae species complex. Acta Trop. 2008;107:50–3.
Walker ED, Thibault AR, Thelen AP, Bullard BA, Huang J, Odiere MR, et al. Identification of field caught Anopheles gambiae s.s. and Anopheles arabiensis by TaqMan single nucleotide polymorphism genotyping. Malar J. 2007;6:23.
Bass C, Nikou D, Donnelly MJ, Williamson MS, Ranson H, Ball A, et al. Detection of knockdown resistance (kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111.
WHO. WHO child growth standards. Geneva: World Health Organization; 2006.
MOH. National Malaria Guidelines. 3rd ed. Addis Ababa: Federal Democratic Republic of Ethiopia Ministry of Health; 2012.
Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–82.
Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–13.
Brass W. Uses of census and survey data for the estimation of vital rates. United Nations Economic Commission for Africa; 1964. https://repository.uneca.org/handle/10855/9560.
Rajaratnam JK, Tran LN, Lopez AD, Murray CJ. Measuring under-five mortality: validation of new low-cost methods. PLoS Med. 2010;7:e1000253.
Hill K, Figueroa M-E. National Malaria Guidelines. Baltimore: Johns Hopkins University; 1999.
Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun. 2014;5:3136.
Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25.
Abeku TA, van Oortmarssen GJ, Borsboom G, de Vlas SJ, Habbema JD. Spatial and temporal variations of malaria epidemic risk in Ethiopia: factors involved and implications. Acta Trop. 2003;87:331–40.
Kilian AH, Langi P, Talisuna A, Kabagambe G. Rainfall pattern, El Nino and malaria in Uganda. Trans R Scoc Trop Med Hyg. 1999;93:22–3.
Fornadel CM, Norris LC, Franco V, Norris DE. Unexpected anthropophily in the potential secondary malaria vectors Anopheles coustani s.l. and Anopheles squamosus in Macha, Zambia. Vector Borne Zoonotic Dis. 2011;11:1173–9.
Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6:114.
Okia M, Ndyomugyenyi R, Kirunda J, Byaruhanga A, Adibaku S, Lwamafa DK, Kironde F. Bioefficacy of long-lasting insecticidal nets against pyrethroid-resistant populations of Anopheles gambiae s.s. from different malaria transmission zones in Uganda. Parasit Vectors. 2013;6:130.
Verhaeghen K, Bortel WV, Roelants P, Okello PE, Talisuna A, Coosemans M. Spatio-temporal patterns in kdr frequency in permethrin and DDT resistant Anopheles gambiae s.s. from Uganda. Am J Trop Med Hyg. 2010;82:566–73.
Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol. 2013;27:276–83.
Asale A, Getachew Y, Hailesilassie W, Speybroeck N, Duchateau L, Yewhalaw D. Evaluation of the efficacy of DDT indoor residual spraying and long-lasting insecticidal nets against insecticide resistant populations of Anopheles arabiensis Patton (Diptera: Culicidae) from Ethiopia using experimental huts. Parasit Vectors. 2014;7:131.
Nabyonga Orem J, Mugisha F, Okui AP, Musango L, Kirigia JM. Health care seeking patterns and determinants of out-of-pocket expenditure for malaria for the children under-five in Uganda. Malar J. 2013;12:175.
Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–505.
Woyessa A, Deressa W, Ali A, Lindtjorn B. Evaluation of CareStart malaria Pf/Pv combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, south-central Ethiopia. Malar J. 2013;12:218.
WHO. WHO policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva: World Health Organization; 2014.
Abeku TA, Helinski M, Kirby MJ. Monitoring malaria epidemiology and control in Ethiopia and Uganda: baseline survey October–November 2012. London: Malaria Consortium; 2015.
WHO. WHO guidance for countries on combining indoor residual spraying and long-lasting insecticidal nets. Geneva: World Health Organization; 2014.
UNICEF. Improving child nutrition: the achievable imperative for global progress. New York: United Nations Children’s Fund; 2013.
Authors’ contributions
TAA, MJK, MEHH, JS, GT and EB designed the study with contributions from SRM, TK, TA, and TH. TAA, MEHH and MJK wrote the manuscript with contributions from SN. TAA, MEHH, MJK and SN carried out statistical analyses. IK contributed to data management. TK, TA, TH, TAA, SB, SCA, MJK and MEHH coordinated field studies and trained field staff. EB, JS, GT, AT, ZK, GM and SRM provided managerial and technical oversight. ANu trained field staff and provided management oversight. AA and VK supervised field work. ANa carried out laboratory analysis of serological samples. LE and MJK carried out laboratory analysis of entomological samples. SC and CB supervised laboratory analyses. SM, GT, TK, TA, CB, AN, GM, EB, SN and AS reviewed the manuscript and provided comments. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the Federal Ministry of Health of Ethiopia and South Nations, Nationalities and Peoples Regional Health Bureau, and the Ministry of Health of Uganda for facilitating the study. We thank the Health Offices of Apac and Kyankwanzi Districts in Uganda and Boloso Sore District and Halaba Special District in Ethiopia for their cooperation and providing technical and management oversight. We thank the communities in all four study sites for their cooperation. We would like to thank Ruth Ashton for her advice on serological analyses. We are grateful to all our field staff and consultants for their technical contributions and assistance, and the staff of Aduku Health Centre IV, Butemba Health Centre III, Hembecho Health Centre and Guba Health Centre for their inputs and for facilitating the surveys. We thank the staff of Malaria Consortium Uganda and Malaria Consortium Ethiopia offices for providing administrative and technical support. We would like to thank the Management of Malaria Consortium Head Office and Africa Regional Office for their support and for facilitating the implementation of the project. We are indebted to the communities in which we work for participating in the study. Funding for this study was provided by UK aid through the Programme Partnership Arrangement (PPA).
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Abeku, T.A., Helinski, M.E.H., Kirby, M.J. et al. Monitoring changes in malaria epidemiology and effectiveness of interventions in Ethiopia and Uganda: Beyond Garki Project baseline survey. Malar J 14, 337 (2015). https://doi.org/10.1186/s12936-015-0852-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-015-0852-7