Abstract
A growing body of evidence indicates that the anticancer effect of the immune system can be activated by the immunogenic modulation of dying cancer cells. Cancer cell death, as a result of the activation of an immunomodulatory response, is called immunogenic cell death (ICD). This regulated cell death occurs because of increased immunogenicity of cancer cells undergoing ICD. ICD plays a crucial role in stimulating immune system activity in cancer therapy. ICD can therefore be an innovative route to improve anticancer immune responses associated with releasing damage-associated molecular patterns (DAMPs). Several conventional and chemotherapeutics, as well as preclinically investigated compounds from natural sources, possess immunostimulatory properties by ICD induction. Natural compounds have gained much interest in cancer therapy owing to their low toxicity, low cost, and inhibiting cancer cells by interfering with different mechanisms, which are critical in cancer progression. Therefore, identifying natural compounds with ICD-inducing potency presents agents with promising potential in cancer immunotherapy. Naturally derived compounds are believed to act as immunoadjuvants because they elicit cancer stress responses and DAMPs. Acute exposure to DAMP molecules can activate antigen-presenting cells (APCs), such as dendritic cells (DCs), which leads to downstream events by cytotoxic T lymphocytes (CTLs) and natural killer cells (NKs). Natural compounds as inducers of ICD may be an interesting approach to ICD induction; however, parameters that determine whether a compound can be used as an ICD inducer should be elucidated. Here, we aimed to discuss the impact of multiple ICD inducers, mainly focusing on natural agents, including plant-derived, marine molecules, and bacterial-based compounds, on the release of DAMP molecules and the activation of the corresponding signaling cascades triggering immune responses. In addition, the potential of synthetic agents for triggering ICD is also discussed.
Similar content being viewed by others
Introduction
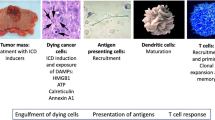
A condition known as cancer is among the most widespread death reasons in the world today. Based on the reported data, the incidence of cancer in 2020 was more than 19.3 million cancer cases word wide that led to approximately 10 million deaths [1]. Cancer is characterized by uncontrollable and excessive cell divisions [2]. Growing evidence has shown that cancer development is considerably affected by immune deficiency. Cancer recurrence and metastasis can be prevented by stimulating and mobilizing the immune system. This process, called immunogenic cell death (ICD), occurs when factors that promote immunity activation are released. In ICD, dying cancer cells release pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Recruitment and activation of dendritic cells (DCs) occur after recognition by pattern recognition receptors (PRRs), which recruit DCs to eradicate cancer cells through phagocytosis. T-cells are activated by DCs and stimulating signals. Therefore, T-cells and DCs play a crucial role in ICD [3]. ICD must be accompanied by a series of immunomodulatory events, including translocation of calreticulin (CRT), the release of high mobility group box-1 (HMGB-1), adenosine triphosphate (ATP), heat shock proteins (HSPs), and ANXA1. These mediators can act by enhancing the immune responses against tumor cells [4]. It is well-known that radiotherapy, chemotherapy, and photodynamic therapy can activate ICD in tumor cells [5]. Amongst the different types of ICD inducers (Fig. 1), natural compounds have gained much attention due to their low toxicity and potent anticancer impacts that are mediated by inhibiting various pathways involved in cancer development. Several reports have shown that natural compounds can alter the immunosuppressive tumor microenvironment (TME) to immunogenic ones and induce ICD when combined with chemotherapy drugs or alone. In other words, natural agents may have the potential to increase efficacy of cancer immunotherapy [6, 7]. This review explores various ICD-inducing natural compounds to show their efficacy in anticancer responses to prevent cancer metastases and recurrences.
Immunogenic cell death: an innovative approach to cancer therapy
Globally, cancer remains one of the most significant barriers to extending life expectancy. As estimated by the World Health Organization (WHO) in 2019, cancer has become one of the biggest causes of death among those aged 70 and younger [8, 9]. The immune system plays a crucial role in regulating tumor progression by detecting and eradicating tumor cells. Notably, immune cells are trained to not respond to normal cells. In contrast, tumor cells sometimes exhibit mutations that produce tumor-specific antigens, recognized by the immune system as a foreign agent that leads to the death of cancer cells. “Antitumor immunity” relates to adaptive and innate responses that regulate the growth of tumors Antitumor effects result from the interaction of various innate and adaptive immune factors like T and B cells, APCs, and NKs [10]. Despite the antitumor activity of immune cells, tumor-induced immunosuppression is a major obstacle in this regard. Tumors can disrupt the balance between the compartments of the regulatory and effector cells to escape immune recognition and subsequent eradication [11, 12]. In recent years, many anticancer therapies have been developed to induce ICD that alert the immune system to dying cancer cells [13, 14]. T cell-mediated immunity is triggered by ICD, a unique type of cell death that responds to antigens produced by dead cells. Several mechanisms contribute to the stimulation of ICD through the translocation of or release of DAMPs by dying cells to trigger an immune response [14]. Many studies have shown that ICD is an interesting approach to activating anticancer immunity. Upon exposure to a variety of specific stressors, cancer cells undergoing ICD and dying cells release DAMPs that produce neoantigens and stimulate adaptive immunity. In this context, ICDs can mediate vaccine-like traits in cancer. As a result, the inducement of ICD can mimic cancer vaccination [15]. This mechanism is mediated by enhancing the immune response against cancer by maturing DCs, activating CTLs, and increasing NK cell function [16]. DAMPs are present in living cells and show immunostimulatory activities in dying cells. As a result, a wide range of DAMPs is being investigated as potent diagnostic or therapeutic agents in cancer therapy [14].
ICDs’ key hallmarks
As discussed earlier, the ICD process involves the release of intracellular molecules, DAMPs. DAMPs have immunostimulatory properties when exposed to or secreted by dying cells. DAMPs are excreted during necrosis under inflammatory or pathological conditions. Several lines of evidence show that cancer cells exposed to chemotherapy or radiotherapy can produce DAMPs, recruit, and activate immune cells. Additionally, DAMPs stimulate phagocytosis and serve as triggers for CTLs to kill cancer cells [3, 17, 18]. In ICD, DAMPs are produced under endoplasmic reticulum (ER) stress and triggered by reactive oxygen species (ROS) [19]. Based on their localization or release site, three significant subclasses of DAMPs exist [1] cell surface-appearing DAMPs (e.g., HSP 90, HSP70, CRT), [2] extracellular appearing DAMPs (e.g., proinflammatory cytokines (like tumor necrosis factor-α (TNF-α), interleukin 10 (IL-10), IL-8, HMGB1, and uric acid), and [3] end-stage degradation products (e.g., ATP, DNA, and RNA) [20, 21]. Several receptors contribute to the recognition of DAMPs, including the toll-like receptor (TLR) family, retinoic acid-inducible gene-I (RIG-I) receptors, and NOD-like receptors (NLRs) [22, 23] (Fig. 2). Over the past decade, several clinical trials have been initiated to determine the efficacy of DAMPs in cancer treatment. Clinical studies have shown that patients with higher HSP and CRT exposure in response to ICD inducers are more likely to survive [24]. Higher levels of HMGB1 in patients with esophageal squamous cell carcinoma were associated with increased survival in patients receiving chemoradiotherapy [25].
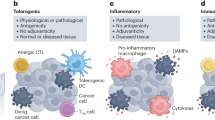
Immunogenic cell death (ICD) inducer causes regulated kind of cell death through ROS production and endoplasmic reticulum (ER) stress in tumor cells. Dead tumor cells expose or release damage associated molecular patterns (DAMPs) such as adenosine Triphosphate (ATP), calreticulin (CRT), High mobility group box protein 1(HMGB1) and Heat shock proteins (HSPs). DAMPs cause functional maturation of dendritic cells through binding to their specific receptors. Mature DCs present cancer specific antigens to T cells and activate anticancer immune response against remained cancer cells
Calreticulin (CRT)
CRT is the most well-known DAMPs found in the ER lumen. CRT plays various roles in immunity, such as maintaining Ca2 + homeostasis and chaperone activity. CRT also regulates cell proliferation, protein synthesis, cell invasion, cell adhesion, and nuclear transport. Gardai et al. showed that CRT acts as a general recognition ligand on the surface of apoptotic cells [26]. There is increasing evidence that CRT contributes significantly to antitumor immunity and the immunogenicity of dying cells [27]. It was found that the induction of ICD causes ER stress and leads to the release or exposure of immune cells to DAMP molecules such as CRT. Upon ICD induction, CRT exposure requires downstream ER stress-responsive genes, including caspase-8-mediated cleavage of ER-resident protein 57 (ERp57), stimulation of BCL2-associated X protein (Bax), Bcl-2 homologous antagonist/killer (Bak), and B cell receptor-associated protein 31 (BAP31). ERp57 mediates the transport of CRT to the plasma membrane; thereby, the deletion of ERp57 prevents CRT translocation [27,28,29]. ICD inducers are divided into two types: Type I, which elicits danger signaling through non-lethal ER stress such as chemotherapy, and Type II, which triggers danger signaling by ER stress such as hypericin-photodynamic therapy (Hyp-PDT) [30]. In the case of type II ICD inducers, fewer demands are needed because of the reliance of this type of inducer only on Bax, Bak, protein kinase-like ER kinase (PERK), and secretory pathways [28]. Following ER stress, CRT translocates to the outer surface of the cell and binds to specific receptors (including CD91) on the DCs surface, causing phagocytosis of dying tumor cells. To act as an “eat me signal,” CRT activates APCs [21]. According to Obeid et al., CRT induces apoptosis via anthracycline and mitoxantrone, and a positive correlation was observed between CRT exposure and inducer-mediated immunogenicity [31].
Moreover, mRNA expression of CRT in cancer cells affects the composition and density of infiltrating immune cells. Indeed, CRT expression is mainly linked to CTLs and DCs infiltration in various types of cancer, such as colorectal, ovarian, and breast cancers [32]. CRT is also targeted by miR-27a in colorectal cancer, as recently discovered. MiR-27a is a negative mediator of drug-induced ICD. This was mediated by reducing CRT levels and exposure to the cell surface. Exposure to CRT enhances the detection of tumor cells by CTLs and DCs [33, 34].
Heat-shock proteins (HSPs)
Another ICD hallmark is the heat-shock protein (HSP), which contributes to protein folding and refolding in stress states [35]. Upon exposure to stress (e.g., oxidative stress, irradiation, or chemotherapy agents), HSPs are overexpressed in the intracellular environment and transported to the plasma membrane [36]. There are many HSPs, but HSP70 and HSP90 are the most associated with ICD. HSP70 and HSP90 have different functions in cancer, depending on their location. Intracellularly located HSPs have defensive properties, whereas extracellular or membrane-bound HSPs have immunological functions [37]. Mobilizing intracellular HSP to the plasma membrane results in potent immunostimulant activity [38]. HSP70 and HSP90 display immunostimulatory activities under exposure to the extracellular layer of dying cells Ecto-HSP70 and HSP90 can interact with receptors on the surface APCs (e.g., CD40, CD91, and LOX1) and control the immunogenicity of dying cells. This leads to the cross-presentation of cancer cell antigens to MHC class I molecules and subsequent activation of CD8 + T-cells. Collectively, the surface exposure of HSPs to cancer cells facilitates anticancer immune responses by enhancing the immunogenicity of cancer cells [39].
