Abstract
Nuclear factor of activated T-cells, cytoplasmic 4 (NFATc4), a transcription factor of NFAT family, which is activated by Ca2+/calcineurin signaling. Recently, it is reported that aberrantly activated NFATc4 participated and modulated in the initiation, proliferation, invasion, and metastasis of various cancers (including cancers of the lung, breast, ovary, cervix, skin, liver, pancreas, as well as glioma, primary myelofibrosis and acute myelocytic leukemia). In this review, we cover the latest knowledge on NFATc4 expression pattern, post-translational modification, epigenetic regulation, transcriptional activity regulation and its downstream targets. Furthermore, we perform database analysis to reveal the prognostic value of NFATc4 in various cancers and discuss the current unexplored areas of NFATc4 research. All in all, the result from these studies strongly suggest that NFATc4 has the potential as a molecular therapeutic target in multiple human cancer types.
Similar content being viewed by others
Introduction
The nuclear factor of activated T-cells (NFAT) consists of transcription factors that regulate the expression of proinflammatory cytokines and related genes during the immune response. NFAT proteins comprise of five members, NFAT1 (NFATp or NFATc2), NFAT2 (NFATc or NFATc1), NFAT3 (NFATc4), NFAT4 (NFATx or NFATc3), and NFAT5 (TonEBP) [1]. Although the NFAT proteins have been extensively investigated in the immune system, their roles in oncogenesis and cancer progression have been less studied relatively. Recent researches showed that NFAT proteins are involved in the initiation, progression, and the prognosis of cancer. The change of NFAT proteins expression level and their post-translational modification (PTM) have been detected in various human solid tumors and cell lines, as well as haematological malignancies.
NFATc4 is encoded by a gene located on human chromosome 14 at q12 and was firstly isolated using a Jurkat T-cells and human peripheral blood lymphocytes (PBLs) cDNA library in 1995 [2]. NFATc4 is involved in several physiological processes, including the development and function of the immune, cardiovascular, musculoskeletal, and nervous systems [3,4,5,6]. It is also reported that NFATc4 could take part in cell growth, cell migration, tumor metastasis, and patient survival recently.
Over the past decades, NFATc4 remains relatively less explored when comparing with other NFAT proteins, in which there are still a huge research potential. In this review, we would like to discuss the recent knowledge on the expression, PTM and epigenetic regulations of NFATc4, its interaction partners and downstream components, as well as its biological function in cancers and other diseases.
NFATc4 structure and expression
NFATc4 has 24 isoforms produced by alternative splicing (Table 1, Fig. 1). Among these isoforms, isoform 1 is regarded as the canonical sequence and all the reported researches are based on isoform 1. As the schematic structure shown (Fig. 2), NFATc4 contains a conserved Rel homology domain (RHD) which includes the DNA-binding motif, and two transactivation domains (TADs) which locate at the N-terminus and C-terminus respectively. Between the TAD in the N-terminus and the RHD is the regulatory domain (NHR) where most SP motifs located, and many biological functions of NFATc4 are mediated by the phosphorylation and dephoshporylation of the SP motifs. In addition, there are two nuclear localization signals located in the NHR and the RHD.
Amino acid sequence alignment of 24 human NFATc4 isoforms. Alignment was done by Multalin interface (http://multalin.toulouse.inra.fr/multalin/). Red color for high consensus (90%), blue color for low consensus (50%), and black color for unaligned residues. The symbol “#” for D, E, N, or Q. Dashes for optimal alignment
Schematic structure of NFATc4. The calcium-regulated NFATc4 protein composed of the NHR, the RHD and the N- and C-terminal transactivation domains. The NHR displays a calcineurin-binding site, a nuclear localization sequence (NLS). Another NLS is located in the RHD. Most of the NFATc4 serine-proline residues that are dephosphorylated upon calcineurin activation and phosphorylated by several kinases are located in NHR. The most conserved domain, RHD, also called DNA binding domain (DBD) that directly contacts with DNA is indicated as the DNA-binding loop. Amino acids marked with red circles indicate reported phosphorylation sites; amino acid marked with green pentagon indicates ADP-ribosylation site; purple lines indicate serine-proline residues. There are 21 serine-proline repeats in the NFATc4 amino acid sequence while 16 serine-proline repeats located in NHR and RHD
At first, NFATc4 was reported to be rarely expressed in the immune system [2], which was different from other NFAT proteins. With the increasing researches of NFATc4, its expression in many tissues can be detected (Table 2) including the immune system. The reason of NFATc4 down-regulation in immune cells is the lack of expression of T-box transcription factor TBX5 [7]. Today, studies show that NFATc4 played important roles in cardiovascular, muscular, skeletal, and nervous systems. Also, NFATc4 is involved in oncogenesis and some immune-related conditions like asthma [8], graft rejection [9], colitis [10] and osteoarthritis [11]. In TCGA and GTEx database, 16 types of cancer show lower mRNA levels of NFATc4 when comparing with normal tissues, while 3 types of cancer show higher mRNA levels (Fig. 3). Yet, there are only a few researches cover the protein level of NFATc4 in cancer and normal tissues.
mRNA level analysis of NFATc4 in various cancers. The NFATc4 mRNA analysis are performed on matched TCGA tumor tissues and normal tissues (TCGA normal and GTEx data) using GEPIA (http://gepia.cancer-pku.cn/index.html). Cancers in green letters have lower NFATc4 expression; Cancers in red letters have higher NFATc4 expression. The NFATc4 mRNA is at a low level in 16 types of cancer, while it is at a high level in 3 types of cancer
Modulation of NFATc4 activation
Epigenetics
Epigenetics is the study of heritable changes in gene expression without alteration of the underlying DNA sequence that are mediated by epigenetic factors like methylation of DNA, modification of histone and non-coding RNA regulation [29]. It is reported that NFATc4 protein expression can be suppressed by several microRNA: miR-34a-5p [30], miR-29a-3p [31], miR-133a [32], miR-182 [14], miR-145-5p [33], miR-7134-5p [34], and miR-140 [11]. However, DNA and histone modification haven’t been explored yet.
