Abstract
Background
As an oncogene, SETD8 can promote tumour growth and tumour cell proliferation. This study aims to reveal the relationship between SETD8 and ferroptosis in pancreatic cancer and its role in pancreatic cancer to provide a possible new direction for the comprehensive treatment of pancreatic cancer.
Methods
The downstream targets were screened by RNA sequencing analysis. Western blot, Real-time Quantitative PCR (qPCR) and immunohistochemistry showed the relationship between genes. Cell proliferation analysis and cell metabolite analysis revealed the function of genes. Chromatin immunoprecipitation (CHIP) assays were used to study the molecular mechanism.
Results
The potential downstream target of SETD8, RRAD, was screened by RNA sequencing analysis. A negative correlation between SETD8 and RRAD was found by protein imprinting, Real-time Quantitative PCR (qPCR) and immunohistochemistry. Through cell proliferation analysis and cell metabolite analysis, it was found that RRAD can not only inhibit the proliferation of cancer cells but also improve the level of lipid peroxidation of cancer cells. At the same time, chromatin immunoprecipitation analysis (CHIP) was used to explore the molecular mechanism by which SETD8 regulates RRAD expression. SETD8 inhibited RRAD expression.
Conclusions
SETD8 interacts with the promoter region of RRAD, which epigenetically silences the expression of RRAD to reduce the level of lipid peroxidation in pancreatic cancer cells, thereby inhibiting ferroptosis in pancreatic cancer cells and resulting in poor prognosis of pancreatic cancer.
Similar content being viewed by others
Background
Pancreatic cancer is a highly malignant tumour. It is the seventh leading cause of death among human cancers [1, 2]. With the development of medical technology, targeted drugs have been applied to breast cancer, lung cancer and other tumours and have achieved good clinical results [3, 4]. However, no effective molecular target for targeted therapy has been found for pancreatic cancer. Pancreatic cancer is resistant to most chemotherapeutic agents, so surgical treatment remains the only way to cure pancreatic cancer. However, due to its late discovery, most patients have lost best opportunity for operation by the time they see a doctor [5]. Therefore, it is particularly important to explore the molecular biological mechanism of the occurrence and development of pancreatic cancer. Understanding the molecular biological mechanisms of pancreatic cancer can provide new therapeutic targets and directions for the comprehensive treatment of pancreatic cancer. Although the overall 5-year survival rate of pancreatic cancer is less than 10%, in clinical practice and other authors' literature, a small proportion of pancreatic cancer patients have a relatively good prognosis [6].
Lysine methyltransferase SETD8 (also known as PR-SET7, SET8 or KMT5A) is a member of the SET domain family. Its important and most common function is to regulate the cell cycle and tumour growth [7, 8]; SETD8 is unique among the KMTs discovered thus far. It is the only methyltransferase that can monomethylate histone H4 lysine 20 (H4K20). At the same time, the most important and common type of histone modification is methylation, and histone methylation is a common form of epigenetic disorder in tumorigenesis and development [9]. In addition, the methylation marker histone H4 lysine 20 (H4K20) is also considered to be an inhibitory signal of gene transcription, which plays a key role in DNA replication, DNA damage repair and silencing heterochromatin [10]. Therefore, enzymes that target the methylation of lysine residues in modified substrates are a potential direction of antitumour drug research. In our study, we found that SETD8 negatively regulates glucose metabolism and redox reactions in pancreatic cancer cells. Through gene expression profiling, we noticed that SETD8 reduces the expression of RRAD, a closely related gene of glycometabolism (RAS associated with diabetes) [11]. Therefore, we speculate that SETD8 may be involved in the regulation of lipid peroxidation in pancreatic cancer cells.
Ferroptosis is a new form of iron dependent programmed cell death that is different from apoptosis, necrosis and autophagy. It is characterized by lipid peroxidation of unsaturated fatty acids highly expressed on the cell membrane [12]. There is evidence that ferroptosis is associated with biological redox reactions and health. At the same time, inducing ferroptosis in cancer cells has great potential in cancer treatment. This is especially true in refractory malignant tumours that are resistant to traditional treatments such as radiotherapy and chemotherapy [13, 14]. Ferroptosis is closely related to RAS mutation of oncogenes. First, Brent R. Stockwell found that some small molecules, such as RAS-selective lethal 3 (RSL3) and erastin, can induce iron-dependent regulatory cell death different from other forms of cell death (such as apoptosis and necrosis) [15, 16]. Interestingly, nearly 95% of all pancreatic cancers have mutations in the KRAS gene [17]. However, research on pancreatic cancer and ferroptosis is insufficient. At the same time, we can analyse the level of lipid peroxidation in pancreatic cancer cells by SETD8. Therefore, we speculate that SETD8 may be involved in the regulation of ferroptosis in pancreatic cancer.
In this study, we studied the effect of methylation of histone H4 lysine 20 by lysine methyltransferase SETD8 on ferroptosis in pancreatic cancer. We found that SETD8 inhibited the occurrence of ferroptosis in pancreatic cancer. We proved that RRAD (RAS associated with diabetes) is a key target gene for SETD8 and that RRAD can promote lipid peroxidation in pancreatic cancer cells. Mechanistically, SETD8 inhibits the transcriptional activity of RRAD by binding to the promoter region of RRAD, thus downregulating the expression of RRAD and resulting in a decrease in the incidence of ferroptosis in pancreatic cancer cells. Therefore, high levels of SETD8 and low levels of RRAD are closely related to poor prognosis in pancreatic cancer patients. At the same time, we found that the SETD8-RRAD-ferroptosis axis may be a potential target for the treatment of pancreatic cancer and provide a new strategy for the comprehensive treatment of pancreatic cancer.