High mobility group box 1 release (HMGB1)
Accumulating evidence has shown that extracellular HMGB1 can elicit anticancer immune responses during ICD. The HMGBs family is composed of three members: HMGB1, HMGB2, and HMGB3, all of which have the potential to bind and distort DNA molecules [40]. HMGB1 is a non-histone chromatin-binding protein, the first family member to be identified. The function of HMGB1 depends on its subcellular localization. The nucleus HMGB1 mediates various functions, including DNA repair, nucleosome maintenance, transcription, and recombination, whereas extracellular HMGB1 participates in angiogenesis and chemotaxis [41, 42]. Upon binding to the corresponding receptors located extracellularly, HMGB1 produces endogenous danger signals in adjacent cells and induces immunity and inflammation. HMGB1 is broadly expressed and acts as an extracellular signal upon active secretion by immune-associated cells or upon passive secretion by injured, dying, and dead cells. Indeed, HMGB1 has intracellular activities and contributes to multiple extracellular functions that are mediated by corresponding receptors, including TLR2, TLR4, and RAGE (receptors for advanced glycation end-products). HSPs and HMGB1 can induce a Th1 type of immune response against cancer cells through binding to TLR4 receptors on DCs [43]. Following its release during the death process, HMGB1 acts as an anti-inflammatory and immune-regulating DAMP. Anticancer agents, apoptosis, and ICD stimulation factors also influence the release of HMGB1. In immature DCs, extracellular HMGB1 binds to TLR4, causing DC maturation and CTL activation [44].
Adenosine triphosphate (ATP)
ATP is another DAMP released during ICD. ATP secretion from dying cells is a crucial factor for the effective initiation of ICD. In response to ICD inducers, ATP is redistributed from lysosomes to autolysosomes and is released by a mechanism that requires lysosomal-associated membrane protein 1 (LAMP 1), which ultimately translocates to the plasma membrane in a caspase-dependent manner. In addition, ATP secretion is mediated by caspases and pannexin 1 (PANX1). ICD requires PANX1 to translocate LAMP1 to the cell surface and exocytose lysosomes [45]. Extracellular ATP acts as a powerful chemotactic agent for APCs and their precursors by binding to purinergic receptors P2RY2 and P2RX7. Therefore, dying cells lose their immunogenic properties not only when ATP does not assemble in their microenvironment but also when P2RY2 or P2RX7 is not present in the myeloid compartment. P2RX7 signaling also activates the NLR domain-containing protein 3 (NLRP3) inflammasome, which in turn elicits the release of IL-1β, a cytokine involved in the extension of an antitumor immune response. Studies have shown that ATP acts as a chemoattractant for DC precursors. La Sala et al. reported that incubation of immature DCs with ATP (250 µM) for 24 h enhanced the level of CD54, CD80, CD83, and CD86 [46]. In the process of cell death, the apoptotic phase that stimulates ICD is necessary for ATP secretion [47].
Type I IFN
Recent studies have indicated that IFN type I is a crucial component of ICD. It plays a substantial role in innate and adaptive immunities [48]. In addition to stimulating antigen presentation, type I IFN enhances the antitumor activity of T cells [49]. Type I IFN response pathway genes are upregulated in tumor cells by factors that promote ICD. These include anthracyclines, oncolytic viruses, and radiotherapy. In addition, a type I IFN-related profile can predict the clinical responses to anthracycline-based chemotherapeutics. The chemokine ligand 10 (CXCL10) is an IFN-I-induced gene that acts as a chemotactic factor and recruits immune cells to eradicate cancer cells selectively [50, 51].
Annexin A1 (ANXA1)
ANXA1 binds phospholipids in the presence of calcium [52]. The immunosuppressive properties of ANXA1 can enhance DC function during ICD implantation. As previously mentioned, APCs interact with dying cancer cells, and ANXA1 is released from dying cancer cells. It binds to the formyl peptide receptor 1 (FPR1) on APCs [53, 54]. In other words, ANXA1 mediates the uptake of tumor antigens for presentation [55]. Neither anthracycline nor oxaliplatin showed therapeutic effects in tumor cells lacking ANXA1 or immune cells lacking FPR1 [56].
ICD inducers: focusing on natural compounds
The first classification system for ICD inducers was proposed by Agostinis et al. in 2013. ICD inducers are classified into types I and II, according to their direct or indirect targeting of the ER [57]. As briefly described in previous sections, Type I ICD inducers (e.g., anthracyclines) do not function by targeting the ER directly but rather by targeting a variety of cellular compartments. In contrast, the ER is the primary target of Type II ICD inducers and directly enhances the immunogenicity of ICD. Only a few Type II inducers are available; for example, hypericin resides mainly in the ER and, when irradiated, generates ROS-driven ER stress, leading to ICD. Type II inducers stimulate ICD and result in a more effective immune response. Type II inducers usually generate higher levels of DAMPs. Most of the discovered ICD-induced compounds belong to Type I [58].
Natural compounds
There is growing interest in natural compounds and their derivatives for developing new anticancer agents. Natural compounds have a wide variety of properties, such as low toxicity, relative cheapness, and availability [59]. Therefore, discovering natural anticancer compounds with ICD-inducing capacity is a promising approach to cancer therapy. Figure 3 demonstrates how natural compounds participate in signaling cascades of ICD. Generally, ICD-inducing natural compounds are categorized into plant-, marine-, and bacteria-based groups. In the following sections, the specific properties of each compound are discussed in detail [60].
Natural compounds induce ICD through different mechanism leading to apoptotic type of cell death. Induction of oxidative stress in mitochondria and endoplasmic reticulum (RT) is the most common phenomena in ICD induced by natural agents. Releasing cytochrome C, activation of PERK/eIF2α/ATF4/CHOP pathway, prevention of NF-κB, increasing TNFα expression and inhibiting STAT3 activation have been reported as the involving signaling pathways in ICD by natural agents
Plant-derived compounds
Capsaicin, a highly aromatic alkaloid in chili peppers, is known as one of the natural ICD inducers. It has several pharmacological functions, including improved immunity, decreased blood pressure, and reduced inflammation and pain. Capsaicin can induce ICD through surface exposure to CRT and the release of HSP90, HSP70, and ATP [61, 62]. Capsaicin has been shown to enhance ROS generation and ER stress in various cancer cell lines [63]. Capsaicin activates DCs by binding to vanilloid receptor 1 or TRPV1 [64] and affects cytokine secretion within the tumor environment, reducing immunosuppressive cells at the tumor site. Furthermore, apoptosis and autophagy can be induced by capsaicin through the reduction of phosphorylated signal transducer and activator of transcription 3 (P-STAT3), as p-STAT3 contributes to the downregulation of antiapoptosis molecules [62].
Alternol is a microbial fermentation product obtained from microorganisms in the bark of the yew tree. Alternol exposure has been found to cause cancer cells to develop a profound oxidative stress state, in addition to apoptosis. It has been shown that the xanthine dehydrogenase/ Xanthine oxidase (XDH/XO) can be activated in alternol-treated cancer cells, resulting in ROS accumulation and apoptosis. Alternatively, alternol-driven ICD can be inhibited by inhibiting ROS production [65]. Researchers have found that alternol induces ICD in prostate cancer cell lines by increasing DAMPs levels (HMGB1, ATP release, and CRT translocation; pro-inflammatory cytokines such as IL-1 A, IL-1B, IL-8, and IL-6) and stimulating the immune response against tumors in prostate cancer cells. Treating cancer cells with alternol activates the uptake of DCs cross-presentation and tumor-associated antigen, leading to an antitumor immune response [66].
Curcumin (also called curry powder) has been used for centuries as a treatment for inflammatory diseases [67]. Combination immunotherapy with curcumin is a potent inducer of ICD in tumor cells, which enhances the immunogenicity of tumors and makes cancer more susceptible to the antitumor T cell effect. Researchers have identified curcumin as an ER stressor and a strong inducer of ICD [68]. Curcumin-treated CT26 cells undergo apoptosis and release DAMPs, particularly CRT, HSP90, HMGB1, ATP, and IL-1. A combination of irinotecan and curcumin demonstrated synergistic antitumor effects on CT-26 colon carcinoma cells, accompanied by upregulation of ICD hallmarks, such as CRT and HMGB1. This combination treatment is more effective than individual treatments [69].
Silibinin is a flavonoid extracted from milk thistle that stimulates apoptosis and inhibits p-STAT3 in most cancerous cells [70]. Previous studies have revealed that inhibition of p-STAT3 can enhance chemotherapy-induced ICD [71]. In a recent survey, ICD was effectively induced in B16F10 and CT26 cells by silibinin and was enhanced in cells treated with a combination of silibinin and DOX. Induction of ICD is accompanied by the production of DAMPs, including HSP70, HMGB1, CRT, and the secretion of IL-12 from functionally matured DCs [43].
Wogonin is a flavonoid compound found in different plants, such as Scutellaria baicalensis Georgi. It possesses anti-inflammatory, antioxidant, antitumor, and immunomodulatory properties. Wogonin was demonstrated to have potent antitumor immunity in vivo [72]. Wogonin can induce ICD via CRT translocation, annexin A1, HMGB1, and ATP release [73].
Ginsenoside is a natural compound derived from ginseng that has been used in ancient times to treat some diseases. Rg3 is a ginsenoside that has been found to eradicate tumor cells. Additionally, it exerts modulatory effects on the immune system [74]. Son et al. reported that Rg3 could kill tumor cells via immunogenic (melanoma cell line) and non-immunogenic (lung carcinoma cell line) mechanisms by inducing apoptosis. Surface exposure to ICD markers, including CRT and HSPs, was enhanced in the Rg3-dying tumor cells. The increased expression of CRT was attributed to the uptake of dying tumor cells by DCs [75]. In addition, quercetin has been shown to significantly increase ICD efficacy induced by Rg3 by generating ROS [76].
Resveratrol is a natural non-flavonoid polyphenol compound found in grape leaves that possesses numerous beneficial effects, including anti-aging, anticancer, antioxidant, cardioprotective, and neuroprotective [77]. Moreover, another study showed that resveratrol exerted antitumor effects against ovarian carcinoma. Resveratrol treatment suppresses proliferation and induces apoptosis in ovarian carcinoma cells. This is mediated by cell surface exposure to CRT, secretion of HMGB1, and release of ATP. Vaccination of ID8 cells (mouse ovarian carcinoma cell line) with resveratrol significantly suppressed tumor growth in inoculated xenograft tumors. In addition, an increase in mature DCs and cytotoxic T cells was observed in xenograft tumors following resveratrol treatment, which inhibited TGF-β expression and triggered IL12 and IFN-γ secretion [78].