Post-translational modification
At the resting state, a balance between the tonic activity of calcineurin phosphatase and the protein kinase is required to maintain cytosolic localization of NFATc4 [35]. Upon an increase of intracellular calcium, activated calcineurin dephosphorylates NFATc4 and promotes its nuclear translocation. Transcription termination promotes rephosphorylation of NFATc4, then NFATc4 can be translocated to the cytosol. Except phosphorylation and dephosphorylation, NFATc4 can also be subjected to ADP-ribosylation, ubiquitination, and deacetylation (Table 3). Specifically, ADP-ribosylation at NFATc4 DNA binding domain could augment the transcriptional activity of NFATc4 [36] whereas ubiquitination of NFATc4 promoted NFATc4 degradation and thus suppressed its transcriptional activity [37]. Also, it has been shown that the deacetylation of NFATc4 by SIRT6 could suppress NFATc4 dephosphorylation [38].
Interaction proteins
Besides post-translational modification, NFATc4 transcriptional activity is also modulated by the interaction proteins (Table 4).
NFATc4 functions in cancer
Lung cancer
Lung cancer is the leading cause of cancer induced death worldwide. The past two decades of research have yielded encouraging results about the disease mechanism and therapy of lung cancer. Nonetheless, the need of new molecular targets remain to be intensively elucidated to prompt the development of diagnosis and new therapies. Chen’s research revealed that the NFATc4 positive rate is 28.3% in tumor tissues and 1.3% in adjacent normal tissues from 159 non-small cell lung cancer patients [12]. They found a positive correlation between the expressions of NFATc4 and COX-2 which were both overexpressed in non-small cell lung carcinoma. In addition, NFATc4 transcriptionally regulated COX-2 expression and prevented human bronchial epithelial cells BEAS-2B from apoptosis caused by arsenite and vanadium treatment [55, 56]. Another research reported that knockdown of NFATc4 expression resulted in a radioprotective effect on A549 lung cancer cell line [57]. The survival analysis of GSE31210 dataset from GEO database indicates that high NFATc4 level tends to a favorable prognosis (Fig. 4A).
Survival analysis of NFATc4 in various cancers. A–C The data is from GEO database and analyzed by KM plotter (https://kmplot.com/analysis/index.php?p=background). A The GSE31210, a lung cancer dataset, shows that the high expression of NFATc4 is significantly related to favorable prognosis. B The GSE12276, a breast cancer dataset, shows that the high expression of NFATc4 is significantly related to poor prognosis. C The GSE26193, a ovarian cancer dataset, shows that the high expression of NFATc4 is significantly related to poor prognosis. D–F The data is from TCGA database and analyzed by GEPIA (http://gepia.cancer-pku.cn/index.html). D The CESC, a cervical cancer dataset, shows that the high expression of NFATc4 is significantly related to poor prognosis. E The SKCM, a skin cancer dataset, shows that the high expression of NFATc4 is significantly related to poor prognosis. F The COAD, a colon cancer dataset, shows that the high expression of NFATc4 is significantly related to poor prognosis
Although the research of NFATc4 in lung carcinoma is a bit less, NFATc4 has presented relatively high expression in lung cancer and involved in radiotherapy and the prognosis.
Breast cancer
Accumulated evidences have indicated that NFATc4 plays essential roles in regulating the proliferation, invasion and migration of breast cancer cells. It has been shown that the expression of NFATc4 can be detected in breast cancer cells and a subset of breast cancer patients. NFATc4 is overexpressed in breast cancer with 65% positive in cancerous tissue and 15% positive in noncancerous tissue. Notably, it was found that NFATc4 not only interacted with the ligand-independent transactivation domain of ER (ERα and ERβ) but also increased its binding to the estrogen-responsive elements resulting in the up-regulation of pS2 and cathepsin D expression. Meanwhile, knockdown of NFATc4 reduced the cell growth rate in ZR-75-1 cells which showed high NFATc4 expression level [48].
Tumor invasion and metastasis may lead to cancer progression and poor outcomes. NFATc4 also shows potential regulation of these aspects in breast cancer cells. Interestingly, NFATc4 expression is restricted to ERα positive cell lines at both transcriptional and translational levels. Remarkably, NFATc4 inhibited cell invasion and migration through regulating actin redistribution. Furthermore, research identified that NFATc4/ERα decreased cell migration capacity through transcriptionally repression of LCN2 expression [13]. Meanwhile, overexpression of ERα promoted the nuclear translocation of NFATc4. NFATc4/ERα and NFATc4/ERβ inhibited the transcriptional activity of IL-2 in breast cancer cells, especially ERα. Phosphorylation of ERα at different sites affected the regulation of ERα on NFATc4 transcriptional activity [47]. Additionally, NFATc4 is highly co-expressed with androgen receptor (AR) which is expressed in 88% ER-positive breast tumors [58].
