Abstract
Purpose
To evaluate the efficacy and safety of early oral feeding (EOF) in patients after upper gastrointestinal surgery through meta-analysis of randomized controlled trials (RCTs).
Methods
We analyzed the endpoints of patients including the length of stay (LOS), time of first exhaust, anastomotic leakage and pneumonia from included studies. And we retrieved RCTs from medical literature databases. Weighted mean difference (WMD), risk ratios (RR) and 95% confidence intervals (CI) were calculated to compare the endpoints.
Results
In total, we retrieved 12 articles (13 trial comparisons) which contained 1771 patients. 887 patients (50.1%) were randomized to EOF group whereas 884 patients (49.9%) were randomized to delay oral feeding group. The result showed that compared with the delay oral feeding group, EOF after upper gastrointestinal surgery significantly shorten the LOS [WMD = − 1.30, 95% CI − 1.79 to − 0.80, I2 = 0.0%] and time of first exhaust [WMD = − 0.39, 95% CI − 0.58 to − 0.20, I2 = 62.1%]. EOF also reduced the risk of pneumonia (RR: 0.74, 95% CI 0.55 to 0.99, I2 = 0.0%). There is no significant difference in the risk of anastomotic leak, anastomotic bleeding, abdominal abscess, reoperation, readmission and mortality.
Conclusions
Overall, compared with the traditional oral feeding, EOF could shorten the LOS and time of first exhaust without increasing complications after upper gastrointestinal surgery.
Similar content being viewed by others
Introduction
Upper gastrointestinal surgery mainly refers to the operation of esophagus, stomach, duodenum, liver or pancreas [1]. Its common indication is cancer. Anastomotic leakage after upper gastrointestinal surgery is a major source of morbidity as it can lead to severe infection and increase the risk of fatal sequelae in the absence of reasonable treatment [2]. After upper gastrointestinal surgery, patients often have difficulty in eating, increased catabolism, weakened anabolism and decreased immune function, which will result in (or aggravating) malnutrition [3]. It may increase the incidence of postoperative complications and mortality.
As for the timing of eating after upper gastrointestinal surgery, most surgeons still follow the traditional principle of restoring intestinal function after anal exhaust and then eating gradually. This approach is based on avoiding complications that may be caused by excessive gastrointestinal volume or early gastrointestinal stimulation, including nausea, vomiting, aspiration pneumonia, anastomotic leakage and so on [4]. However, fasting from postoperative to anal exhaust will lead to insufficient enteral nutrition and inhibit the secretion of saliva and digestive glands [5]. It will also delay the recovery of digestive system function and increase the risk of potential pathogen infection and microbial translocation which seriously affect postoperative recovery and wound healing [6]. After all, nutritional status is an important factor affecting postoperative recovery.
Recently, enhanced recovery after surgery (ERAS) has attracted more and more attention, which requires multidisciplinary teamwork to accelerate recovery during perioperative care [7]. Early oral feeding (EOF) is one of the most important elements of ERAS [8]. A considerable number of literatures have confirmed that EOF can effectively reduce the length of stay and accelerate the recovery of gastrointestinal function without increasing postoperative complications [9,10,11,12]. In 2016, Willcutts et al. [13] made a meta-analysis to compare the effect of EOF on clinical outcome after upper gastrointestinal surgery. They concluded that compared with conventional feeding, postoperative EOF was associated with a shorter length of hospital stay and is not associated with an increase in clinically relevant complications. In the past 5 years, more and more randomized controlled trials (RCTs) about the effect of EOF in upper gastrointestinal surgery have been continuously published. However, the clinical application of EOF is still controversial, and it is not widely used. It remains to be seen whether the conclusion of Willcutts’s study is still applicable. It is necessary to make an updated meta-analysis.