Camphene is an essential oil from the branches of Piper cernuum that induces apoptosis in melanoma cancer models. Camphene may induce ICD in apoptotic tumor cells by inducing ER stress and increasing HMGB1 and CRT expression [79].
Alantolactone is a natural product that is extracted from Chinese medicinal plants. This compound promoted antitumor responses through ICD induction. According to Zhang et al., alantolactone alone induces ICD in microsatellite-stable colorectal cancer. In contrast, quercetin enhances this process through ROS production and interference with the protein kinase and nuclear factor kappa B (NF-κB) pathways. The combination of alantolactone and quercetin at a molar ratio of 1:4 induced synergistic ICD. The micellar delivery of alantolactone and quercetin results in prolonged blood circulation and enhanced tumor accumulation. Indeed, combination therapy increased CRT exposure in tumor cells and the release of HMGB1. This formulation prevents the release of IL-1β, IL-10, CCL2, and TGF-β. Collectively, this formulation significantly inhibited tumor growth in a colorectal cancer model, which was mediated by ICD induction, cell toxicity, extended anti-tumor immune effects, and modulation of the immune-suppressive tumor environment [80].
Gallotannin-rich fractions extracted from Caesalpinia Spinosa are natural compounds that promote apoptosis through the activation of caspases 3 and 9 and externalization of annexin V. This compound has antiproliferative effects on melanoma cells. Additionally, it induces ICD markers such as ATP and HMGB1 and activates autophagy. Gallotannin can result in a reduction in the B16 melanoma tumor volume through DCs activation and an increase in CD8+ IFN-γ+ T cells [81, 82].
Shikonin is a phenolic compound extracted from Lithospermum erythrorhizon. Shikonin, an anti-inflammatory and antitumor phytochemical, can be used as an adjuvant for DC-based cancer vaccines via ICD induction and can enhance the expression levels of all five DAMPs in tumor cell lysates. Shikonin efficiently activated apoptotic pathways. In this context, Shikonin-treated B16F10 cells induced caspase 8 and 9, Bax, and cytochrome C apoptotic death pathways [83]. Lin et al. provided evidence that shikonin can effectively induce ICD, and this effect may serve as an adjuvant for use in DC-based cancer vaccines. In other words, shikonin can enhance the immunogenicity of vaccines via ICD [84].
Lentinan is a natural polysaccharide extracted from shiitake mushroom. This compound has been shown to have apoptotic effects on tumor cells. Wang et al. reported ICD induction in murine H22 Cells upon incubating with lentinan. Lentinan induced the expression of CRT, HMGB1, ATP, and HSP70. Indeed, the antitumor effect of lentinan may be correlated with the regulation of ICD-related markers, which may be beneficial for the development of liver cancer vaccines [85].
Chalcones are considered precursors of all flavonoids in plants that possess numerous biological activities [86]. It has been reported that JA3 and JA7, two aldehyde biphenyl chalcones, have cytotoxic effects on solid cancers and hematological malignancies. In this context, CRT exposure and the release of ATP lead to immunogenic-related responses and apoptosis. These compounds can induce ICD with severe mitochondrial damage downstream of ER and oxidative stress. They also improved the anti-leukemic efficacy of cytarabine and vincristine in different leukemic cells [87].
Celastrol is a promising medicinal compound with various properties such as anti-obesity, anticancer, and anti-inflammatory properties. It has been reported that celastrol at a very low dose could effectively induce ICD (without inducing toxicity) by promoting autophagy, modulating TME, and enhancing systemic immunotherapy [88, 89].
Bullatacin is one of the most promising antitumor agents isolated from Annona atemoya’s fruit. The compound is effective against lung, liver, breast, bladder, cervical cancer, and lymphoma. Fan et al. showed that low concentrations of bullatacin led to significant accumulation of CRT and HSP90, (as biomarkers of ICD) on the cell surface of colon cancer cell lines SW480 and HT-29 cells, indicating that bullatacin can stimulate immunogenic tumor cell death by activating ER stress [90].
OT52 is a natural compound that belongs to coumarin compound extracted from various plants. Coumarins are natural agents with antioxidant, anticancer, and anti-inflammatory properties that regulate the inflammatory response. OT52 has shown antiproliferative effects in non-small cell lung cancer (NSCLC) cells. This cytostatic effect is attributed to ER and Golgi stress, which leads to metabolic alterations and the inhibition of STAT3 transactivation. A dose-dependent increase in CRT expression was detected with OT52. Additionally, combining BH3 protein inhibitors with OT52 resulted in a significant increase in HMGB1 release compared with OT52 alone [91].
Hemidesmus indicus is a widely used medicinal plant that can stimulate ICD. Turrini et al. showed that hemidesmus triggers tumor cell cytotoxicity, which is characterized by surface exposure to calreticulin and increased levels of HSP70, ATP, and HMGB1. These findings show that Hemidesmus indicus, an inducer of ICD, has the potential to be used in innovative cancer immunotherapy [92].
Plumbagin is a plant-derived naphthoquinone. The phenanthraquinone compound dihydrotanshinone I (derived from Salvia miltiorrhiza) enhanced ICD by producing ROS. It has been reported that nano co-delivery of these compounds significantly enhanced the half-life and tumor-targeting ability of these two drugs in orthotopic hepatocellular carcinoma (HCC) mice; consequently, this nanoformulation loaded with low doses of plumbagin and dihydrotanshinone I resulted in longer survival of HCC mice without cytotoxicity signs [93].
Cardiac glycosides (CGs), also known as type 1 ICD inducers, are classified into two groups, cardenolides and bufadienolides. Most CGs have natural sources and are found in plants. CGs exert several immunomodulatory functions associated with the suppression of T-helper cell activity or modulation of immune response-related genes by inhibiting NF-κB. The FDA has approved CGs such as digoxin to treat arrhythmias and heart failure. Recent studies have shown that CGs are powerful anticancer agents. Four CGs have been identified as the most effective ICD inducers: digoxin, digitoxin, ouabain, and lanatoside [94]. In 2012, Menger et al. reported that CGs could induce ICD. CGs have been shown to induce ICD biomarkers, such as CRT, HMGB1, and ATP, in several human cancer cell lines. It has also been confirmed that CGs induces ER stress. Moreover, antioxidants can inhibit the cytotoxic effects of CGs, indicating a strong correlation between their cytotoxicity and ICD induction [95]. Another report by Xiang et al. showed that co-administration of digoxin and cisplatin prodrug effectively led to a series of events in the B16F10 cell line, including ICD induction, DCs maturation, CD8 + T cell activation, and complete tumor elimination [96]. CGs show synergistic effects owing to their impact on ICD. One of the primary factors contributing to this feature is their capacity to modulate Mcl-1 and their moderate effect on Bcl-xL and Bcl-2 expression [97]. Researchers have demonstrated that digitoxin synergistically activates thapsigargin and simvastatin in estrogen-positive breast cancer cells [98]. Oleandrin treatment, a natural compound belonging to the CGs family results in secretion of ATP, HMGB1, HSP70, and HSP90 as ICD markers. As a result of oleandrin treatment, DCs were more likely to mature and activate, which further increase the efficiency of CD8+ T T-cell cytotoxicity. Animal models have shown that oleandrin prevents tumor growth. Oleandrin stimulated ER stress and ICD primarily through the PERK/elF2α/ATF4/CHOP pathway in the breast cancer cell lines [99].
Vesiculated Α-Tocopheryl Succinate is non-toxic vitamin E analog extracted from various type of seeds. Ramanathapuram et al. showed that Vα-TOS might utilize a dual approach to enhance DC-mediated cancer immunotherapy as follows: destroying tumor cells directly and maturing DC via HSPs as a danger signal [100].
Micheliolide is a natural guaianolide sesquiterpene lactone that induces ICD-associated DAMP molecules, such as CRT exposure, HMGB1 release, and ATP secretion. Micheliolide induced ICD by DCs maturation and activation of CD4 + and CD8 + T-cells responses in a mouse model. Indeed, the ICD-associated effects of micheliolide rely on the generation of ROS-mediated ER stress [101].
Norcantharidin is the most important analogs of cantharidin. Cantharidin is a natural toxin with potent anti-tumor properties. Norcantharidin was shown to induce ICD in bladder cancer cells that accompanied by promoting DC maturation and CRT exposure, but not ATP secretion [102, 103].
Schweinfurthin was found in Macaranga schweinfurthii (an African plant). Schweinfurthin can stimulate ICD without inducing ER stress or caspase-related mechanisms. This compound induced cell surface exposure to CRT and enhanced phagocytosis of tumor cells by DCs in vitro. Schweinfurthin does not require PERK to induce CRT exposure. It did not elicit ERp57 exposure, and a lack of ERp57 expression did not decrease CRT exposure. For this phenomenon to occur, the ER-Golgi transport system must be intact [104].
Withania somnifera, mostly known as Ashwagandh, belongs to the Solanaceae family. It possesses anti-inflammatory, immunomodulatory, and anticancer cancer properties [105]. It has been shown that withania has a strong potential to induce ICD in lung adenocarcinoma cancer cells [106].
Colchicine is a microtubule-depolymerizing drug extracted from Colchicum autumnale. This compound can induce ICD in cancer cells by affecting the expression of DAMPs, such as HSP70, HSP90, and HMGB1, without affecting the expression of CRT [107].
Marine-based compounds
The sea covers a large part of the Earth’s surface and is a large reservoir of biological diversity. Because of the challenging and dynamic environment in which these organisms live, they are a great reservoir of biologically active molecules that are uncommon on land. We have summarized several marine-based compounds mentioned as having ICD-inducing properties. These compounds may be a helpful approach for preventing and treating cancer, alone or in combination with other immunotherapy strategies. In addition to the various compounds produced by microalgae, some other compounds may have health benefits. There is evidence of immunomodulatory and anticancer effects of compounds derived from microalgal sources; however, ICD induction remains unknown. Various studies have been performed on human cancer cell lines, indicating that fractions and microalgae extracts can stimulate cell death through specific signaling pathways [63].
Sulfavants are a group of synthetic sulfoglycolipids that mimic the natural α-sulfoquinovosides found in the diatom Thalassiosira weissflodgi. At micromolar concentrations, Sulfavant A prototype of the sulfavant family, is a potent stimulator of DC maturation [108]. This agent stimulates the expression of co-stimulatory molecules and MHC II, especially CD54, CD86, and CD83, leading to T-cell differentiation. These properties demonstrate the potential of Sulfavant A as an ICD inducer. In a murine model of a melanoma vaccine, Sulfavant A has already been shown to be effective, and its efficacy has already been confirmed in preclinical studies [109].
Alexandrium minutum is an isolated glycopeptide from the marine dinoflagellate that has been shown to induce mitophagy in cancer cells without affecting normal cells. This form of microautophagy promotes a cascade of ICD via lysosomal ATP secretion. Researchers indicated that this compound had a potent cytotoxic effect on the A549 lung adenocarcinoma cell line, with an IC50 = 1.3 µg·mL − 1 [110].