Extracellular vesicles (EVs) are kinds of two-layer secreted vesicles which play pivotal roles in mediating cell signal transduction and pathological process. The tumor-derived EVs are thought as the promising therapeutic approaches through mediating intercellular communication in the tumor microenvironment. EVs produced by breast cancer cells expressing NFATc4 inhibited cell invasion effectively among different types of cancer cell lines (triple negative breast cancer, invasive melanoma, glioblastoma, and pancreatic cancer). Intra-tumor EVs injection presented a significant inhibition of the tumor volume and metastasis in tumor-bearing mice. To further explain EVs function, de Camargo et al. demonstrated EVs from the NFATc4-expressing cells decreased the cell proliferation rate and induced cell apoptosis through macrophages recruitment in the 2D cell culture system [59]. In addition, NFATc4 down-regulated during (E)-4-chloro-2-((3-ethoxy-2-hydroxybenzylidene) amino) phenol (ACES) induced apoptosis through p38-β/SAPK in MCF-7 breast cancer cells, indicating that NFATc4 could be a latent cancer therapeutic target [60].
Based on survival analysis, NFATc4 is an unfavorable prognostic factor in breast cancer (Fig. 4B). Thus, these observations collectively highlighted the critical role of NFATc4 in breast cancer development. On the one hand, NFATc4 could interact with ER and transcriptionally regulate downstream genes, and promote the cell growth. On the another hand, NFATc4 presenting high expression at mRNA and protein levels in ERα-positive cells repressed LCN2 and led to inhibition of invasion and migration capacities of breast cancer cells. NFATc4 could also play an important role in tumor immune microenvironment through EVs from NFATc4-expressing cells.
Ovarian cancer
Ovarian cancer ranks the fifth cancer-induced death among women. Patients diagnosed with ovarian cancer are treated with complete resection surgery and neoadjuvant or adjuvant chemotherapy. Recently, it is considered that many patients were overtreated especially those at early stage. It is important to develop the molecular signature to indicate more suitable individual therapeutic strategy. Accumulating studies identified that NFATc4 is involved in the ovarian cancer, too. NFATc4 is overexpressed in ovarian cell lines 3D spheroids comparing with adherent cells [16]. In ovarian quiescent cancer stem-like cells (CSCs), NFATc4 is also overexpressed [15]. NFATc4 directly bound to the CXCR4 promoter at the DNA sequence of -114 to + 59 relative to the transcriptional start site and correspondingly increased CXCR4 expression. When the HeyA8 ovarian cell line 3D spheroids were treated with Cyclosporine A (a usual NFAT inhibitor), the cells dissociated from the 3D spheroids. CXCR4 also presented as a key driver in tumor progression in HeyA8 xenograft model. Moreover, phospho-ERK participated in modulating CXCR4 expression through inhibiting NFAT transactivation [16]. Interestingly, NFATc4 is enriched in ovarian quiescent CSCs and inhibited the cell growth through arresting cells at G0 phase. It was found that NFATc4-involved quiescence could play a role in cisplatin resistance. Cisplatin treatment activated NFATc4 and promoted NFATc4 translocation into the nucleus in multiple ovarian cell lines. Remarkably, MYC overexpression may partially inhibit NFATc4-regulated quiescence, which indicated a potential therapy for cisplatin resistance patients [15]. Besides, NFATc4 acted as a risk factor in the survival of ovarian cancer analyzed from the GSE26193 dataset (Fig. 4C).
As a transcriptional factor, activated NFATc4 took part in ovarian cancer progression in various regulatory ways. NFATc4 could promote tumor formation through CXCR4 expression up-regulation whereas NFATc4 inhibited cell proliferation by down-regulating MYC during cisplatin resistance-associated quiescence.
Skin cancer
Skin-derived malignancies include squamous cell carcinoma, basal cell carcinoma, and malignant melanoma. These malignancies partially related to harmful factors exposure. NFATc4, a stress response transcriptional factor, was found overexpressing in many skin cancer cell lines comparing with HaCaT cells. Consistently, NFATc4 showed high protein level in various tissues of skin malignancies. The ectopic expression and activation of NFATc4 may markedly facilitate the progress of skin cancer. Overexpressing NFATc4 increased the cell proliferation, colony formation, migration, invasion, as well as tumor volume [19], while knockdown of NFATc4 by siRNA or inhibiting NFATc4 activity by tacrolimus (FK506) or ascomycin (FK520) suppressed skin cancer cell migration and invasion [18]. Further study elucidated that CDK3 phosphorylated NFATc4 at Ser259 and enhanced NFATc4 effect on skin cancer, and EGF treatment could promote CDK3/NFATc4-mediated cell transformation and proliferation. Of note, both CDK3 and NFATc4 are highly expressed among various types of skin tumor tissues compared with normal tissues [19]. Survival curve drawn with TCGA-SKCM indicates a poor outcome in patients with high NFATc4 level.
Pancreatic cancer
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignances for its dramatically low 5-year survival rate. A recent study showed that NFATc4 is positively expressed in cell nucleus of human chronic pancreatitis and it is highly induced during pancreatic acinar-to-ductal metaplasia. NFATc4 could play a pivotal role in caerulein-induced pancreatic cancer initiation through NFATc4 transcriptional overexpression of SOX9 [20].