Methods
Search strategy
Two investigators searched published articles according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to compare the efficacy and safety of EOF in patients after upper gastrointestinal surgery [14]. We conducted a systematic search for RCTs in databases such as the Cochrane Library, Embase, Baidu Schilar, PubMed, and Google Scholar with language restrictions to English and publication dates restricted to April 14, 2021. The following keywords and MeSH terms were used to search: (“early oral feeding” or “EOF” or “enhanced recovery after surgery” or “ERAS” or “direct oral feeding”) and (“upper gastrointestinal surgery” or “esophagectomy” or “gastrectomy” or “anastomosis” or “gastrointestinal” or “surgery”). The retrieved studies were screened by one author and double-reviewed by another author. When there was a dispute, a third author was involved in the discussion and made a decision together. All data was extracted from published studies, therefore patient consent and ethical approval were not required.
Exclusion and inclusion criteria
Exclusion criteria: (1) Semi-randomized or non-randomized trials; (2) Animal experiments; (3) Nonclinical trials or case reports; and (4) Articles with incorrect or incomplete data or articles whose data could not be extracted.
Inclusion criteria: (1) Studies that compared EOF and delay oral feeding for patients after upper gastrointestinal surgery; (2) The study was a RCT; (3) Baseline characteristics (e.g., age, gender and type of surgery) were not statistically different between two groups; (4) The study subjects were patients undergoing upper gastrointestinal surgery; (5) one of groups was applied EOF; (6) The language of the studies was restricted to English; and (7) Included studies provided sufficient data for the analysis.
Endpoints
The primary effective endpoints were length of stay (LOS) and time of first exhaust. The safety endpoints were anastomotic leak, anastomotic bleeding, abdominal abscess, reoperation, readmission, mortality and pneumonia.
Data extraction
The content of the included studies was independently reviewed by two authors. Two authors extracted the primary endpoints and a third author verified endpoints. The following main information was extracted from the included studies: year of publication, first author's name, time period, country of patients, population, mean age, operation type, endpoints in each study and intervention. If the included studies required clarification, we contacted the first author of the included study. When there was a disagreement, we resolved it by consensus or consultation with a third author.
Assessment of risk of bias
Two authors independently evaluated the quality of the methodology according to the Cochrane Risk of Bias criteria [15]. Each quality items were classified as high risk, low risk, and some concerns. There are 5 items were used to estimate bias for each included studies, including bias due to deviations from intended interventions, bias arising from the randomization process, bias in measurement of the outcome, bias due to missing outcome data, bias in selection of the reported result.
Statistical analysis
We used Stata (version 12.0) to analyze and pool the included studies results. We recorded pooled results by weignted mean difference (WMD), risk ratios (RR), and 95% confidence intervals (CI) with two-sided P-values. There were considered to be statistically significant when P-values < 0.05. I2 test was used to evaluate heterogeneity. The heterogeneity was considered to be substantial and the random effect model was used when I2 > 50%, while the fixed effect model was used when I2 < 50%. If there were more than ten studies assessed one endpoint, we examined the publication bias and explored sources of heterogeneity by funnel plot [16, 17].
Results
Features of the studies included and retrieved data
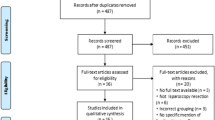
According to PRISMA guidelines, 478 studies were enrolled. We then eliminated a portion of the articles by screening the abstracts, and identified the final articles for inclusion by reading the full text. Finally, 12 studies [9, 18,19,20,21,22,23,24,25,26,27,28] (13 trial comparisons) were included which contained 1771 patients as shown in Fig. 1. 887 patients (50.1%) were randomized to EOF group whereas 884 patients (49.9%) were randomized to delay oral feeding group. All included studies were RCTs. The basic characteristics of the included studies were described in Table 1.
Assessment of quality of the studies
Two authors evaluated the quality of the retrieved studies by The Cochrane Risk of Bias criteria [15]. 12 studies [9, 18,19,20,21,22,23,24,25,26,27,28] described random sequence generation and allocation concealment. None of the studies described other biases. The included studies were all RCTs. The literature quality score was shown in Table 2.