Docosahexaenoic acid (DHA) is a ω-3 polyunsaturated fatty acid present in fish oil. It increases the cytotoxicity of numerous anticancer agents, especially by generating ROS and increasing cancer cells’ sensitivity. Additionally, cardio-protective effects of DHA have been revealed, and these can be very beneficial when used in combination with DOX [111, 112]. It can also induce ICD. DHA-treated human multiple myeloma cell line (OPM-2 cells) stimulates immunogenic apoptosis and autophagy and inhibits STAT3 activation in both tumor and DCs. Immunogenic apoptosis was associated with the expression of DAMP molecules (CRT, HMGB1, HSP90) and the activation of pro-apoptotic autophagy [113].
Polyunsaturated aldehydes isolated from three diatoms, Skeletonema costatum, Thalassiosira rotula, and Pseudonitzschia delicatissima, can stimulate necroptosis in colon and lung cell lines The receptor-interacting protein kinase 3 (RIPK3) can be activated by immune ligands, which can initiate necroptosis. This leads to increased ATP and HMGB1 levels, which are hallmarks of ICD [114].
Thalassia testudinum is a polyphenol extracted from marine seagrass. Pharmacological studies have proven this compound has potent anti-inflammatory and antioxidant properties. ROS-induced apoptosis of cancer cells is one of the most widely recognized mechanisms underlying the cytotoxicity of polyphenols. Prior reports state that T. Testudinum extract raises the cytosolic Ca2 + level, producing ROS and DNA fragmentation [115]. This compound inhibits colorectal cancer growth, motility, and angiogenesis by inducing ICD pathways and autophagic stress [116].
Lepadin A is a marine alkaloid that possesses the potential to induce ICD. At micromolar concentrations, leptin A displayed cytotoxic effects against cancer cells, which were associated with the maturation of mouse DCs. This alkaloid over-expresses MHC-II and its co-stimulatory molecules, which play a crucial role in differentiating naïve T cells by DCs, together with an increase in the effective immune response [117].
MHO7 is a marine-derived molecule that acts as a potent ICD inducer via the ER stress-C/EBP-homologous protein (CHOP) cascade to treat triple-negative breast cancer (TNBC). MHO7 alters the expression of genes associated with ribosomes and ER proteins, resulting in ROS generation and glutathione reduction. TNBC cells are affected by MHO7 through the induction of ER stress and ROS production, release ICD-related DAMPs, and stimulate in vivo immunity by the production of antitumor cytokines, including TNF-α, IFN-γ, IL-1β, and IL-6 [118].
Spirulina maxima and Schizochytrium sp. microalgal compounds modulate the microbiota. Recent studies have revealed that administering microalgal species like Spirulina sp as a food supplement exhibits immunomodulation properties. Spirulina-derived modified pectin can induce mucin, IFN-α, and IL-6 releases during inflammation, triggering ICD activation [119]. The microbiota is stimulated, and mucin is released by Schizochytrium’s polyunsaturated fatty acids (PUFAs). Recent studies showed how PUFAs disrupt the organization of membrane domains by altering lipid rafts and activating particular signaling networks through stimulating microbiota, cytokine release, and anti-inflammatory activities [63].
Bacterial-based compounds
Several studies have demonstrated the potential of bacterial compounds to induce ICD in cancer cells.
Lactaptin is a proteolytic fragment of human milk kappa-casein, which is produced recombinantly in bacteria. Troitskaya et al. reported that recombinant lactaptin-induced death in cancer cells with is associated with ICD biomarkers in vitro, including external cell exposure of CRT and HSP70 and the release of ATP and HMGB1 [120].
Septacidin is an antibiotic produced by Streptomyces fibriatus with the potential to induce ICD.
Engineered human osteosarcoma cells and murine fibrosarcoma cells responded to septacidin by increasing CRT exposure, ATP secretion, and HGMB1 expression [121].
Patupilone is one of the most important therapeutic compounds isolated from the bacterium, Sorangium cellulosum. Epothilones showed strong cytotoxic effects both in vitro and in vivo. In a mouse model, patupilone has the potential to trigger ICD and translocate CRT; therefore, it can be used to vaccinate immunocompetent mice [122].
It has been shown that the gut microbiome contributes to ICD induction (Table 1). Gut microbiota can influence the ICD procedure, but how it works remains unclear [123]. Various studies have indicated that gut microbes are promising targets for improving the efficacy of cancer therapy and reducing its harmful effects [124, 125]. Based on this review, gut bacteria have a modulatory impact on immunity and are critical for the effectiveness of anticancer drugs [126,127,128]. Indeed, gut microbiota may influence ICD by activating T cells and DCs. Recently, the gut microbiota has been extensively studied in cancer and immunotherapy. Due to the critical role the gut microbiota plays in cancer therapy by modulating immune responses, antibiotics decrease the anticancer activity of drugs by lowering gut microbiota levels. Therefore, gut microbiota can be used as an adjuvant for tumor immunotherapy through fecal microbiota transplantation (FMT) and probiotics [129,130,131].
Classification of natural ICD-inducing agents tested in animal and human models
The substantial numbers of natural ICD-inducing agents have been assessed preclinically for anticancer and ICD-inducing potency in in vitro and in vivo studies. Low bioavailability and water solubility is the most important obstacle limits the oral or systematic use of several types of natural agents (such as silibinin, septacidin, alternol, curcumin, lentinan, alantolactone, oleandrin, Norcantharidin, withania somnifera) as a standard treatment. At present, in order to achieve the high efficacy, the anticancer effect of low water-soluble agents is studied in in vivo animal tumor models through insitu injection in tumor site or intraperitoneally. Formulation of these compounds in nano carriers is one of the promising strategies could enhance their bioavailability and possible toxicity caused by high doses [70, 132,133,134,135]. Table 2 presents animal models and immune related antitumor effect of each compound in details.
Most of naturally compounds with known mechanisms of action are now either just entering or about to enter clinical studies. Capsaicin, curcumin, shikonin, silibinin, ginsenoside, resveratrol, lentinan, celastrol, withania somnifera, norcantharidin, digoxin, colchicine are among the natural agents used in the clinical trials as dietary supplement.
As of yet, there is no any clinical trials evaluating the effects of natural agents on inducing of ICD in cancer patients. However, several natural agents introduced as an ICD inducer have been used in clinical trials for their safety and anti-cancer studies. Table 3 has listed the clinically tested natural agents in human with different conditions.
Combination of natural compound with each other or with chemotherapeutic agents
Researchers are paying more attention to an increasing number of naturally immunogenic-enhancing substances. Combining traditional chemotherapeutic drugs with plant extracts is a common practice that increases the potency of chemotherapeutics while decreasing their tumor-specific resistance. Natural plant extracts are now widely used to treat a variety of cancers as an effective substance to enhance drug efficacy and decrease its toxicity. Combination therapy has other important advantages in the case of direct-acting natural compounds that are required in excessive and unsafe doses to inhibit cancer cell growth or induce ICD if used alone. Besides, for inducing the adequate level of ICD often a high dosage of ICD stimulus is needed which may lead to the toxicity of normal cells [136].
Curcumin is one of the robust ICD inducers and most studied natural compounds, which is mainly known for its chemopreventive effects. It has been reported that curcumin acts as an anticancer agent through multiple pathways and can enhance the anticancer effect and ICD hallmarks induced by chemotherapeutics (irinotecan and paclitaxel) and radiotherapy when used in combination therapies [137, 138]. A combination of low doses of shikonin with anthracyclines in a liposomal form has been reported to induce synergistic chemo-immunotherapy and strong ICD [134]. Ginsenoside Rg3 is the other agent studied for its influential role in increasing ICD when used in combination with PDT, agents such as doxorubicin and quercetin, and immune checkpoint inhibitors (PD-L1) [76, 139, 140]. Immune checkpoints play a crucial role in the induction of TME immunosuppression, which causes lower efficacy of immunotherapeutic agents. It has been revealed that a combination of immune checkpoint inhibitors such as anti-PDL-1, anti-CTLA-4, and anti-PD-1 can increase treatment response of patients with different cancer types [141]. Table 4 presents different combination therapies by natural agents used to enhance the ICD inducing potency of drugs.
Synthetic chemotherapeutic agents
Conventional chemotherapeutics are generally categorized based on their mechanism of action. Some agents can increase local immunity against tumors by inducing ICD. The co-administration of chemicals that produce ICD-associated DAMPs can improve the efficacy of conventional chemotherapeutics [18]. Indeed, chemotherapy has been employed as a conventional paradigm for cancer therapy. Some of the antitumor chemotherapeutics, such as cyclophosphamide (CPA), oxaliplatin (OXA), doxorubicin (DOX), and paclitaxel (PTX), can tackle tumors by inducing ICD in cancer cells and now are using as a standard treatment in the clinic. The ability of various synthetic chemotherapeutic agents to induce ICD has been identified in detail [142]. Cyclophosphamide is one of the most broadly used alkylating agents for cancer therapy due to its immunomodulatory functions. Several mechanisms have been attributed to cyclophosphamide-mediated immunomodulatory effects, such as triggering Th2/Th1 switch, the increasing proliferation and long-term survival of lymphocytes, and enhancement of antitumor efficacy by producing soluble mediators such as cytokines. IFN-I is induced by cyclophosphamide and mediate most of the effects attributed to this drug such as preferential expansion of memory T cells [143].
It has been shown that doxorubicin could elicit immunogenic apoptosis in a caspase-dependent manner in tumor models. Tumor cells dying in response to doxorubicin showed an effective immune response, suppression of the growth of inoculated tumors, and regression of established tumors in animal models [144].
Lau et al. reported that paclitaxel, as an antitumor drug could induce ICD in ovarian cancer cells and elicit significant antitumor responses in a TLR4-independent manner [145]. Table 5 summarizes several chemotherapeutic agents that have been described as ICD inducers.
Metal-based agents inducing ICD
There has been a significant increase in the discovery of small molecules as potential inducers of ICD. Metal-based agents have been shown to induce ICD. Anticancer metal compounds have been shown to elicit an immune-related response to ICD induction in tumor cells. There is a substantial increase in the number of molecules being tested as potential ICD inducers such as metal-based complexes. Metal-based drugs are based on Ru, Pt, Ir, Cu, and Au, which have the potential to induce ICD and elicit an immune response against tumor cells. The success of platinum containing molecules (e.g., cisplatin, carboplatin and oxaliplatin) as an anticancer agent leads to the investigation in this field. Several non-platinum metal-based agents such as ruthenium-based compounds can be used as an alternative to platinum-anticancer agents [58]. With this notion, the employment of novel anticancer metal agents with the potential to trigger ICD shows promise as new immunotherapies for cancer as an emerging approach in the treatment of neoplasia. Table 6 shows the metals and their mechanisms of action in tumor cell death mediated by ICD.