Brain cancer
Since the blood-brain barrier and the crucial function of brain, malignant brain tumors remain a therapeutic challenge, in spite of surgical and medical advancement. NFATc4 was required and activated in doxorubicin-mediated cell death as well as doxorubicin-induced inhibition of cell migration and invasion in glioblastoma cell lines [22]. In Schwannoma, NFATc4 and SOX10 synergistically enhanced KROX20 activity and induced P0 transcription to regulate the cell proliferation. When overexpressing SOX10 in Merlin-null Schwannoma cells in which the SOX10 level was reduced to restore Merlin tumor suppressor, NFATc4 was activated with nuclear translocation and the cell growth was alleviated [23, 53].
Other cancers
Besides from the above mentioned, the involvement of NFATc4 in cancer was gradually discovered in some other types of cancer, including hepatic cell carcinoma, colon cancer, cervical cancer, and leukemia.
It is well-known that certain patients with non-alcoholic fatty liver disease, the most common chronic liver disorder, may also develop non-alcoholic steatohepatitis (NASH). NASH is a disease associated with hepatocyte injury and inflammation, which could then lead to liver fibrosis, and subsequently cirrhosis and/or hepatic cell carcinoma (HCC). NFATc4 was activated and translocated into nucleus during methionine-choline diet-induced NASH, while knockdown of NFATc4 inhibited both methionine-choline diet-induced NASH and obesity-related NASH in mice. NFATc4 directly bound to PPARα and interfered with lipid metabolism through negatively regulating PPARα transcriptional activity, and then contributed to the development of NASH [24, 61]. These findings provided a potential therapeutic target in preventing NASH progression through inhibiting NFATc4 expression and activation. Remarkably, it was found that the inhibition of calcineurin/NFATc4 signaling mediated by RCAN1.4 could retard liver fibrosis [62].
NFATc4 is also involved in colon cancer, another digestive system neoplasm. It has been reported that arsenic sulfide (As4S4) and cyclosporine A (CsA) synergistically decreased colon cancer cell proliferation through the regulatory PML and p53 mediated NFAT signal pathway. CsA is a well-known NFAT inhibitor, meanwhile, As4S4 could inhibit the transcriptional expression of NFATc1, NFATc3, and NFATc4, except NFATc2 [63].
Accumulating bioinformatical analyses about immune-related genes (IRGs) indicated that NFATc4 was recognized as the key immune gene to predict the poor prognosis in cervical cancer [64, 65] and acute myelocytic leukemia (AML) [66]. In AML, NFATc4, an immune-related transcriptional factor, was co-expressed with immune gene set of T cell co-stimulation and was involved in Tregs recruitment. Simultaneously, NFATc4 was co-expressed with ATP-binding cassette transporter signaling pathway [66]. In addition, NFATc4 played a role in primary myelofibrosis (PMF), a stem cell-derived clonal myeloproliferative neoplasm. In PMF megakaryocytic cells, NFATc4 was up-regulated by activation of FL/Flt3 axis, while the Flt3 inhibition reinforced the Flt3/p38-MAPK axis effect on PMF dysmegakaryopoiesis [67].
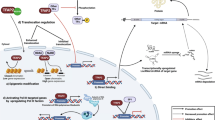
In summary, these emerging observations in the past ten years emphasized the significant roles of NFATc4 in the progression of varieties of human malignancies (Table 5). NFATc4 may perform a dual-function during tumor progression like other NFAT proteins (Fig. 5). In the aspect of oncogenesis, NFATc4 activation promoted the EGFR-stimulated process of acinar-to-ductal metaplasia in pancreas and NFATc4 was activated in the NASH and liver fibrosis. NFATc4 also provided as a novel potential molecular target for cancer therapies, since NFATc4 participated in cell proliferation, migration, invasion, colony formation, 3D spheroid formation, and cell apoptosis. Furthermore, NFATc4 was related to the tumor immune microenvironment through recruiting macrophage and Tregs. Finally, the expression of NFATc4 could effectively evaluate the risk and outcome of the patients. Hence, the further study of NFATc4 function in various cancers could help to prevent the precancerous development and improve the individualized cancer therapy.
NFATc4 functions in other diseases
In the past three decades, the researchers have mainly focused on the function of NFATc4 in cardiac hypertrophy, neurodegenerative disease, immune-related disease, and ischemia–reperfusion injury. In hypertrophic cardiomyocytes, overexpressed and activated NFATc4 transcriptionally up-regulated downstream genes like ANP and BNP [69]. In the cortex of Alzheimer’s disease patients, increased expression of NFATc4 and calcineurin were detected, and activation of NFATc4 regulated the downstream factor Aβ protein to modulate the neurodegeneration [70]. Furthermore, hepatic ischemia–reperfusion injury could be alleviated by pre-treatment with NFATc4 inhibitor FK506, while the Bcl-2/Bax ratio increased [71].
Conclusions and future prospects
Taken together, growing evidences revealed the molecular regulatory mechanism of NFATc4 and its crucial role in the cancer development. Transcription activity of NFATc4 is mainly regulated by the calcineurin and phosphokinase, meanwhile acetylation could facilitate the dephosphorylation of NFATc4. Specifically, ADP-ribosylation at NFATc4 DNA binding domain could augment the transcriptional activity of NFATc4 whereas ubiquitination of NFATc4 promoted NFATc4 degradation and thus suppressed its transcriptional activity. According to recent decade of researches, NFATc4 protein has been recognized as an important transcription factor during the tumor initiation, cell growth, migration, invasion, metastasis, and drug resistance.
However, the regulation of NFATc4 is not deeply studied compared with other NFAT proteins. Since the activated NFATc4 has been detected in the precancerous lesions and cancers, its downstream genes are noteworthy to be elucidated.