Materials and methods
Cell culture
The human pancreatic cancer cell lines MIAPACA-2 and SW1990 were obtained from the American Type Culture Collection Center (ATCC, VA, USA) and cultured according to the ATCC-provided program. All cells used in the experiment were within 10 generations after thawing. All cell culture media contained 100 U/ml penicillin and 100 mg/ml streptomycin.
Chemicals
The ferroptosis inducer RAS-selective lethal 3 (RSL3) and ferroptosis inhibitor ferrostatin-1 (Fer-1) were purchased from Selleckchem.
Plasmids
The coding sequences of human RRAD and SETD8 were cloned into the whole virus vector p pCDH-CMV-MCS-EF1-puro (SBI, USA) to produce the expression plasmids of RRAD and SETD8. To inhibit the expression of the target gene, the pLKO.1 TRC cloning vector (Addgene plasmid 10878, Watertown, MA, USA) was used. The 21 bp targets for SETD8 were CCGAGGAACAGAAGATCAAAG and CGCAACAGAATCGCAAACTTA; the 21 bp targets for RRAD were CGTAGCTCGTAACAGCCGCAA and CACACCTATGATCGCTCCATT. The control interference shRNA (Addgene plasmid 1864) was used as a knockout control vector. The corresponding overexpression structures of SETD8 and RRAD were obtained by using the pCDH-CMV-MCS-EF1-Puro vector (System Biosciences, Palo Alto, CA, USA), and empty body (EV) was used as a control. Lentiviruses are composed of the target gene expression vectors psPAX2 and pMD2. 293 T cells were added at a ratio of 4:3:1. Lentivirus particles were used to infect MIAPACA-2 and SW1990 cells, followed by puromycin screening to obtain stable cell lines.
Western blot
Cells were first collected and washed twice with PBS, RIPA buffer mixed with protease and phosphatase inhibitors (Beyotime Biotechnology, Shanghai, China) was added, and the cells were lysed on ice for 30 min. Then, the protein concentration of the lysate was determined by a BCA protein analysis kit (Beyotime Biotechnology, Shanghai, China). Approximately 20 μg protein samples were separated on 10% SDS–polyacrylamide gels and then transferred to PVDF membranes (Millipore, Billerica, USA), and incubated with specific antibodies against SETD8 (Proteintech, 1:1000) and RRAD (Abcam, 1:1000). Next, the membrane was detected with a secondary antibody bound to HRP (protein, 1:5000). Finally, immunoblotting was incubated with an enhanced chemiluminescence detection kit (Millipore) and displayed by an imaging system (Clinx). The original figures of the western blots are all in Additional file 1.
RNA extraction and real-time quantitative PCR
In summary, First, the cells were suspended in a 15 ml centrifuge tube and washed twice with PBS. Then, 2 × 10^7 cells were taken into a 1.5 ml EP tube and total RNA was extracted with Trizol reagent (Invitrogen, USA). cDNA was obtained by reverse transcription using the Takara primescript RT kit. Quantitative real-time PCR was used to determine the expression level of the target gene by an ABI 7900ht real-time PCR system (American Applied Biological Systems Company). β-Actin was used as the control, and the relative mRNA level was expressed by multiple changes compared with the control. The primers used were as follows: human SETD8: 5′-AAGATGTCCAAGCCCCGC-3′ (forward), 5′-TGTTCCTCGGACTTCATGGC-3′ (reverse); Human RRAD: 5′-ACATTTGGGAGCAGGACGG-3′ (forward), 5′-CTCTTGTTGCCCACGAGGAT-3′ (reverse); people β-Actin: 5′-CTACGTCGCCCTGGACTTCGAGC-3′ (forward), 5′-GATGGAGCCGCCGATCCACACGG-3′ (reverse). Subsequently, the delta-delta Ct method was used for data statistics. All tests were carried out in triplicate.
Chromosome immunoprecipitation assay
Chromosome immunoprecipitation analysis was performed to evaluate the occupancy of SETD8 on the RRAD promoter according to the instructions provided by the Magna CHIP A/G Chromatin Immunoprecipitation Kit (Darmstadt Millipore, Germany). A pair of primers was used to amplify the chromatin region of RRAD. The primer sequences were as follows: forward primer (5′–3′): AGTTGCTGCTTTTGGCTGATTGGGTT, reverse primer (5′–3′): AGTTGCTGCTTTTGGCTGATTGGGTT. Simply put, the cells were crosslinked with 1% formaldehyde for 10 min and then lysed and sonicated to an average size of 500 bp. The cross-linked protein/DNA complex was immunoprecipitated by anti-SETD8 antibody (Santa Cruz Biotechnology, USA) and isotype control IgG (Cell Signaling Technology, 3900), incubated at 4 °C, bound to protein magnetic beads, eluted from the complex and purified for DNA. CHIP-ReCHIP was carried out basically the same as primary CHIP. The target DNA sequence was finally analysed on agarose gel for CHIP experiments.
C11-BODIPY staining
Half a million cells were seeded into each well in a six-well plate (Corning) and then pretreated with a ferroptosis inducer for 24 h. Before flow cytometry, the cells were separated, resuspended and washed, and then stained with 2 μmol/l C11 BODIPY for 30 min. Then, the fluorescence intensity was detected by flow cytometry (Beckman).
MDA and GSH/GSSG determination
The cells were placed in a 6-well cell culture plate (Corning). The treatment conditions were consistent with c11-bodipy analysis. After obtaining the cell homogenate, the protein concentration was measured using a BCA protein analysis kit (Beyotime), and then MDA was detected using the lipid peroxidation MDA Analysis Kit (Beyotime). After obtaining the MDA content, the ratio of MDA to protein concentration was calculated. The ratio of GSH/GSSG was measured according to the Beyotime GSH/GSSG assay kit and standardized using the protein concentration of the cell lysate.