Conclusion
Based on the known mechanisms of ICD induction and the related molecular mechanisms, ICD induction is a promising area of research. Natural compounds that act as ICD inducers could represent a new frontier in cancer therapy. Among them, plant-derived, marine molecules and bacteria-based compounds may represent attractive modalities for ICD induction.
Indeed, only a small number of anticancer chemicals or natural compounds have been found to induce ICD. The identification of new compounds that induce ICD will be important in the future. Most of the known phytochemical compounds are able to induce apoptosis, ER stress, and ROS production in cancer cells. We believe that these phytochemicals are promising agents in immunotherapy of cancers due to the broad spectrum of anticancer effects and are potential candidates to be studied for their ICD capability. Thus, there is an urgent need to explore the potential of novel natural compounds to induce ICD, whether alone or in combination. A combination of natural ICD inducers with immune checkpoint inhibitors or other chemotherapeutic drugs could be one of the combinational therapy strategies for tumor regression through overcoming TME immunosuppression and enhancing tumor immunogenicity. Moreover, several types of plant- and/or marine-based compounds, indicated outward properties of ICD induction in vitro, need to be observed for their antitumor immune response in vivo. Herbal formulations mainly consist of several bioactive molecules, which have been shown to be beneficial for various types of diseases. However, they have not been fully accepted as standard treatment, mainly due to their complexity and lack of strict quality control during preparation. Moreover, most of the naturally derived compounds have low water solubility and bioavailability that limit their clinical usage. In this regard, nano-formulation of low water-soluble agents could be the promising approach and not only enhances the agent‘s water solubility and bioavailability but also could increase the ICD potency of natural agents. In the case of drug combinations, molecular interactions in complex phytochemicals can be antagonistic or dangerous because herbal formulations may contain toxic or even lethal compounds. In addition, only a few natural cytotoxic drugs capable of inducing ICD are being investigated in clinical trials. Therefore, the identification of other chemicals and phytochemicals that may cause ICD is clinically important. It seems that more researches are desired to determine the guidelines for the clinical application of natural ICD inducers.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ICD:
-
Immunogenic cell death
- DAMPs:
-
Damage-associated molecular patterns
- DCs:
-
Dendritic cells
- NKs:
-
Natural killer cells
- APCs:
-
Antigen-presenting cells
- CTL:
-
Cytotoxic T lymphocytes
- PAMPs:
-
Pathogen-associated molecular patterns
- PRRs:
-
Pattern recognition receptors
- CRT:
-
Calreticulin
- HMGB-1:
-
High mobility group box-1
- ATP:
-
Adenosine triphosphate
- HSPs:
-
Heat shock proteins
- TME:
-
Tumor microenvironment
- WHO:
-
World Health Organization
- TNF-α:
-
Tumor necrosis factor-α
- TLR:
-
Toll-like receptor
- ERp57:
-
ER-resident protein 57
- Hyp-PDT:
-
Hypericin-photodynamic therapy
- PERK:
-
Protein kinase-like ER kinase
- LAMP 1:
-
Lysosomal-associated membrane protein 1
- PANX1:
-
Pannexin 1
- NLRP3:
-
NLR domain-containing protein 3
- CXCL10:
-
Chemokine ligand 10
- FPR1:
-
Formyl peptide receptor 1
- ANXA1:
-
Annexin A1
- XDH/XO:
-
The xanthine dehydrogenase/ Xanthine oxidase
- P-STAT3:
-
Phosphorylated signal transducer and activator of transcription 3
- NSCLC:
-
Non-small cell lung cancer
- HCC:
-
Hepatocellular carcinoma
- CGs:
-
Cardiac glycosides
- RIPK3:
-
Receptor-interacting protein kinase 3
- TNBC:
-
Triple-negative breast cancer
References
Chhikara BS, Parang K. Global Cancer Statistics 2022: the trends projection analysis. Chem Biology Lett. 2023;10(1):451.
Matthews HK, Bertoli C, de Bruin RA. Cell cycle control in cancer. Nat Rev Mol Cell Biol. 2022;23(1):74–88.
Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111.
Zhang S, Wang J, Kong Z, Sun X, He Z, Sun B, et al. Emerging photodynamic nanotherapeutics for inducing immunogenic cell death and potentiating cancer immunotherapy. Biomaterials. 2022;282:121433.
Yadollahvandmiandoab R, Jalalizadeh M, Buosi K, Garcia-Perdomo HA, Reis LO. Immunogenic cell death role in Urothelial Cancer Therapy. Curr Oncol. 2022;29(9):6700–13.
Xiang J, Zhang Y, Liu X, Zhou Q, Piao Y, Shao S, et al. Natural polyphenols-platinum nanocomplexes stimulate Immune System for Combination Cancer Therapy. Nano Lett. 2022;22(13):5615–25.
Molavi O, Torkzaban F, Jafari S, Asnaashari S, Asgharian P. Chemical compositions and anti-proliferative activity of the aerial parts and rhizomes of squirting cucumber, Cucurbitaceae. Jundishapur J Nat Pharm Prod. 2020;15(1).
by Cause GD, Age S. By Country and by Region, 2000-2019. World Health Organization; 2020.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–84.
Russo V, Protti MP. Tumor-derived factors affecting immune cells. Cytokine Growth Factor Rev. 2017;36:79–87.
Tormoen GW, Crittenden MR, Gough MJ. Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiation Oncol. 2018;3(4):520–6.
Showalter A, Limaye A, Oyer JL, Igarashi R, Kittipatarin C, Copik AJ, et al. Cytokines in immunogenic cell death: applications for cancer immunotherapy. Cytokine. 2017;97:123–32.
Birmpilis AI, Paschalis A, Mourkakis A, Christodoulou P, Kostopoulos IV, Antimissari E, et al. Immunogenic cell death, DAMPs and Prothymosin α as a putative Anticancer Immune Response Biomarker. Cells. 2022;11(9):1415.
Jin M-Z, Wang X-P. Immunogenic cell death-based cancer vaccines. Front Immunol. 2021;12:697964.
Rahman M, Alam K, Beg S, Chauhan D, Kumar V, Hafeez A et al. Cancer vaccines: past, present, and future. Nanotherapeutics in Cancer Vaccination and Challenges: Elsevier; 2022. p. 1–12.
Bao X, Xie L. Targeting purinergic pathway to enhance radiotherapy-induced immunogenic cancer cell death. J Experimental Clin Cancer Res. 2022;41(1):1–18.
Wang Y-J, Fletcher R, Yu J, Zhang L. Immunogenic effects of chemotherapy-induced tumor cell death. Genes & Diseases. 2018;5(3):194–203.
Collett GP, Redman CW, Sargent IL, Vatish M. Endoplasmic reticulum stress stimulates the release of extracellular vesicles carrying danger-associated molecular pattern (DAMP) molecules. Oncotarget. 2018;9(6):6707.
Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. OncoImmunology. 2014;3(9):e955691.
Montico B, Nigro A, Casolaro V, Dal Col J. Immunogenic apoptosis as a Novel Tool for Anticancer Vaccine Development. Int J Mol Sci. 2018;19(2):594.
Ming B, Zhu Y, Zhong J, Dong L, editors. Immunopathogenesis of Sjogren’s syndrome: current state of DAMPs. Seminars in arthritis and Rheumatism. Elsevier; 2022.
Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–36.
Garg AD, Elsen S, Krysko DV, Vandenabeele P, de Witte P, Agostinis P. Resistance to anticancer vaccination effect is controlled by a cancer cell-autonomous phenotype that disrupts immunogenic phagocytic removal. Oncotarget. 2015;6(29):26841.
Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res. 2012;72(16):3967–76.
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123(2):321–34.
Kielbik M, Szulc-Kielbik I, Klink M. Calreticulin—multifunctional chaperone in immunogenic cell death: potential significance as a prognostic biomarker in ovarian cancer patients. Cells. 2021;10(1):130.
Serrano-del Valle A, Anel A, Naval J, Marzo I. Immunogenic cell death and immunotherapy of multiple myeloma. Front Cell Dev Biology. 2019;7:50.
Liu C-C, Leclair P, Pedari F, Vieira H, Monajemi M, Sly LM, et al. Integrins and ERp57 coordinate to regulate cell surface calreticulin in immunogenic cell death. Front Oncol. 2019;9:411.
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med. 2019;23(8):4854–65.
Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini J-L, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61.
Stoll G, Iribarren K, Michels J, Leary A, Zitvogel L, Cremer I, et al. Calreticulin expression: interaction with the immune infiltrate and impact on survival in patients with ovarian and non-small cell lung cancer. Oncoimmunology. 2016;5(7):e1177692.
Colangelo T, Polcaro G, Ziccardi P, Muccillo L, Galgani M, Pucci B, et al. The miR-27a-calreticulin axis affects drug-induced immunogenic cell death in human colorectal cancer cells. Cell Death Dis. 2016;7(2):e2108–e.
Mansoori B, Mohammadi A, Shirjang S, Baradaran B. MicroRNAs in the diagnosis and treatment of Cancer. Immunol Investig. 2017;46(8):880–97.
Yang S, Xiao H, Cao L. Recent advances in heat shock proteins in cancer diagnosis, prognosis, metabolism and treatment. Biomed Pharmacother. 2021;142:112074.
Lyon MS, Milligan C. Extracellular heat shock proteins in neurodegenerative diseases: new perspectives. Neurosci Lett. 2019;711:134462.
Joly A-L, Wettstein G, Mignot G, Ghiringhelli F, Garrido C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J Innate Immun. 2010;2(3):238–47.
Tanguy J, Pommerolle L, Garrido C, Kolb M, Bonniaud P, Goirand F, et al. Extracellular heat shock proteins as therapeutic targets and biomarkers in fibrosing interstitial lung diseases. Int J Mol Sci. 2021;22(17):9316.
Asadzadeh Z, Safarzadeh E, Safaei S, Baradaran A, Mohammadi A, Hajiasgharzadeh K, et al. Current approaches for combination therapy of cancer: the role of immunogenic cell death. Cancers. 2020;12(4):1047.
Fang J, Ge X, Xu W, Xie J, Qin Z, Shi L, et al. Bioinformatics analysis of the prognosis and biological significance of HMGB1, HMGB2, and HMGB3 in gastric cancer. J Cell Physiol. 2020;235(4):3438–46.
Andersson U, Wang H, Palmblad K, Aveberger A-C, Bloom O, Erlandsson-Harris H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192(4):565–70.
Zhang Y, You B, Liu X, Chen J, Peng Y, Yuan Z. High-mobility group box 1 (HMGB1) induces migration of endothelial progenitor cell via receptor for advanced glycation end-products (RAGE)-dependent PI3K/Akt/eNOS signaling pathway. Med Sci Monitor: Int Med J Experimental Clin Res. 2019;25:6462.
Jafari S, Heydarian S, Lai R, Aghdam EM, Molavi O. Silibinin induces immunogenic cell death in cancer cells and enhances the induced immunogenicity by chemotherapy. BioImpacts: BI. 2023;13(1):51.