Thus, there are compelling reasons to believe that it is necessary to further study the unrevealed mechanisms of NFATc4 in cancer. NFATc4 may be considered as a new target to prevent the malignant cancer progression.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- NFATc4:
-
Nuclear factor of activated T-cells, cytoplasmic 4
- PTM:
-
Post-translational modification
- RHD:
-
Rel homology domain
- TAD:
-
Transactivation domain
- EVs:
-
Extracellular vesicles
- PBMC:
-
Peripheral blood mononuclear cell
- NASH:
-
Non-alcoholic steatohepatitis
- HCC:
-
Hepatic cell carcinoma
- PMF:
-
Primary myelofibrosis
- AML:
-
Acute myelocytic leukemia
- ACC:
-
Adrenocortical carcinoma
- BLCA:
-
Bladder urothelial carcinoma
- BRCA:
-
Breast invasive carcinoma
- CESC:
-
Cervical squamous cell carcinoma and endocervical adenocarcinoma
- CHOL:
-
Cholangio carcinoma
- COAD:
-
Colon adenocarcinoma
- DLBC:
-
Lymphoid neoplasm diffuse large B-cell lymphoma
- ESCA:
-
Esophageal carcinoma
- GBM:
-
Glioblastoma multiforme
- HNSC:
-
Head and neck squamous cell carcinoma
- KICH:
-
Kidney chromophobe
- KIRC:
-
Kidney renal clear cell carcinoma
- KIRP:
-
Kidney renal papillary cell carcinoma
- LAML:
-
Acute myeloid leukemia
- LGG:
-
Brain lower grade glioma
- LIHC:
-
Liver hepatocellular carcinoma
- LUAD:
-
Lung adenocarcinoma
- LUSC:
-
Lung squamous cell carcinoma
- MESO:
-
Mesothelioma
- OV:
-
Ovarian serous cystadenocarcinoma
- PAAD:
-
Pancreatic adenocarcinoma
- PCPG:
-
Pheochromocytoma and paraganglioma
- PRAD:
-
Prostate adenocarcinoma
- READ:
-
Rectum adenocarcinoma
- SARC:
-
Sarcoma
- SKCM:
-
Skin cutaneous melanoma
- STAD:
-
Stomach adenocarcinoma
- TGCT:
-
Testicular germ cell tumors
- THCA:
-
Thyroid carcinoma
- THYM:
-
Thymoma
- UCEC:
-
Uterine corpus endometrial carcinoma
- UCS:
-
Uterine carcinosarcoma
References
Müller MR, Rao A. NFAT, immunity and cancer: a transcription factor comes of age. Nat Rev Immunol. 2010;10(9):645–56.
Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2(5):461–72.
Giri PS, Dwivedi M, Laddha NC, Begum R, Bharti AH. Altered expression of nuclear factor of activated T cells, forkhead box P3, and immune-suppressive genes in regulatory T cells of generalized vitiligo patients. Pigment Cell Melanoma Res. 2020;33(4):566–78.
Su F, Shi M, Zhang J, Li Y, Tian J. Recombinant high–mobility group box 1 induces cardiomyocyte hypertrophy by regulating the 14–3–3η, PI3K and nuclear factor of activated T cells signaling pathways. Mol Med Rep. 2021;23(3):214.
Emrani R, Rébillard A, Lefeuvre L, Gratas-Delamarche A, Davies KJ, Cillard J. The calcineurin antagonist RCAN1-4 is induced by exhaustive exercise in rat skeletal muscle. Free Radic Biol Med. 2015;87:290–9.
Zhang Q, Li X, He R, Ma Q, Sun R, Ji S, Wang B, Tian Y. The effect of brain-derived neurotrophic factor on radiation-induced neuron architecture impairment is associated with the NFATc4/3 pathway. Brain Res. 2018;1681:21–7.
Kaminuma O, Kitamura N, Nishito Y, Nemoto S, Tatsumi H, Mori A, Hiroi T. Downregulation of NFAT3 due to lack of T-box transcription factor TBX5 is crucial for cytokine expression in T cells. J Immunol. 2018;200(1):92–100.
Yan F, Li W, Zhou H, Wu Y, Ying S, Chen Z, Shen H. Interleukin-13-induced MUC5AC expression is regulated by a PI3K-NFAT3 pathway in mouse tracheal epithelial cells. Biochem Biophys Res Commun. 2014;446(1):49–53.
Petra H, Eva H, Irena B, Petra H, Ondřej V. Molecular profiling of acute and chronic rejections of renal allografts. Clin Dev Immunol. 2013;2013:509259.
Yang M, Lin HB, Gong S, Chen PY, Geng LL, Zeng YM, Li DY. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine. 2014;70(2):81–6.
Tardif G, Pelletier JP, Fahmi H, Hum D, Zhang Y, Kapoor M, Martel-Pelletier J. NFAT3 and TGF-β/SMAD3 regulate the expression of miR-140 in osteoarthritis. Arthritis Res Ther. 2013;15(6):R197.
Chen ZL, Zhao SH, Wang Z, Qiu B, Li BZ, Zhou F, Tan XG, He J. Expression and unique functions of four nuclear factor of activated T cells isoforms in non-small cell lung cancer. Chin J Cancer. 2011;30(1):62–8.
Fougère M, Gaudineau B, Barbier J, Guaddachi F, Feugeas JP, Auboeuf D, Jauliac S. NFAT3 transcription factor inhibits breast cancer cell motility by targeting the Lipocalin 2 gene. Oncogene. 2010;29(15):2292–301.