CCK-8 and colony formation test
We used the Cell Counting Kit-8 (Beyotime) for cell proliferation and toxicity experiments. The cells were inoculated into 96-well plates (1 per well) × 103 cells), and then 10 μl the Cell Counting Kit-8 (CCK-8) solution was added to each well at 0, 24, 48, 72, 96 and 120 h and incubated at 37 °C in 5% CO2 for 1.5 h, and then, the absorbance of each sample was measured at 450 nm wavelength using a microplate reader. In the cytotoxicity experiment, the cells were inoculated into 96-well plates (5000 cells per well) and then treated with a ferroptosis inducer and inhibitor. After 48 h, 10 μl CCK-8 solution was added to each well, incubated in 5% CO2 at 37 °C for 1 h, and finally, the absorbance of each sample was measured at 450 nm using a microplate reader. We tested cell viability according to the instructions of the Cell Counting Kit-8 reagent (Beyotime). For colony formation, pancreatic cancer cells (150 cells per pore) were inoculated in 6-well plates for 7–10 days. The colonies were fixed with 4% paraformaldehyde for 15 min, stained with 1% crystal violet for 30 min, and then counted.
Tissue samples and immunohistochemical (IHC) staining
The clinical tissue samples used in this study were clinical PDAC tissue samples from 80 patients confirmed by surgery and pathology and approved by the ethics committee of the Affiliated Tumour Hospital of Fudan University (FUSCC). The immunohistochemical staining of paraffin-embedded tissues adopts a two-step scheme. First, The antigen was extracted by dewaxing hydration and antigen retrieval, and then the slide was incubated with the following primary antibodies: anti-SETD8 (Proteintech) and anti-RRAD (Abcam). HRP binds affinity-purified sheep anti rabbit IgG (Proteintech) as a secondary antibody. Three different views were randomly selected under the microscope, and each slide was scored. According to the total area and intensity of staining, the protein expression level score was (1), < 5% of the total cells; (2), 5–25%; (3), 25–50%; (4), 50–75 and > 75%: (5). The final score was the average of the three views and was classified as follows: low (1 ≤ score < 3) and high (3 ≤ score ≥ 5).
Statistical analysis
The experiment was repeated at least three times. All data were analysed by GraphPad Prism 8. Two-tailed unpaired Student’s t tests were used to compare the differences between the two groups. The χ2 test was used to analyse the relationship between the expression of SETD8 or RRAD and the corresponding clinicopathological features. Survival curves were drawn using the Kaplan‒Meier method and compared by the log-rank test. Differences were considered significant at *P < 0.05; **P < 0.01. NS means there was no significant difference.
Results
SETD8 inhibits ferroptosis in pancreatic cancer cells
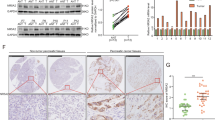
The most common and important function of SETD8 is to regulate the cell cycle and tumour growth. We silenced the expression of SETD8 in the SW1990 cell line using two SETD8-specific shRNA expression lentiviruses (shSETD8#1 and shSETD8#2). At the same time, the expression of SETD8 was enhanced in the Mia PaCa-2 cell line. The efficiency of SETD8 knockout and overexpression was confirmed by qPCR and Western blotting (Fig. 1a, b). We found that the level of lipid oxidation increased in the SW1990 cell line with SETD8 silencing, but decreased with the Mia PaCa-2 cell line’s overexpression of SETD8 (Fig. 1c). Therefore, we speculated that the expression level of glutathione as the main antioxidant [18] decreased in the cell lines with SETD8 knockout and increased in the Mia PaCa-2 cell lines with increased SETD8 expression. To confirm this hypothesis, we tested the GSH/GSSG ratio in the SW1990 cell line with silenced SETD8 expression and the Mia PaCa-2 cell line overexpressing SETD8 (Fig. 1d). As expected, SETD8 increased the expression of GSH. At the same time, GSH is closely related to GPX4, a key substance regulating ferroptosis [19]. Therefore, we used the BODIPY 581/591C11 probe to detect the level of lipid peroxidation in the SW1990 cell line with silenced SETD8 expression and the Mia PaCa-2 cell line overexpressing SETD8 (Fig. 1f). The results showed that SETD8 inhibited the lipid peroxidation of cells. Lipid peroxidation is the most important marker of ferroptosis [20], and SETD8 inhibits lipid peroxidation. Therefore, SETD8 may inhibit ferroptosis. To verify this, we added the ferroptosis inhibitor ferrostatin-1 to the SW1990 cell line, which silenced SETD8 expression. The ferroptosis inhibitor ferrostatin-1 (Fer) reversed the lipid peroxidation induced by SETD8 silencing (Fig. 1e). A ferroptosis inducer (RSL3) was added to the Mia PaCa-2 cell line overexpressing SETD8. The results showed that a ferroptosis inducer (RSL3) could inhibit the increased cell viability of the cells overexpressing SETD8 (Fig. 1e). This indicates that SETD8 inhibits the occurrence of ferroptosis in pancreatic cancer cells.