Chen R, Kang R, Tang D. The mechanism of HMGB1 secretion and release. Exp Mol Med. 2022;54(2):91–102.
Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala A, Shen S, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death & Differentiation. 2014;21(1):79–91.
la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166(3):1611–7.
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12(12):860–75.
Blaauboer A, Sideras K, van Eijck CH, Hofland LJ. Type I interferons in pancreatic cancer and development of new therapeutic approaches. Crit Rev Oncol/Hematol. 2021;159:103204.
Vacchelli E, Sistigu A, Yamazaki T, Vitale I, Zitvogel L, Kroemer G. Autocrine signaling of type 1 interferons in successful anticancer chemotherapy. Oncoimmunology. 2015;4(8):e988042.
Melero I, Quetglas JI, Reboredo M, Dubrot J, Rodriguez-Madoz JR, Mancheno U, et al. Strict requirement for Vector-Induced Type I Interferon in efficacious antitumor responses to virally encoded IL12IFNα/β in Cancer Virotherapy Encoding for IL12. Cancer Res. 2015;75(3):497–507.
Wang X, Schoenhals JE, Li A, Valdecanas DR, Ye H, Zang F, et al. Suppression of type I IFN Signaling in Tumors mediates resistance to Anti-PD-1 treatment that can be overcome by RadiotherapyAnti-PD-1–Resistant Cancer Model and Radiotherapy. Cancer Res. 2017;77(4):839–50.
Han P-F, Che X-D, Li H-Z, Gao Y-Y, Wei X-C, Li P-C. Annexin A1 involved in the regulation of inflammation and cell signaling pathways. Chin J Traumatol. 2020;23(02):96–101.
Álvarez-Teijeiro S, Menéndez ST, Villaronga M, Pena-Alonso E, Rodrigo JP, Morgan RO, et al. Annexin A1 down-regulation in head and neck squamous cell carcinoma is mediated via transcriptional control with direct involvement of miR-196a/b. Sci Rep. 2017;7(1):1–12.
Sousa SOd S, MRd, Teixeira SC, Ferro EAV, Oliani SM. ANNEXIN A1: roles in Placenta, Cell Survival, and Nucleus. Cells. 2022;11(13):2057.
Cruickshank B, Giacomantonio M, Marcato P, McFarland S, Pol J, Gujar S. Dying to be noticed: epigenetic regulation of immunogenic cell death for cancer immunotherapy. Front Immunol. 2018;9:654.
Vacchelli E, Ma Y, Baracco EE, Sistigu A, Enot DP, Pietrocola F, et al. Chemotherapy-induced antitumor immunity requires formyl peptide receptor 1. Science. 2015;350(6263):972–8.
Dudek AM, Garg AD, Krysko DV, De Ruysscher D, Agostinis P. Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev. 2013;24(4):319–33.
Sen S, Won M, Levine MS, Noh Y, Sedgwick AC, Kim JS, et al. Metal-based anticancer agents as immunogenic cell death inducers: the past, present, and future. Chem Soc Rev. 2022;51(4):1212–33.
Agarwal G, Carcache PJB, Addo EM, Kinghorn AD. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol Adv. 2020;38:107337.
Diederich M. Natural compound inducers of immunogenic cell death. Arch Pharm Res. 2019;42(7):629–45.
Wang F, Xue Y, Fu L, Wang Y, He M, Zhao L, et al. Extraction, purification, bioactivity and pharmacological effects of capsaicin: a review. Crit Rev Food Sci Nutr. 2022;62(19):5322–48.
Li X. The inducers of immunogenic cell death for tumor immunotherapy. Tumori J. 2018;104(1):1–8.
Sansone C, Bruno A, Piscitelli C, Baci D, Fontana A, Brunet C, et al. Natural compounds of marine origin as inducers of immunogenic cell death (ICD): potential role for cancer interception and therapy. Cells. 2021;10(2):231.
Bagood MD, Isseroff RR. TRPV1: role in skin and skin Diseases and potential target for improving Wound Healing. Int J Mol Sci. 2021;22(11):6135.
Li C, Zhang Y, Yan S, Zhang G, Wei W, Qi Z, et al. Alternol triggers immunogenic cell death via reactive oxygen species generation. Oncoimmunology. 2021;10(1):1952539.
Liu X, Wang J, Sun B, Zhang Y, Zhu J, Li C. Cell growth inhibition, G2M cell cycle arrest, and apoptosis induced by the novel compound Alternol in human gastric carcinoma cell line MGC803. Investig New Drugs. 2007;25(6):505–17.
Ganassin R, Oliveira GRT, da Rocha MCO, Morais JAV, Rodrigues MC, Motta FN, et al. Curcumin induces immunogenic cell death in murine colorectal carcinoma CT26 cells. Pharmacol Research-Modern Chin Med. 2022;2:100046.
Liu X, Feng Z, Wang C, Su Q, Song H, Zhang C, et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials. 2020;230:119649.
Zhu C, Fang Z, Peng L, Gao F, Peng W, Song F. Curcumin suppresses the progression of colorectal Cancer by improving immunogenic cell death caused by Irinotecan. Chemotherapy. 2022:1–12.
Rad AH, Asiaee F, Jafari S, Shayanfar A, Lavasanifar A, Molavi O. Poly (ethylene glycol)-poly (ε-caprolactone)-based micelles for solubilization and tumor-targeted delivery of silibinin. BioImpacts: BI. 2020;10(2):87.
Jafari S, Lavasanifar A, Hejazi MS, Maleki-Dizaji N, Mesgari M, Molavi O. STAT3 inhibitory stattic enhances immunogenic cell death induced by chemotherapy in cancer cells. DARU J Pharm Sci. 2020;28(1):159–69.
Li-Weber M. New therapeutic aspects of flavones: the anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat Rev. 2009;35(1):57–68.
Yang Y, Li X-J, Chen Z, Zhu X-X, Wang J, Zhang L-b, et al. Wogonin induced calreticulin/annexin A1 exposure dictates the immunogenicity of cancer cells in a PERK/AKT dependent manner. PLoS ONE. 2012;7(12):e50811.
Liu Z, Liu T, Li W, Li J, Wang C, Zhang K. Insights into the antitumor mechanism of ginsenosides Rg3. Mol Biol Rep. 2021;48:2639–52.
Son K-j, Choi ryung, Lee K, Lee SJ. Immunogenic cell death induced by ginsenoside Rg3: significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. 2016;16(1):75–84.
Sun D, Zou Y, Song L, Han S, Yang H, Chu D, et al. A cyclodextrin-based nanoformulation achieves co-delivery of ginsenoside Rg3 and quercetin for chemo-immunotherapy in colorectal cancer. Acta Pharm Sinica B. 2022;12(1):378–93.
Huang X-t, Li X, Xie M-l, Huang Z, Huang Y-x, Wu G-x, et al. Resveratrol: review on its discovery, anti-leukemia effects and pharmacokinetics. Chemico-Biol Interact. 2019;306:29–38.
Zhang Y, Yang S, Yang Y, Liu T. Resveratrol induces immunogenic cell death of human and murine ovarian carcinoma cells. Infect Agents Cancer. 2019;14(1):1–9.
Girola N, Figueiredo CR, Farias CF, Azevedo RA, Ferreira AK, Teixeira SF, et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem Biophys Res Commun. 2015;467(4):928–34.
Zhang J, Shen L, Li X, Song W, Liu Y, Huang L. Nanoformulated codelivery of quercetin and alantolactone promotes an antitumor response through synergistic immunogenic cell death for microsatellite-stable colorectal cancer. ACS Nano. 2019;13(11):12511–24.
Gomez-Cadena A, Urueña C, Prieto K, Martinez-Usatorre A, Donda A, Barreto A, et al. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis. 2016;7(6):e2243–e.
Prieto K, Lozano MP, Urueña C, Alméciga-Díaz CJ, Fiorentino S, Barreto A. The delay in cell death caused by the induction of autophagy by P2Et extract is essential for the generation of immunogenic signals in melanoma cells. Apoptosis. 2020;25(11):875–88.
Chen H-M, Wang P-H, Chen S-S, Wen C-C, Chen Y-H, Yang W-C, et al. Shikonin induces immunogenic cell death in tumor cells and enhances dendritic cell-based cancer vaccine. Cancer Immunol Immunother. 2012;61(11):1989–2002.
Lin T-J, Lin H-T, Chang W-T, Hsiao P-W, Yin S-Y, Yang N-S. Shikonin-enhanced cell immunogenicity of tumor vaccine is mediated by the differential effects of DAMP components. Mol Cancer. 2015;14(1):1–13.
Wang W, Yang X, Li C, Li Y, Wang H, Han X. Immunogenic cell death (ICD) of murine H22 cells Induced by Lentinan. Nutr Cancer. 2022;74(2):640–9.
Michalkova R, Mirossay L, Gazdova M, Kello M, Mojzis J. Molecular mechanisms of antiproliferative effects of natural chalcones. Cancers. 2021;13(11):2730.
Maioral MF, Stefanes NM, Neuenfeldt PD, Chiaradia-Delatorre LD, Nunes RJ, Santos-Silva MC. Aldehyde biphenyl chalcones induce immunogenic apoptotic-like cell death and are promising new safe compounds against a wide range of hematologic cancers. Future Med Chem. 2020;12(8):673–88.
Qiu N, Liu Y, Liu Q, Chen Y, Shen L, Hu M, et al. Celastrol nanoemulsion induces immunogenicity and downregulates PD-L1 to boost abscopal effect in melanoma therapy. Biomaterials. 2021;269:120604.
Geng Y, Xiang J, Shao S, Tang J, Shen Y. Mitochondria-targeted polymer-celastrol conjugate with enhanced anticancer efficacy. J Controlled Release. 2022;342:122–33.
Fan F, Shen P, Ma Y, Ma W, Wu H, Liu H, et al. Bullatacin triggers immunogenic cell death of colon cancer cells by activating endoplasmic reticulum chaperones. J Inflamm. 2021;18(1):1–10.
Lee J-Y, Talhi O, Jang D, Cerella C, Gaigneaux A, Kim K-W, et al. Cytostatic hydroxycoumarin OT52 induces ER/Golgi stress and STAT3 inhibition triggering non-canonical cell death and synergy with BH3 mimetics in lung cancer. Cancer Lett. 2018;416:94–108.
Turrini E, Catanzaro E, Muraro MG, Governa V, Trella E, Mele V, et al. Hemidesmus indicus induces immunogenic death in human colorectal cancer cells. Oncotarget. 2018;9(36):24443.
Han S, Bi S, Guo T, Sun D, Zou Y, Wang L, et al. Nano co-delivery of Plumbagin and Dihydrotanshinone I reverses immunosuppressive TME of liver cancer. J Controlled Release. 2022;348:250–63.
Škubník J, Pavlíčková V, Rimpelová S. Cardiac glycosides as immune system modulators. Biomolecules. 2021;11(5):659.
Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med. 2012;4(143):143ra99–ra99.