Soheilifar MH, Vaseghi H, Seif F, Ariana M, Ghorbanifar S, Habibi N, Papari Barjasteh F, Pornour M. Concomitant overexpression of mir-182-5p and mir-182-3p raises the possibility of IL-17-producing Treg formation in breast cancer by targeting CD3d, ITK, FOXO1, and NFATs: a meta-analysis and experimental study. Cancer Sci. 2021;112(2):589–603.
Cole AJ, Iyengar M, Panesso-Gómez S, O’Hayer P, Chan D, Delgoffe GM, Aird KM, Yoon E, Bai S, Buckanovich RJ. NFATC4 promotes quiescence and chemotherapy resistance in ovarian cancer. JCI Insight. 2020;5(7):e131486
Huang K, Kiefer C, Kamal A. Novel role for NFAT3 in ERK-mediated regulation of CXCR4. PLoS One. 2014;9(12):e115249.
Ram BM, Dolpady J, Kulkarni R, Usha R, Bhoria U, Poli UR, Islam M, Trehanpati N, Ramakrishna G. Human papillomavirus (HPV) oncoprotein E6 facilitates calcineurin-nuclear factor for activated T cells 2 (NFAT2) signaling to promote cellular proliferation in cervical cell carcinoma. Exp Cell Res. 2018;362(1):132–41.
Xiao T, Chen W, Wang S, Huang S, Chiang C, Zou Y, Zhao Y, Zheng D. Tacrolimus and ascomycin inhibit melanoma cell growth, migration and invasion via targeting nuclear factor of activated T-cell 3. Melanoma Res. 2020;30(4):325–35.
Xiao T, Zhu JJ, Huang S, Peng C, He S, Du J, Hong R, Chen X, Bode AM, Jiang W, et al. Phosphorylation of NFAT3 by CDK3 induces cell transformation and promotes tumor growth in skin cancer. Oncogene. 2017;36(20):2835–45.
Hessmann E, Zhang JS, Chen NM, Hasselluhn M, Liou GY, Storz P, Ellenrieder V, Billadeau DD, Koenig A. NFATc4 regulates Sox9 gene expression in acinar cell plasticity and pancreatic cancer initiation. Stem Cells Int. 2016;2016:5272498.
Wang Q, Zhou Y, Jackson LN, Johnson SM, Chow CW, Evers BM. Nuclear factor of activated T cells (NFAT) signaling regulates PTEN expression and intestinal cell differentiation. Mol Biol Cell. 2011;22(3):412–20.
Gopinath S, Vanamala SK, Gujrati M, Klopfenstein JD, Dinh DH, Rao JS. Doxorubicin-mediated apoptosis in glioma cells requires NFAT3. Cell Mol Life Sci. 2009;66(24):3967–78.
Doddrell RD, Dun XP, Shivane A, Feltri ML, Wrabetz L, Wegner M, Sock E, Hanemann CO, Parkinson DB. Loss of SOX10 function contributes to the phenotype of human Merlin-null schwannoma cells. Brain. 2013;136(Pt 2):549–63.
Du M, Wang X, Yuan L, Liu B, Mao X, Huang D, Yang L, Huang K, Zhang F, Wang Y, et al. Targeting NFATc4 attenuates non-alcoholic steatohepatitis in mice. J Hepatol. 2020;73(6):1333–46.
Minematsu H, Shin MJ, Celil Aydemir AB, Kim KO, Nizami SA, Chung GJ, Lee FY. Nuclear presence of nuclear factor of activated T cells (NFAT) c3 and c4 is required for Toll-like receptor-activated innate inflammatory response of monocytes/macrophages. Cell Signal. 2011;23(11):1785–93.
Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature. 2010;465(7299):808–12.
Al-Daraji WI, Tugrul S, Dempsey E, Zelger B, Abdellaoui A, Prescott R, Zelger B. A preliminary examination of the role of NFAT 3 in human skin, cultured keratocytes and dermal fibroblasts. J Cutan Pathol. 2010;37(9):e21–36.
Santamaría MH, Ríos LD, Corral RS. Trypanosoma cruzi down-regulates adiponectin expression in mouse adipocytes via the NFAT signaling pathway. Microbes Infect. 2021;23(1):104757.
Xu YM, Yu FY, Lau ATY. Discovering epimodifications of the genome, transcriptome, proteome, and metabolome: the quest for conquering the uncharted epi(c) territories. Curr Pharmacol Rep. 2017;3:286–93.
Diener C, Hart M, Alansary D, Poth V, Walch-Rückheim B, Menegatti J, Grässer F, Fehlmann T, Rheinheimer S, Niemeyer BA, et al. Modulation of intracellular calcium signaling by microRNA-34a-5p. Cell Death Dis. 2018;9(10):1008.
Li M, Wang N, Zhang J, He HP, Gong HQ, Zhang R, Song TF, Zhang LN, Guo ZX, Cao DS, et al. MicroRNA-29a-3p attenuates ET-1-induced hypertrophic responses in H9c2 cardiomyocytes. Gene. 2016;585(1):44–50.
Su Q, Liu M, Jiang M, Wang Y, Ma X, Li S, Xie J. Involvement of calcineurin/NFATc4 pathway in a single-prolonged stress-based rat model of post-traumatic stress disorder. Mol Biol Rep. 2019;46(6):6197–204.