SETD8 declines ferroptosis in pancreatic cancer cells. A, B The shRNAs against SETD8 plasmid were transfected into SW1990 cell line. Plasmid overexpressing SETD8 was transfected into Mia PaCa-2 cell line. Western blot analysis and qPCR analysis were performed to exam SETD8 protein and mRNA levels, respectively. C The MDA was detected in SETD8-silenced SW1990 cell line and SETD8-overexpressed Mia PaCa-2 cell line. The results showed that SETD8 could inhibit the expression of MDA. D The GSH/GSSG ratio was detected in SETD8-silenced SW1990 cell line and SETD8-overexpressed Mia PaCa-2 cell line. It was found that silencing SETD8 could decrease the GSH/GSSG ratio and overexpression of SETD8 could increase the GSH/GSSG ratio. E Cell viability was detected in the SETD8-silenced SW1990 cell line in the presence or absence of 2 μmol/l Fer and the SETD8-overexpressed Mia PaCa-2 cell line in the presence or absence of 2 μmol/l RSL3. The results showed that the cell viability decreased by SETD8 knockdown could be reversed by the ferroptosis inhibitor Fer and the cell viability increased by SETD8 overexpression could be reversed by the ferroptosis inducer RSL3. F Flow cytometry analyzed the fluorescence of BODIPY581/591C11 (lower) and the relative content was calculated (upper). The results showed that SETD8 could reduce the level of intracellular lipid peroxidation. Two tailed unpaired Student t-test was used in the above experiments. *P < 0.05; **P < 0.01
RNA expression profiling identified RRAD as a downstream target of SETD8 regulating ferroptosis
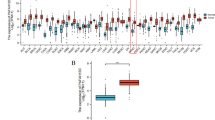
To study the molecular mechanism by which SETD8 regulates ferroptosis in pancreatic cancer cells, we examined the effect of SETD8 knockout on gene expression profiles. Specifically, two different shRNAs targeting SETD8 (shSETD8 # 1 and shSETD8 # 2) were used to silence the expression of SETD8 in SW1990 cells, and SETD8 was overexpressed in Mia PaCa-2 cells. After that, we compared the mRNA expression level differences between the above specific treatment cells by RNA sequencing. The results showed that a series of genes were upregulated and downregulated in SW1990 cells silenced by SETD8 and Mia PaCa-2 cells overexpressed by SETD8. (Fig. 2a). The total number of genes in the final list is relatively limited, in part because two different shSETD8s were transfected into the cell line to minimize the off-target effects and false-positive results of shRNAs. These include the RAS-related GTPase subfamily member RRAD. RRAD is also known as the RAS family diabetes-related gene, can inhibit the proliferation and migration of tumour cells, and has been identified as a tumour suppressor gene in many tumours [21,22,23,24]. It has been reported that epigenetic genes usually play a key role in cancer cells by inhibiting the expression of tumour suppressor genes [25]. Therefore, we confirmed the gene RRAD through qPCR and found that its expression was significantly upregulated with SETD8 knockout, which was consistent with the sequencing data (Fig. 2b). At the same time, the expression of RRAD was also downregulated with SETD8 overexpression (Fig. 2b). In addition, we further examined the effect of SETD8 on RRAD at the protein level by Western blotting (Fig. 2c). Coincidentally, the result was consistent with that of qPCR. The expression of RRAD was significantly upregulated in SETD8-silenced cells and downregulated in SETD8-overexpressing cells. To further verify the relationship between SETD8 and RRAD expression, IHC was performed in human PDAC tissue (Fig. 2d). The results showed that there was a negative correlation between the expression of SETD8 and RRAD (r = − 0.344; P = 0.0018; n = 80).
The downstream target gene of SETD8 is RRAD and negatively regulates RRAD expression. A In the gene heatmap of knockdown of SETD8 in SW1990 cells, RRAD was significantly overexpressed. In the heatmap of genes overexpressing SETD8 in Mia PaCa-2 cells, RRAD was significantly decreased. These results indicated that RRAD was a downstream target gene of SETD8 and was negatively regulated by SETD8. B qPCR analysis was performed to exam RRAD mRNA level. These results confirmed that SETD8 knockdown led to upregulation of the RRAD expression level, while SETD8 overexpression led to downregulation of the RRAD expression level. This further proves that SETD8 negatively regulates RRAD expression. C Western blot was performed to exam RRAD protein level. The results showed that SETD8 knockdown resulted in upregulation of the RRAD protein level, while SETD8 overexpression led to downregulation of the RRAD protein level. This further indicates that SETD8 negatively regulates RRAD expression. D Representative images of IHC staining of SETD8 and RRAD in PDAC tumours and their correlation. P = 0.0018. It was further shown that SETD8 was negatively correlated with RRAD. *P < 0.05; **P < 0.01
RRAD inhibits the proliferation of PDAC cell lines and is associated with a better prognosis in PDAC patients
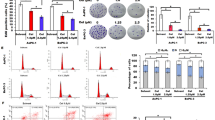
To further study the function of RRAD in the development of pancreatic cancer, we overexpressed RRAD in the MIA PaCa-2 cell line and confirmed the efficiency of RRAD over expression by qPCR and Western blotting (Fig. 3a, b). By analysing the results of the following functional analysis, and we found that compared with the control group, the ability of RRAD-overexpressing cell lines to proliferate and form colonies decreased significantly (Fig. 3c, d), indicating that RRAD inhibited the proliferation of pancreatic cancer cells. At the same time, the expression of RRAD was closely related to the prolongation of overall survival (Fig. 3e, f, g). Overall, the above results indicate that the high expression of RRAD indicates that pancreatic cancer patients have a better prognosis and plays a role in inhibiting the occurrence and development of pancreatic cancer.