Xiang Y, Chen L, Li L, Huang Y. Restoration and enhancement of immunogenic cell death of cisplatin by coadministration with digoxin and conjugation to HPMA copolymer. ACS Appl Mater Interfaces. 2019;12(1):1606–16.
Cerella C, Muller F, Gaigneaux A, Radogna F, Viry E, Chateauvieux S, et al. Early downregulation of Mcl-1 regulates apoptosis triggered by cardiac glycoside UNBS1450. Cell Death Dis. 2015;6(6):e1782–e.
Einbond LS, Wu H-a, Sandu C, Ford M, Mighty J, Antonetti V, et al. Digitoxin enhances the growth inhibitory effects of thapsigargin and simvastatin on ER negative human breast cancer cells. Fitoterapia. 2016;109:146–54.
Li X, Zheng J, Chen S, Meng F-d, Ning J, Sun S-l. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Dis. 2021;12(4):1–15.
Ramanathapuram LV, Hahn T, Dial SM, Akporiaye ET. Chemo-immunotherapy of breast cancer using vesiculated α-tocopheryl succinate in combination with dendritic cell vaccination. Nutr Cancer. 2005;53(2):177–93.
Xu Z, Xu J, Sun S, Lin W, Li Y, Lu Q et al. Mecheliolide elicits ROS-mediated ERS driven immunogenic cell death in hepatocellular carcinoma. Redox Biol. 2022:102351.
Wang G, Dong J, Deng L. Overview of cantharidin and its analogues. Curr Med Chem. 2018;25(17):2034–44.
Xu L, Su B, Mo L, Zhao C, Zhao Z, Li H, et al. Norcantharidin induces immunogenic cell death of bladder cancer cells through promoting autophagy in acidic culture. Int J Mol Sci. 2022;23(7):3944.
Zhang R, Neighbors J, Schell T, Hohl R. Schweinfurthin induces ICD without ER stress and caspase activation. Oncoimmunology. 2022;11(1):2104551.
Singh N, Yadav S, Rao AS, Nandal A, Kumar S, Ganaie S, et al. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J Ethnopharmacol. 2021;270:113704.
Prabhu A. Withania somnifera deploys immunomodulation and exerts anticancer effects on lung adenocarcinoma cells. South Afr J Bot. 2022;151:47–65.
Wen C-C, Chen H-M, Chen S-S, Huang L-T, Chang W-T, Wei W-C, et al. Specific microtubule-depolymerizing agents augment efficacy of dendritic cell-based cancer vaccines. J Biomed Sci. 2011;18(1):44.
Gallo C, Barra G, Saponaro M, Manzo E, Fioretto L, Ziaco M, et al. A new Bioassay platform design for the Discovery of Small Molecules with Anticancer immunotherapeutic activity. Mar Drugs. 2020;18(12):604.
Manzo E, Cutignano A, Pagano D, Gallo C, Barra G, Nuzzo G, et al. A new marine-derived sulfoglycolipid triggers dendritic cell activation and immune adjuvant response. Sci Rep. 2017;7(1):1–10.
Galasso C, Nuzzo G, Brunet C, Ianora A, Sardo A, Fontana A, et al. The marine dinoflagellate Alexandrium minutum activates a mitophagic pathway in human lung cancer cells. Mar Drugs. 2018;16(12):502.
Fabian CJ, Kimler BF, Hursting SD. Omega-3 fatty acids for breast cancer prevention and survivorship. Breast Cancer Res. 2015;17(1):1–11.
Lages EB, Fernandes RS, de Silva JdO ÂM, Cassali GD, de Barros ALB, et al. Co-delivery of doxorubicin, docosahexaenoic acid, and α-tocopherol succinate by nanostructured lipid carriers has a synergistic effect to enhance antitumor activity and reduce toxicity. Biomed Pharmacother. 2020;132:110876.
D’Eliseo D, Di Renzo L, Santoni A, Velotti F. Docosahexaenoic acid (DHA) promotes immunogenic apoptosis in human multiple myeloma cells, induces autophagy and inhibits STAT3 in both tumor and dendritic cells. Genes & cancer. 2017;8(1–2):426.
Martínez Andrade KA, Lauritano C, Romano G, Ianora A. Marine microalgae with anti-cancer properties. Mar Drugs. 2018;16(5):165.
Delgado-Roche L, González K, Mesta F, Couder B, Tavarez Z, Zavala R, et al. Polyphenolic fraction obtained from Thalassia testudinum marine plant and thalassiolin B exert cytotoxic effects in colorectal cancer cells and arrest tumor progression in a xenograft mouse model. Front Pharmacol. 2020;11:592985.
Hernández-Balmaseda I, Guerra IR, Declerck K, Herrera Isidrón JA, Pérez-Novo C, Van Camp G, et al. Marine seagrass extract of Thalassia testudinum suppresses colorectal tumor growth, motility and angiogenesis by autophagic stress and immunogenic cell death pathways. Mar Drugs. 2021;19(2):52.
Nuzzo G, Gallo C, Crocetta F, Romano L, Barra G, Senese G, et al. Identification of the Marine Alkaloid Lepadin A as potential inducer of immunogenic cell death. Biomolecules. 2022;12(2):246.
Wen H, Zhong Y, Yin Y, Qin K, Yang L, Li D, et al. A marine-derived small molecule induces immunogenic cell death against triple-negative breast cancer through ER stress-CHOP pathway. Int J Biol Sci. 2022;18(7):2898–913.
Chandrarathna H, Liyanage T, Edirisinghe S, Dananjaya S, Thulshan E, Nikapitiya C, et al. Marine microalgae, Spirulina maxima-derived modified pectin and modified pectin nanoparticles modulate the gut microbiota and trigger immune responses in mice. Mar Drugs. 2020;18(3):175.
Troitskaya O, Varlamov M, Nushtaeva A, Richter V, Koval O. Recombinant lactaptin induces immunogenic cell death and creates an antitumor vaccination effect in vivo with enhancement by an IDO inhibitor. Molecules. 2020;25(12):2804.
Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, et al. Screening of novel immunogenic cell death inducers within the NCI mechanistic diversity set. Oncoimmunology. 2014;3(4):e28473.
Rogalska A, Marczak A. Therapeutic potential of patupilone in epithelial ovarian cancer and future directions. Life Sci. 2018;205:38–44.
Chen W, Wang S, Wu Y, Shen X, Guo Z, Li Q, et al. Immunogenic cell death: a link between gut microbiota and anticancer effects. Microb Pathog. 2020;141:103983.
Ma W, Mao Q, Xia W, Dong G, Yu C, Jiang F. Gut microbiota shapes the efficiency of cancer therapy. Front Microbiol. 2019;10:1050.
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Reviews Clin Oncol. 2019;16(3):151–67.
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–84.
Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews M, Karpinets T, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103.
Routy B, Le Chatelier E, Derosa L, Duong CP, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–7.
McQuade JL, Daniel CR, Helmink BA, Wargo JA. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019;20(2):e77–e91.
Zhou C-B, Zhou Y-L, Fang J-Y. Gut microbiota in cancer immune response and immunotherapy. Trends in Cancer. 2021;7(7):647–60.
Matson V, Chervin CS, Gajewski TF. Cancer and the microbiome—influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology. 2021;160(2):600–13.
Jafari S, Bakhshaei A, Eskandani M, Molavi O. Silibinin-loaded nanostructured lipid carriers for growth inhibition of cisplatin-resistant ovarian Cancer cells. Assay Drug Dev Technol. 2022;20(8):339–48.
Hong C, Wang D, Liang J, Guo Y, Zhu Y, Xia J, et al. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics. 2019;9(15):4437.
Li J, Zhou S, Yu J, Cai W, Yang Y, Kuang X, et al. Low dose shikonin and anthracyclines coloaded liposomes induce robust immunogenetic cell death for synergistic chemo-immunotherapy. J Controlled Release. 2021;335:306–19.
Oliyapour Y, Dabiri S, Molavi O, Hejazi MS, Davaran S, Jafari S, et al. Chrysin and chrysin-loaded nanocarriers induced immunogenic cell death on B16 melanoma cells. Med Oncol. 2023;40(10):278.
Jafari S, Dabiri S, Mehdizadeh Aghdam E, Fathi E, Saeedi N, Montazersaheb S et al. Synergistic effect of chrysin and radiotherapy against triple-negative breast cancer (TNBC) cell lines. Clin Transl Oncol. 2023:1–10.
Zhu C, Fang Z, Peng L, Gao F, Peng W, Song F. Curcumin suppresses the progression of colorectal cancer by improving immunogenic cell death caused by irinotecan. Chemotherapy. 2022;67(4):211–22.
Ashrafizadeh M, Zarrabi A, Hashemi F, Moghadam ER, Hashemi F, Entezari M, et al. Curcumin in cancer therapy: a novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020;256:117984.
Li J, Wang X, Guo Y, Zhang Y, Zhu A, Zeng W, et al. Ginsenoside Rg3-engineered exosomes as effective delivery platform for potentiated chemotherapy and photoimmunotherapy of glioblastoma. Chem Eng J. 2023;471:144692.
Wu H, Wei G, Luo L, Li L, Gao Y, Tan X, et al. Ginsenoside Rg3 nanoparticles with permeation enhancing based chitosan derivatives were encapsulated with doxorubicin by thermosensitive hydrogel and anti-cancer evaluation of peritumoral hydrogel injection combined with PD-L1 antibody. Biomaterials Res. 2022;26(1):1–21.
Jafari S, Molavi O, Kahroba H, Hejazi MS, Maleki-Dizaji N, Barghi S, et al. Clinical application of immune checkpoints in targeted immunotherapy of prostate cancer. Cell Mol Life Sci. 2020;77:3693–710.
Jiang M, Zeng J, Zhao L, Zhang M, Ma J, Guan X, et al. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo–immunotherapy. Nanoscale. 2021;13(41):17218–35.
Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. 2011;71(3):768–78.
Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202(12):1691–701.
Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, et al. Paclitaxel induces immunogenic cell death in ovarian cancer via TLR4/IKK2/SNARE-dependent exocytosis. Cancer Immunol Res. 2020;8(8):1099–111.
Tian P-J, Li B-L, Shan Y-J, Zhang J-N, Chen J-Y, Yu M, et al. Extraction of peptidoglycan from L. Paracasei subp. Paracasei X12 and its preliminary mechanisms of inducing immunogenic cell death in HT-29 cells. Int J Mol Sci. 2015;16(8):20033–49.
Anker JF, Naseem AF, Mok H, Schaeffer AJ, Abdulkadir SA, Thumbikat P. Multi-faceted immunomodulatory and tissue-tropic clinical bacterial isolate potentiates prostate cancer immunotherapy. Nat Commun. 2018;9(1):1–14.
Groza D, Gehrig S, Kudela P, Holcmann M, Pirker C, Dinhof C, et al. Bacterial ghosts as adjuvant to oxaliplatin chemotherapy in colorectal carcinomatosis. Oncoimmunology. 2018;7(5):e1424676.