Castellanos-Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, Sequeira-Lopez ML, Gomez RA. Recombination signal binding protein for Ig-κJ region regulates juxtaglomerular cell phenotype by activating the myo-endocrine program and suppressing ectopic gene expression. J Am Soc Nephrol. 2015;26(1):67–80.
Zhang Z, Yue L, Wang Y, Jiang Y, Xiang L, Cheng Y, Ju D, Chen Y. A circRNA-miRNA-mRNA network plays a role in the protective effect of diosgenin on alveolar bone loss in ovariectomized rats. BMC Complement Med Ther. 2020;20(1):220.
Yang TT, Yu RY, Agadir A, Gao GJ, Campos-Gonzalez R, Tournier C, Chow CW. Integration of protein kinases mTOR and extracellular signal-regulated kinase 5 in regulating nucleocytoplasmic localization of NFATc4. Mol Cell Biol. 2008;28(10):3489–501.
Olabisi OA, Soto-Nieves N, Nieves E, Yang TT, Yang X, Yu RY, Suk HY, Macian F, Chow CW. Regulation of transcription factor NFAT by ADP-ribosylation. Mol Cell Biol. 2008;28(9):2860–71.
Fan Y, Xie P, Zhang T, Zhang H, Gu D, She M, Li H. Regulation of the stability and transcriptional activity of NFATc4 by ubiquitination. FEBS Lett. 2008;582(29):4008–14.
Li Z, Zhang X, Guo Z, Zhong Y, Wang P, Li J, Li Z, Liu P. SIRT6 suppresses NFATc4 expression and activation in cardiomyocyte hypertrophy. Front Pharmacol. 2018;9:1519.
Yang TT, Xiong Q, Enslen H, Davis RJ, Chow CW. Phosphorylation of NFATc4 by p38 mitogen-activated protein kinases. Mol Cell Biol. 2002;22(11):3892–904.
Wang D, Fasciano S, Li L. The interleukin-1 receptor associated kinase 1 contributes to the regulation of NFAT. Mol Immunol. 2008;45(15):3902–8.
Yao K, Cho YY, Bergen HR 3rd, Madden BJ, Choi BY, Ma WY, Bode AM, Dong Z. Nuclear factor of activated T3 is a negative regulator of Ras-JNK1/2-AP-1 induced cell transformation. Cancer Res. 2007;67(18):8725–35.
Chow CW, Davis RJ. Integration of calcium and cyclic AMP signaling pathways by 14-3-3. Mol Cell Biol. 2000;20(2):702–12.
Cho YY, Yao K, Bode AM, Bergen HR 3rd, Madden BJ, Oh SM, Ermakova S, Kang BS, Choi HS, Shim JH, et al. RSK2 mediates muscle cell differentiation through regulation of NFAT3. J Biol Chem. 2007;282(11):8380–92.
Yang TT, Xiong Q, Graef IA, Crabtree GR, Chow CW. Recruitment of the extracellular signal-regulated kinase/ribosomal S6 kinase signaling pathway to the NFATc4 transcription activation complex. Mol Cell Biol. 2005;25(3):907–20.
Dong W, Li Y, Li X, Hu M, Aodengqimuge, Sun L, Guo N, Yuan S, Song L. p85α mediates NFAT3-dependent VEGF induction in the cellular UVB response. J Cell Sci. 2013;126(Pt 6):1317–22.
Yang T, Davis RJ, Chow CW. Requirement of two NFATc4 transactivation domains for CBP potentiation. J Biol Chem. 2001;276(43):39569–76.
Qin X, Wang XH, Yang ZH, Ding LH, Xu XJ, Cheng L, Niu C, Sun HW, Zhang H, Ye QN. Repression of NFAT3 transcriptional activity by estrogen receptors. Cell Mol Life Sci. 2008;65(17):2752–62.
Zhang H, Xie X, Zhu X, Zhu J, Hao C, Lu Q, Ding L, Liu Y, Zhou L, Liu Y, et al. Stimulatory cross-talk between NFAT3 and estrogen receptor in breast cancer cells. J Biol Chem. 2005;280(52):43188–97.
Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93(2):215–28.
Oka T, Dai YS, Molkentin JD. Regulation of calcineurin through transcriptional induction of the calcineurin A beta promoter in vitro and in vivo. Mol Cell Biol. 2005;25(15):6649–59.
Kim HB, Kumar A, Wang L, Liu GH, Keller SR, Lawrence JC Jr, Finck BN, Harris TE. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol Cell Biol. 2010;30(12):3126–39.
Liu XP, Gao H, Huang XY, Chen YF, Feng XJ, He YH, Li ZM, Liu PQ. Peroxisome proliferator-activated receptor gamma coactivator 1 alpha protects cardiomyocytes from hypertrophy by suppressing calcineurin-nuclear factor of activated T cells c4 signaling pathway. Transl Res. 2015;166(5):459–73.e3.
Kao SC, Wu H, Xie J, Chang CP, Ranish JA, Graef IA, Crabtree GR. Calcineurin/NFAT signaling is required for neuregulin-regulated Schwann cell differentiation. Science. 2009;323(5914):651–4.
Liao W, Wang S, Han C, Zhang Y. 14-3-3 proteins regulate glycogen synthase 3beta phosphorylation and inhibit cardiomyocyte hypertrophy. Febs J. 2005;272(8):1845–54.
Ding J, Li J, Xue C, Wu K, Ouyang W, Zhang D, Yan Y, Huang C. Cyclooxygenase-2 induction by arsenite is through a nuclear factor of activated T-cell-dependent pathway and plays an antiapoptotic role in Beas-2B cells. J Biol Chem. 2006;281(34):24405–13.