RRAD inhibits the proliferation of pancreatic cancer cells and is associated with the prognosis of patients with pancreatic cancer. A, B Western blot analysis and qPCR analysis were performed to exam RRAD protein and mRNA levels, respectively. The overexpression of RRAD was confirmed in Mia PaCa-2 cell line. C CCK-8 assay showed that overexpression of RRAD decreased the proliferation of pancreatic cancer cells. D Colony formation assay showed that overexpression of RRAD reduced the proliferation of pancreatic cancer cells. E Representative images of IHC staining of RRAD in PDAC tumour tissues and paired adjacent normal tissues. The results showed that RRAD was underexpressed in pancreatic cancer. F lHC analysis further confirmed that RRAD protein levels were lower in cancer tissues. wilcoxon rank test P = 0.0015. G Kaplan–Meier survival rate analysis for PDAC patients showed high RRAD expression was associated with longer over survival. *P < 0.05; **P < 0.01
RRAD promotes ferroptosis in pancreatic cancer
RRAD can play a role in regulating the occurrence and development of pancreatic cancer. To further study its role in pancreatic cancer. We silenced the expression of RRAD in the SW1990 cell line by using two kinds of RRAD-specific shRNA expression lentiviruses (shRRAD#1 and shRRAD#2). At the same time, the expression of RRAD was enhanced in the Mia PaCa-2 cell line. The efficiency of RRAD knockout and overexpression was verified by qPCR and Western blotting (Fig. 4a, b). We found that the level of lipid oxidation decreased in the SW1990 cell line with silenced RRAD expression, while the level of lipid oxidation increased in the Mia PaCa-2 cell line overexpressing RRAD (Fig. 4c). Therefore, we speculate that the expression level of glutathione as the main antioxidant is increased in the cell line with knockout of RRAD expression and decreased in the Mia PaCa-2 cell line with increased RRAD expression. To test this hypothesis, we tested the GSH/GSSG ratio in the SW1990 cell line with silenced RRAD expression and the Mia PaCa-2 cell line overexpressing RRAD (Fig. 4d). As expected, RRAD decreased the expression of GSH. At the same time, GSH is closely related to GPx4 [19], a key substance regulating ferroptosis. Therefore, we used the BODIPY 581/591C11 probe to detect the level of lipid peroxidation in the SW1990 cell line, which silenced RRAD expression, and the Mia PaCa-2 cell line, which overexpressed RRAD (Fig. 4f). The results showed that RRAD promoted lipid peroxidation in pancreatic cancer cells. Lipid peroxidation is the most important marker of ferroptosis [20], and RRAD promotes lipid peroxidation. This suggests that RRAD may promote ferroptosis. To verify this, we added a ferroptosis inducer (RSL3) to the SW1990 cell line to silence RRAD expression. The ferroptosis inducer (RSL3) inhibited the increased cell viability of RRAD-silenced cells. The ferroptosis inhibitor ferrostatin-1 was added to the Mia PaCa-2 cell line to overexpress RRAD. The results showed that the ferroptosis inhibitor ferrostatin-1 partially reversed the lipid peroxidation caused by RRAD overexpression (Fig. 4e). This indicates that RRAD promotes the occurrence of ferroptosis in pancreatic cancer cells.
RRAD enhances ferroptosis in pancreatic cancer cells. A, B The shRNAs against RRAD plasmid were transfected into SW1990 cell line. Plasmid overexpressing RRAD was transfected into Mia PaCa-2 cell line. Western blot analysis and qPCR analysis were performed to exam SETD8 protein and mRNA levels, respectively. C The MDA was detected in RRAD-silenced SW1990 cell line and RRAD-overexpressed Mia PaCa-2 cell line. The results showed that RRAD could promote the expression of MDA. D The GSH/GSSG ratio was detected in RRAD-silenced SW1990 cell line and RRAD-overexpressed Mia PaCa-2 cell line. It was found that silencing RRAD could increase the GSH/GSSG ratio and overexpression of RRAD could decrease the GSH/GSSG ratio. E Cell viability was detected in the RRAD-silenced SW1990 cell line in the presence or absence of 2 μmol/l RSL3 and the RRAD-overexpressed Mia PaCa-2 cell line in the presence or absence of 2 μmol/l Fer. The results showed that the cell viability increased by RRAD knockdown could be reversed by the ferroptosis inducer RSL3 and the cell viability decreased by RRAD overexpression could be reversed by the ferroptosis inhibitor Fer. F Flow cytometry analyzed the fluorescence of BODIPY581/591C11 (lower) and the relative content was calculated (upper). The results showed that RRAD could increase the level of intracellular lipid peroxidation. Two tailed unpaired Student t-test was used in the above experiments. *P < 0.05; **P < 0.01
SETD8 suppresses ferroptosis in pancreatic cancer by downregulating RRAD
To further investigate how SETD8 affects the occurrence of ferroptosis in pancreatic cancer, we speculated that SETD8 may inhibit the occurrence of ferroptosis by inhibiting the expression of RRAD. Therefore, we constructed a stable pancreatic cancer cell line that silenced SETD8 and RRAD and silenced SETD8 and RRAD simultaneously in SW1990 cell lines. We also constructed stable pancreatic cancer cell lines that stably expressed SETD8 and RRAD and overexpressed SETD8 and RRAD in MIA PaCa-2 cell lines. Then, we performed qPCR and Western blotting to verify the efficiency of knockout and overexpression of the above cell lines (Fig. 5a, b). Then, we used the BODIPY 581/591c11 probe to detect the level of lipid peroxidation in the above treated cell lines at the same time (Fig. 5d). The results showed that the increase in lipid peroxidation levels in cancer cells caused by knockout of SETD8 could be reversed by silencing the expression of the RRAD gene. The increase in lipid peroxidation caused by overexpression of RRAD can be reversed by overexpression of the SETD8 gene.