Mu D, Gao Z, Guo H, Zhou G, Sun B. Sodium butyrate induces growth inhibition and apoptosis in human prostate cancer DU145 cells by up-regulation of the expression of annexin A1. PLoS ONE. 2013;8(9):e74922.
Zhang Y, Liu Y, Zhou Y, Zheng Z, Tang W, Song M, et al. Lentinan inhibited colon cancer growth by inducing endoplasmic reticulum stress-mediated autophagic cell death and apoptosis. Carbohydr Polym. 2021;267:118154.
Wang J, Li W, Huang X, Liu Y, Li Q, Zheng Z, et al. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget. 2017;8(1):610.
Xiu Z, Sun T, Yang Y, He Y, Yang S, Xue X et al. Curcumin enhanced ionizing radiation-induced immunogenic cell death in glioma cells through endoplasmic reticulum stress signaling pathways. Oxidative Medicine and Cellular Longevity. 2022;2022.
Zhang S, Gao Q, Li W, Zhu L, Shang Q, Feng S, et al. Shikonin inhibits cancer cell cycling by targeting Cdc25s. BMC Cancer. 2019;19(1):1–9.
Wang Y, Zhou Y, Jia G, Han B, Liu J, Teng Y, et al. Shikonin suppresses tumor growth and synergizes with gemcitabine in a pancreatic cancer xenograft model: involvement of NF-κB signaling pathway. Biochem Pharmacol. 2014;88(3):322–33.
Bagamanshina AV, Troitskaya OS, Nushtaeva AA, Yunusova AY, Starykovych MO, Kuligina EV, et al. Cytotoxic and Antitumor Activity of Lactaptin in Combination with Autophagy inducers and inhibitors. Biomed Res Int. 2019;2019:4087160.
Stan SD, Hahm E-R, Warin R, Singh SV. Withaferin a causes FOXO3a-and bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68(18):7661–9.
Li X, Zheng J, Chen S, Meng F-d, Ning J, Sun S-l. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Dis. 2021;12(4):314.
Qin T, Xu X, Zhang Z, Li J, You X, Guo H, et al. Paclitaxel/sunitinib-loaded micelles promote an antitumor response in vitro through synergistic immunogenic cell death for triple-negative breast cancer. Nanotechnology. 2020;31(36):365101.
Kuo I-M, Lee J-J, Wang Y-S, Chiang H-C, Huang C-C, Hsieh P-J, et al. Potential enhancement of host immunity and anti-tumor efficacy of nanoscale curcumin and resveratrol in colorectal cancers by modulated electro-hyperthermia. BMC Cancer. 2020;20:1–13.
Wu S, Liu D, Li W, Song B, Chen C, Chen D, et al. Enhancing TNBC chemo-immunotherapy via combination reprogramming tumor immune microenvironment with immunogenic cell death. Int J Pharm. 2021;598:120333.
Li Q, He J, Li S, Tian C, Yang J, Yuan H, et al. The combination of gemcitabine and ginsenoside Rh2 enhances the immune function of dendritic cells against pancreatic cancer via the CARD9-BCL10-MALT1 / NF-κB pathway. Clin Immunol. 2023;248:109217.
Dai Y, Wang W, Sun Q, Tuohayi J. Ginsenoside Rg3 promotes the antitumor activity of gefitinib in lung cancer cell lines. Experimental and Therapeutic Medicine. 2019;17(1):953–9.
Wu H, Wei G, Luo L, Li L, Gao Y, Tan X, et al. Ginsenoside Rg3 nanoparticles with permeation enhancing based chitosan derivatives were encapsulated with doxorubicin by thermosensitive hydrogel and anti-cancer evaluation of peritumoral hydrogel injection combined with PD-L1 antibody. Biomaterials Res. 2022;26(1):77.
Hong C, Wang D, Liang J, Guo Y, Zhu Y, Xia J, et al. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics. 2019;9(15):4437–49.
Liu X, Sun G. Shikonin enhances Adriamycin antitumor effects by inhibiting efflux pumps in A549 cells. Oncol Lett. 2017;14(4):4270–6.
Song J, Zhao Z, Fan X, Chen M, Cheng X, Zhang D, et al. Shikonin potentiates the effect of arsenic trioxide against human hepatocellular carcinoma in vitro and in vivo. Oncotarget. 2016;7(43):70504.
Huo X, Pei Z, Wang W, Liu Y, Sun J, Wang H et al. Lentinan Enhances the Function of Oxaliplatin on the Esophageal Tumors by Persuading Immunogenic Cell Death. Computational and Mathematical Methods in Medicine. 2022;2022.
Liu Q, Chen F, Hou L, Shen L, Zhang X, Wang D, et al. Nanocarrier-mediated chemo-immunotherapy arrested cancer progression and induced tumor dormancy in desmoplastic melanoma. ACS Nano. 2018;12(8):7812–25.
Li B, Tan T, Chu W, Zhang Y, Ye Y, Wang S, et al. Co-delivery of paclitaxel (PTX) and docosahexaenoic acid (DHA) by targeting lipid nanoemulsions for cancer therapy. Drug Delivery. 2022;29(1):75–88.
Jeong S, Kim DY, Kang SH, Yun HK, Kim JL, Kim BR, et al. Docosahexaenoic acid enhances oxaliplatin-induced autophagic cell death via the ER stress/Sesn2 pathway in colorectal cancer. Cancers. 2019;11(7):982.
Ma Y, Yu J, Li Q, Su Q, Cao B. Addition of docosahexaenoic acid synergistically enhances the efficacy of apatinib for triple-negative breast cancer therapy. Biosci Biotechnol Biochem. 2020;84(4):743–56.
Wu J, Waxman DJ. Metronomic cyclophosphamide eradicates large implanted GL261 gliomas by activating antitumor Cd8 + T-cell responses and immune memory. Oncoimmunology. 2015;4(4):e1005521.
Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillère R, Hannani D, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6.
Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide Synergizes with Type I Interferons through systemic dendritic cell reactivation and induction of immunogenic tumor ApoptosisCTX and IFN-I synergism in DC-Driven Antitumor Response. Cancer Res. 2011;71(3):768–78.
Du B, Waxman DJ. Medium dose intermittent cyclophosphamide induces immunogenic cell death and cancer cell autonomous type I interferon production in glioma models. Cancer Lett. 2020;470:170–80.
Li F, Zheng X, Wang X, Xu J, Zhang Q. Macrophage polarization synergizes with oxaliplatin in lung cancer immunotherapy via enhanced tumor cell phagocytosis. Translational Oncol. 2021;14(11):101202.
Dudek-Perić AM, Ferreira GB, Muchowicz A, Wouters J, Prada N, Martin S, et al. Antitumor Immunity triggered by Melphalan is potentiated by Melanoma Cell Surface–Associated CalreticulinMelphalan, Antimelanoma immunity, inflammation. Cancer Res. 2015;75(8):1603–14.
Zhao T, Ren H, Jia L, Chen J, Xin W, Yan F, et al. Inhibition of HIF-1α by PX-478 enhances the anti-tumor effect of gemcitabine by inducing immunogenic cell death in pancreatic ductal adenocarcinoma. Oncotarget. 2015;6(4):2250.
De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. Am J Pathol. 2014;184(1):42–54.
Jie Y, Yang X, Chen W. Pulsatilla Decoction combined with 5-Fluorouracil triggers immunogenic cell death in the Colorectal Cancer cells. Cancer Biotherapy & Radiopharmaceuticals; 2021.
Limagne E, Thibaudin M, Nuttin L, Spill A, Derangère V, Fumet J-D, et al. Trifluridine/Tipiracil plus Oxaliplatin improves PD-1 blockade in Colorectal Cancer by Inducing Immunogenic Cell Death and Depleting MacrophagesChemotherapy improves Anti–PD-1 in MSS Colorectal Cancer. Cancer Immunol Res. 2019;7(12):1958–69.
Huang M, Wang S, Chen S, Wang J, Xu C, Liu J, et al. Pegylated liposomal mitoxantrone modulates tumor immune landscape to boost PD-L1 blockade therapy. Nano Today. 2022;44:101500.
Bugaut H, Bruchard M, Berger H, Derangère V, Odoul L, Euvrard R, et al. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS ONE. 2013;8(6):e65181.
Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, et al. Paclitaxel induces immunogenic cell death in Ovarian Cancer via TLR4/IKK2/SNARE-Dependent ExocytosisPaclitaxel induces ICD via TLR4 in Cancer cells. Cancer Immunol Res. 2020;8(8):1099–111.
Flieswasser T, Van Loenhout J, Freire Boullosa L, Van den Eynde A, De Waele J, Van Audenaerde J, et al. Clinically relevant chemotherapeutics have the ability to induce immunogenic cell death in non-small cell lung cancer. Cells. 2020;9(6):1474.
Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71(14):4821–33.
Liu P, Zhao L, Kepp O, Kroemer G. Crizotinib–a tyrosine kinase inhibitor that stimulates immunogenic cell death. Taylor & Francis; 2019. p. e1596652.
Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. 2016;22(6):624–31.
Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene. 2010;29(4):482–91.
Sun F, Cui L, Li T, Chen S, Song J, Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J Recept Signal Transduction. 2019;39(3):208–14.
Wernitznig D, Meier-Menches SM, Cseh K, Theiner S, Wenisch D, Schweikert A, et al. Plecstatin-1 induces an immunogenic cell death signature in colorectal tumour spheroids. Metallomics. 2020;12(12):2121–33.
Wernitznig D, Kiakos K, Del Favero G, Harrer N, Machat H, Osswald A, et al. First-in-class ruthenium anticancer drug (KP1339/IT-139) induces an immunogenic cell death signature in colorectal spheroids in vitro. Metallomics. 2019;11(6):1044–8.
Kaur P, Johnson A, Northcote-Smith J, Lu C, Suntharalingam K. Immunogenic cell death of breast Cancer Stem cells Induced by an endoplasmic reticulum-targeting copper(II) complex. ChemBioChem. 2020;21(24):3618–24.
Wang L, Guan R, Xie L, Liao X, Xiong K, Rees TW, et al. An ER-Targeting Iridium (III) Complex that induces immunogenic cell death in non‐small‐cell lung Cancer. Angew Chem. 2021;133(9):4707–15.
Sen S, Hufnagel S, Maier EY, Aguilar I, Selvakumar J, DeVore JE, et al. Rationally designed redox-active au (I) N-heterocyclic carbene: an immunogenic cell death inducer. J Am Chem Soc. 2020;142(49):20536–41.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
MA, writing first draft of manuscript, OM and Sh.S: reviewing and editing, SJ and SM: conceptualization; reviewing, editing and drawing figures. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Amiri, M., Molavi, O., Sabetkam, S. et al. Stimulators of immunogenic cell death for cancer therapy: focusing on natural compounds. Cancer Cell Int 23, 200 (2023). https://doi.org/10.1186/s12935-023-03058-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-03058-7