Tang H, Sun Y, Xiu Q, Lu H, Han H. Cyclooxygenase-2 induction requires activation of nuclear factor of activated T-cells in Beas-2B cells after vanadium exposure and plays an anti-apoptotic role. Arch Biochem Biophys. 2007;468(1):92–9.
Dittmann K, Mayer C, Czemmel S, Huber SM, Rodemann HP. New roles for nuclear EGFR in regulating the stability and translation of mRNAs associated with VEGF signaling. PLoS ONE. 2017;12(12):e0189087.
Naderi A. Coagulation factor VII is regulated by androgen receptor in breast cancer. Exp Cell Res. 2015;331(1):239–50.
de Camargo LCB, Guaddachi F, Bergerat D, Ourari N, Coillard L, Parietti V, Le Bras M, Lehmann-Che J, Jauliac S. Extracellular vesicles produced by NFAT3-expressing cells hinder tumor growth and metastatic dissemination. Sci Rep. 2020;10(1):8964.
Karaosmanoğlu O. P38-β/SAPK-inhibiting and apoptosis-inducing activities of (E)-4-chloro-2-((3-ethoxy-2-hydroxybenzylidene) amino)phenol. Hum Exp Toxicol. 2020;39(10):1374–89.
Martinez-Chantar ML, Foti M. NFATc4: New hub in NASH development. J Hepatol. 2020;73(6):1313–5.
Pan XY, You HM, Wang L, Bi YH, Yang Y, Meng HW, Meng XM, Ma TT, Huang C, Li J. Methylation of RCAN1.4 mediated by DNMT1 and DNMT3b enhances hepatic stellate cell activation and liver fibrogenesis through Calcineurin/NFAT3 signaling. Theranostics. 2019;9(15):4308–23.
Ding W, Tong Y, Zhang X, Pan M, Chen S. Study of arsenic sulfide in solid tumor cells reveals regulation of nuclear factors of activated T-cells by PML and p53. Sci Rep. 2016;6:19793.
Ding H, Fan GL, Yi YX, Zhang W, Xiong XX, Mahgoub OK. Prognostic implications of immune-related genes’ (IRGs) signature models in cervical cancer and endometrial cancer. Front Genet. 2020;11:725.
Wang Q, Vattai A, Vilsmaier T, Kaltofen T, Steger A, Mayr D, Mahner S, Jeschke U. Hildegard Heidegger H. Immunogenomic identification for predicting the prognosis of cervical cancer patients. Int J Mol Sci. 2021;22(5):2442.
Zhao C, Yang S, Lu W, Liu J, Wei Y, Guo H, Zhang Y, Shi J. Increased NFATC4 correlates with poor prognosis of AML through recruiting regulatory T Cells. Front Genet. 2020;11:573124.
Desterke C, Bilhou-Nabéra C, Guerton B, Martinaud C, Tonetti C, Clay D, Guglielmelli P, Vannucchi A, Bordessoule D, Hasselbalch H, et al. FLT3-mediated p38-MAPK activation participates in the control of megakaryopoiesis in primary myelofibrosis. Cancer Res. 2011;71(8):2901–15.
Song L, Li J, Ye J, Yu G, Ding J, Zhang D, Ouyang W, Dong Z, Kim SO, Huang C. p85alpha acts as a novel signal transducer for mediation of cellular apoptotic response to UV radiation. Mol Cell Biol. 2007;27(7):2713–31.
Pai P, Velmurugan BK, Kuo CH, Yen CY, Ho TJ, Lin YM, Chen YF, Lai CH, Day CH, Huang CY. 17β-Estradiol and/or estrogen receptor alpha blocks isoproterenol-induced calcium accumulation and hypertrophy via GSK3β/PP2A/NFAT3/ANP pathway. Mol Cell Biochem. 2017;434(1–2):181–95.
Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, et al. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30(7):2636–49.
Chen F, Zhang YM, Wang JT, Wang J, Cui ZL, Liu ZR. Pre-treatment with FK506 reduces hepatic ischemia-reperfusion injury in rats. Clin Res Hepatol Gastroenterol. 2019;43(2):161–70.
Acknowledgements
We would like to thank members of the Lau And Xu laboratory for critical reading of this manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 31771582 and 31271445), the Guangdong Natural Science Foundation of China (No. 2017A030313131), the “Thousand, Hundred, and Ten” Project of the Department of Education of Guangdong Province of China, the Basic and Applied Research Major Projects of Guangdong Province of China (2017KZDXM035 and 2018KZDXM036), the “Yang Fan” Project of Guangdong Province of China (Andy T. Y. Lau-2016; Yan-Ming Xu-2015), and the Shantou Medical Health Science and Technology Plan (200624165260857).
Author information
Authors and Affiliations
Contributions
Writing—original draft preparation, Q.-H.Z., S.-W.Z., A.T.Y.L., and Y.-M.X.; writing—review and editing, Q.-H.Z., S.-W.Z., A.T.Y.L., and Y.-M.X.; supervision, A.T.Y.L., and Y.-M.X.; funding acquisition, A.T.Y.L., and Y.-M.X. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhong, QH., Zha, SW., Lau, A.T.Y. et al. Recent knowledge of NFATc4 in oncogenesis and cancer prognosis. Cancer Cell Int 22, 212 (2022). https://doi.org/10.1186/s12935-022-02619-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02619-6