SETD8 influences the occurrence of ferroptosis in pancreatic cancer through RRAD. A, B qPCR and Western blot assay confirmed the efficiency of shRNAs targeting SETD8, RRAD or both in the SW1990 cell line and the overexpression efficiency of SETD8, RRAD or both in the Mia PaCa-2 cell line. C Cell viability was detected in SETD8-, RRAD- or both-silenced SW1990 cell lines and in SETD8-, RRAD- or both-overexpressed Mia PaCa-2 cell line. The results showed that overexpression of SETD8 enhanced cell viability, which was reversed by overexpression of RRAD. The increase in cancer cell activity caused by silencing RRAD expression was reversed by silencing SETD8 expression. D Flow cytometry analyzed the fluorescence of BODIPY581/591C11 (lower) and the relative content was calculated (upper). The results showed that SETD8 knockout induced elevated lipid peroxidation in cancer cells, which could be reversed by silencing RRAD expression. The elevated lipid peroxidation induced by RRAD overexpression was reversed by overexpression of SETD8. *P < 0.05; **P < 0.01
To more directly observe the effect of the SETD8 gene on the viability of pancreatic cancer cells, we detected the viability of these different pancreatic cancer cells. The results showed that overexpression of the SETD8 gene increased the activity of cancer cells, which could be reversed by overexpression of the RRAD gene. The increase in cancer cell viability caused by silencing the expression of the RRAD gene can be reversed by silencing the expression of the SETD8 gene (Fig. 5c). Therefore, the above results suggest that SETD8 can inhibit ferroptosis in pancreatic cancer by downregulating the expression of RRAD.
SETD8 interacts with the promoter region of RRAD to inhibit its expression
To better understand the mechanism by which SETD8 affects RRAD expression, we carried out affinity purification and overexpression of SETD8 labelled with FLAG (FLAG-SETD8) in Mia PaCa-2 cells. Cell extracts were prepared and affinity purified using an anti-flag affinity gel. Double luciferase reporter analysis was performed in HEK293T cells, and SETD8 was overexpressed in the luciferase reporter driven by the RRAD promoter (Fig. 6a). The results showed that RRAD reporter activity decreased gradually with increasing SETD8 overexpression. To further confirm the specific binding of SETD8 to the RRAD promoter, SW1990 and MIA PaCa-2 cells were collected for chromatin immunoprecipitation (CHIP) analysis to verify the occupation of SETD8 on the RRAD promoter. The results confirmed that SETD8 occupied the RRAD promoter region (Fig. 6b, c). In general, SETD8 inhibits RRAD transcription by interacting with the RRAD promoter, thus inhibiting ferroptosis in pancreatic cancer cells (Fig. 6d).
SETD8 binds to the promoter of RRAD to inhibit its expression. A Dual-luciferase assay showed that the fluorescence intensity decreased gradually with the increase of SETD8 expression. SETD8 suppressed RRAD promoter activity in HEK293T cells. B, C CHIP‒qPCR analyses confirmed that SETD8 specifically bound to the RRAD promoter in SW1990 cells and Mia PaCa-2 cells. D Schematic diagram of the mechanism of SETD8 inhibiting cell ferroptosis. SETD8 inhibits the transcription of RRAD by binding to the promoter of RRAD, which leads to a decrease in the level of lipid peroxidation, thus reducing the occurrence of ferroptosis in PDAC. Two tailed unpaired Student t-test was used in the above experiments. *P < 0.05; **P < 0.01
Discussion
Pancreatic cancer is a highly malignant and aggressive tumour. The total survival rate of pancreatic cancer patients in the last 5 years is less than 10% [26, 27]. The RAS superfamily plays an important role in cell physiological activities. Its mutation or abnormal activation will promote the occurrence and progression of cancer [28, 29]. KRAS mutations can be observed in more than 95% of pancreatic cancers [17]. However, no obvious progress has been made in the treatment strategy of pancreatic cancer. Currently, the chemotherapy regimen is gemcitabine, anaxx, Forfield and albumin-bound paclitaxel. However, pancreatic cancer has strong chemoresistance, the prognosis of chemotherapy is still very poor [30]. Therefore, it is urgent to find compounds that can selectively kill RAS mutant cancer cells. Brent R. Stockwell found specific compounds that can kill RAS mutant cancer cells among tens of thousands of small compounds. In addition, they found that these compounds killed cancer cells in a manner different from apoptosis and necrosis [15, 16]; this method of death is named ferroptosis. Increasing evidence shows that ferroptosis has broad application prospects in the clinic. Ferroptosis holds great promise in cancer therapy, especially in treating tumors that have developed resistance to traditional therapies [31]. There has been evidence that drug-resistant cancer cells easily undergo ferroptosis. Therefore, ferroptosis can be used as a targeted therapy for cancer [14, 32]. At the same time, using nanomaterials as drug carriers to induce ferroptosis in cancer cells also provides another option [33]. In addition, studies have shown that the induction of ferroptosis in pancreatic cancer cells can enhance their sensitivity to chemotherapy, such as gemcitabine and cisplatin [34, 35]. Therefore, further study of the role of ferroptosis in pancreatic cancer will help to provide a new direction for the treatment of pancreatic cancer.
In this study, we found that lysine methyltransferase SETD8, a member of the SET domain family, plays an important and common role in regulating the cell cycle and tumour growth. It can increase the GSH/GSSG ratio in pancreatic cancer and reduce the level of lipid peroxidation. A high GSH/GSSG ratio and low lipid peroxidation can inhibit ferroptosis [36, 37]. We further demonstrated that SETD8 can inhibit the occurrence of ferroptosis in pancreatic cancer. To elucidate the underlying mechanisms, we screened gene expression profiles. In SETD8 downregulated genes, we found that RRAD, a member of the RAS-related GTPase subfamily and also known as the RAS family diabetes-related gene, can inhibit tumour cell proliferation and migration and has been identified as a tumour suppressor gene in many tumours [21,22,23,24]. We further found that RRAD could inhibit the proliferation of pancreatic cancer. In addition, RRAD can also reduce the GSH/GSSG ratio in pancreatic cancer and increase the level of lipid peroxidation. This indicates that RRAD can promote the occurrence of ferroptosis in pancreatic cancer. Our further experiments show that SETD8 inhibits RRAD transcription and that the SETD8 knockout-induced increase in lipid peroxidation levels in pancreatic cancer cells can be reversed by silencing RRAD gene expression. The increase in lipid peroxidation caused by overexpression of RRAD can be reversed by overexpression of the SETD8 gene. Accordingly, ferroptosis inhibitors can save the cell viability reduced by low expression of SETD8. At the same time, based on the results of IHC analysis, the higher the level of SETD8 or the lower the RRAD level, the worse the prognosis of pancreatic cancer patients. In order to further clarify how SETD8 inhibits RRAD transcription. Through CHIP experiments, we demonstrated that SETD8 regulates RRAD expression by specific binding to the RRAD promoter region.
SETD8 has been shown to affect the progression of diabetic nephropathy by regulating bach1 transcription [38]. It has also been found that SETD8 can promote tumour cell growth and metastasis through the receptor tyrosine kinase ROR1 [39]. Meanwhile, SETD8 promoted the development of endometrial cancer by inhibiting the function of tumour suppressor genes through H4K20 methylation and p53 expression [40]. In this study, we demonstrated that SETD8 can also promote tumor cell growth by inhibiting ferroptosis. Studies have found that RRAD can inhibit tumour cell proliferation, migration and Warburg effect by downregulating ACTG1 expression [41, 42]. We demonstrate for the first time an association of RRAD with ferroptosis. RRAD can inhibit tumour cell growth through ferroptosis. The expression of RRAD was closely related to the prognosis of pancreatic cancer patients. Pancreatic cancer patients with high RRAD expression have a better prognosis than those with low RRAD expression. Furthermore, we found that SETD8 inhibited the ferroptosis in pancreatic cancer by binding to the promoter region of RRAD, revealing the role of SETD8-RRAD-ferroptosis axis in the regulation of pancreatic cancer.
However, the limitation of this study is that it does not explain how SETD8 combines with the promoter region of RRAD to regulate the transcription of RRAD. We speculate that SETD8 may inhibit the transcription of RRAD by binding a transcription factor to the promoter of RRAD. GPx4 is a key gene in the regulation of ferroptosis and we did not further investigate the relationship between RRAD and GPx4. At the same time, the role of SETD8-RRAD-ferrodeath axis in pancreatic cancer was not further verified in animal experiments.
Taken together, SETD8 promotes the growth of pancreatic cancer cells by inhibiting ferroptosis. RRAD inhibits ferroptosis in pancreatic cancer cells. Meanwhile, low expression of RRAD is closely related to poor prognosis of pancreatic cancer patients. STED8 inhibits the transcription of RRAD by binding to the promoter region of RRAD and thus reduces the expression of RRAD. Finally, it inhibits the ferroptosis of pancreatic cancer cells and promotes the proliferation of pancreatic cancer. These results reveal that the SETD8-RRAD-ferroptosis axis may provide potential therapeutic targets and predictors for the treatment of pancreatic cancer.
Conclusions
Our study reveals the role of SETD8-RRAD in the occurrence of ferroptosis in pancreatic cancer. The combination of SETD8 and the RRAD promoter subregion results in the inhibition of RRAD transcription, thereby affecting the occurrence of erroptosis in pancreatic cancer. These results may provide new strategies for the induction of ferroptosis in pancreatic cancer and provide a new direction for the comprehensive treatment of pancreatic cancer.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CHIP:
-
Chromatin immunoprecipitation assay
- H4K20:
-
Monomethylate histone H4 lysine 20
- Fer:
-
Ferrostatin-1
- RSL3:
-
RAS-selective lethal 3
- CCK-8:
-
Cell counting kit-8
- IHC:
-
Immunohistochemistry
- shRNAs:
-
Short hairpin RNAs
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Schmid S, Früh M, Peters S. Targeting MET in EGFR resistance in non-small-cell lung cancer-ready for daily practice? Lancet Oncol. 2020;21(3):320–2.
Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37(4):496–513.
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet (London). 2011;378(9791):607–20.
Dal Molin M, Zhang M, de Wilde RF, Ottenhof NA, Rezaee N, Wolfgang CL, Blackford A, Vogelstein B, Kinzler KW, Papadopoulos N, et al. Very long-term survival following resection for pancreatic cancer is not explained by commonly mutated genes: results of whole-exome sequencing analysis. Clin Cancer Res. 2015;21(8):1944–50.
Abbas T, Shibata E, Park J, Jha S, Karnani N, Dutta A. CRL4(Cdt2) regulates cell proliferation and histone gene expression by targeting PR-Set7/Set8 for degradation. Mol Cell. 2010;40(1):9–21.
Chen X, Ding X, Wu Q, Qi J, Zhu M, Miao C. Monomethyltransferase SET8 facilitates hepatocellular carcinoma growth by enhancing aerobic glycolysis. Cell Death Dis. 2019;10(4):312.
Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823–37.
van Nuland R, Gozani O. Histone H4 lysine 20 (H4K20) methylation, expanding the signaling potential of the proteome one methyl moiety at a time. Mol Cell Proteom MCP. 2016;15(3):755–64.
Reynet C, Kahn CR. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science (New York). 1993;262(5138):1441–4.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–72.
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–85.
Liang C, Zhang X, Yang M, Dong X. Recent progress in ferroptosis inducers for cancer therapy. Adv Mater (Deerfield Beach). 2019;31(51):e1904197.
Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15(3):234–45.
Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3(3):285–96.
Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer. 2016;16(9):553–65.
Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1–2):1–12.
Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–81.
Hadian K, Stockwell BR. SnapShot: ferroptosis. Cell. 2020;181(5):1188-1188.e1181.
Liu J, Zhang C, Wu R, Lin M, Liang Y, Liu J, Wang X, Yang B, Feng Z. RRAD inhibits the Warburg effect through negative regulation of the NF-κB signaling. Oncotarget. 2015;6(17):14982–92.
Suzuki M, Shigematsu H, Shames DS, Sunaga N, Takahashi T, Shivapurkar N, Iizasa T, Minna JD, Fujisawa T, Gazdar AF. Methylation and gene silencing of the Ras-related GTPase gene in lung and breast cancers. Ann Surg Oncol. 2007;14(4):1397–404.
Mo Y, Midorikawa K, Zhang Z, Zhou X, Ma N, Huang G, Hiraku Y, Oikawa S, Murata M. Promoter hypermethylation of Ras-related GTPase gene RRAD inactivates a tumor suppressor function in nasopharyngeal carcinoma. Cancer Lett. 2012;323(2):147–54.
Jin Z, Feng X, Jian Q, Cheng Y, Gao Y, Zhang X, Wang L, Zhang Y, Huang W, Fan X, et al. Aberrant methylation of the Ras-related associated with diabetes gene in human primary esophageal cancer. Anticancer Res. 2013;33(11):5199–203.
Zhang Q, Wang HY, Marzec M, Raghunath PN, Nagasawa T, Wasik MA. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci USA. 2005;102(19):6948–53.
Balachandran VP, Beatty GL, Dougan SK. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156(7):2056–72.
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–705.
Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118(Pt 5):843–6.
Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE Signal Transduct Knowl Environ. 2004;2004(250):Re13.
Kim MP, Gallick GE. Gemcitabine resistance in pancreatic cancer: picking the key players. Clin Cancer Res. 2008;14(5):1284–5.
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551(7679):247–50.
Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–49.
Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater (Deerfield Beach). 2018;30(12):e1704007.
Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43(11):1245–56.
Yang J, Xu J, Zhang B, Tan Z, Meng Q, Hua J, Liu J, Wang W, Shi S, Yu X et al. Ferroptosis: at the crossroad of gemcitabine resistance and tumorigenesis in pancreatic cancer. Int J Mol Sci. 2021;22(20):1.
Busciglio J, Yankner BA. Apoptosis and increased generation of reactive oxygen species in Down’s syndrome neurons in vitro. Nature. 1995;378(6559):776–9.
Malla JA, Umesh RM, Yousf S, Mane S, Sharma S, Lahiri M, Talukdar P. A glutathione activatable ion channel induces apoptosis in cancer cells by depleting intracellular glutathione levels. Angew Chem Int Ed Engl. 2020;59(20):7944–52.
Li X, Lu L, Hou W, Wang F, Huang T, Meng Z, Zhu M. The SETD8/ELK1/bach1 complex regulates hyperglycaemia-mediated EndMT in diabetic nephropathy. J Transl Med. 2022;20(1):147.
Liu M, Shi Y, Hu Q, Qin Y, Ji S, Liu W, Zhuo Q, Fan G, Ye Z, Song C, et al. SETD8 induces stemness and epithelial-mesenchymal transition of pancreatic cancer cells by regulating ROR1 expression. Acta Biochim Biophys Sin. 2021;53(12):1614–24.
Kukita A, Sone K, Kaneko S, Kawakami E, Oki S, Kojima M, Wada M, Toyohara Y, Takahashi Y, Inoue F et al. The histone methyltransferase SETD8 regulates the expression of tumor suppressor genes via H4K20 methylation and the p53 signaling pathway in endometrial cancer cells. Cancers. 2022;14(21):1.
Yan Y, Xu H, Zhang L, Zhou X, Qian X, Zhou J, Huang Y, Ge W, Wang W. RRAD suppresses the Warburg effect by downregulating ACTG1 in hepatocellular carcinoma. Onco Targets Ther. 2019;12:1691–703.
Yan Y, Xie M, Zhang L, Zhou X, Xie H, Zhou L, Zheng S, Wang W. Ras-related associated with diabetes gene acts as a suppressor and inhibits Warburg effect in hepatocellular carcinoma. Onco Targets Ther. 2016;9:3925–37.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (81602054) and the Applied Basic Research of Changzhou Technology Bureau (CJ20190093). Major Science and Technology Project of Changzhou Health Commission (ZD201906); The “Six One Project” top-notch talent research project of high-level health talents of Jiangsu Provincial Health Commission (LGY2019022).
Author information
Authors and Affiliations
Contributions
XMC and XWX designed and guided the study. ZKL, QSH, YQ, HY, BKX, SRJ, GCZ, ZLW, and GXF carried out experiments and prepared diagrams; ZKL and QSH contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Cancer Hospital of Fudan University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The original figures of the western blot.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lu, Z., Hu, Q., Qin, Y. et al. SETD8 inhibits ferroptosis in pancreatic cancer by inhibiting the expression of RRAD. Cancer Cell Int 23, 50 (2023). https://doi.org/10.1186/s12935-023-02899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-023-02899-6