Endpoints
Length of stay (LOS)
Four studies [19,20,21,22] (4 trial comparisons) reported LOS. Compared to the control group, the LOS in the EOF group was significantly shorter with statistical differences [WMD = − 1.30, 95% CI − 1.79 to − 0.80, I2 = 0.0%] as showed in Fig. 2. The fixed effect model was applied.
Time of first exhaust
Four studies [20,21,22, 26] (4 trial comparisons) reported time of first exhaust. Compared to the control group, time of first exhaust in the EOF group was significantly shorter with statistical differences [WMD = − 0.39, 95% CI − 0.58 to − 0.20, I2 = 62.1%] as showed in Fig. 3. The random effect model was applied. And we performed a subgroup analysis by type of surgery. The results of the subsequent subgroup analysis showed that compared to patients undergoing other type of surgery (such as bilioenteric anastomosis) [RR = − 0.26, 95% CI − 0.42 to − 0.10], the effect in those patients undergoing gastrectomy was more significant [RR = − 0.48, 95% CI − 0.77 to − 0.19] as shown in Fig. 4.
Safety endpoint
EOF could reduce the risk of pneumonia compared with delay oral feeding (8.4% vs 11.5%) (RR: 0.74, 95% CI 0.55 to 0.99, I2 = 0.0%) (Fig. 5). And there was no significant difference between EOF group and delay oral feeding group in the risk of anastomotic leak (RR: 0.91, 95% CI 0.60 to 1.38, I2 = 0.0%), anastomotic bleeding (RR: 1.47, 95% CI 0.53 to 4.03, I2 = 0.0%), abdominal abscess(RR: 0.54, 95% CI 0.27 to 1.07, I2 = 0.0%), reoperation (RR: 0.81, 95% CI 0.53 to 1.26, I2 = 0.0%), readmission (RR: 1.08, 95% CI 0.72 to 1.61, I2 = 0.0%) and mortality(RR: 0.71, 95% CI 0.36 to 1.39, I2 = 0.0%) as shown in Figs. 6, 7, 8, 9, 10, 11.
Sensitivity analysis and publication bias
The funnel plot shows a low probability of publication bias for the included studies, as shown in Figs. 12, 13. The results of the sensitivity analysis are shown in Fig. 14.
Discussion
ERAS promotes global evidence-based treatment in perioperative period [29]. It was first described and performed in patients after elective colorectal surgery in European countries [30], and now it gradually extends to any type of surgery, including some major upper gastrointestinal surgery [31]. The program aims to reduce injury caused by surgery, support the recovery of intestinal function and promote early activity of patients [32]. It has adopted a variety of strategies in the postoperative process, such as avoiding nasotracheal intubation, epidural analgesia, early exercise and early oral nutrition, most of which show obvious effects. Among them, early oral feeding is different from the traditional oral feeding. EOF program recommends to begin to oral fluid food within postoperative day 1, and gradually transition to semi-fluid and solid diet [33]. The traditional treatment is “nil by mouth” until intestinal function recovers naturally. It is due to fear of postoperative complications, such as anastomotic fistula and aspiration pneumonia [34].
Nowadays, there are only a few meta-analyses to study the efficacy and safety of EOF in patients after upper gastrointestinal surgery. Zhang et al. [12] assessed the effect of EOF on the incidence of anastomotic leakage after esophagectomy through a meta-analysis. And they found that EOF did not increase anastomotic leakage rate. However, due to significant heterogeneity, bias and small samples, the results are unreliable. Another study by Liu et al. [11] made a meta-analysis based on RCTs to evaluate the feasibility of EOF after gastrectomy for gastric cancer. And they suggested that EOF after gastric cancer surgery seems to be feasible and safe regardless of the scope and type of gastrectomy. However, only patients from China and Korea were included, which is not representative of a broad population. Li et al. [35] evaluate the effect of EOF on anastomotic leakage rate after esophagectomy. They concluded that anastomotic leakage in open esophagectomy is related to the timing of oral feeding, and delayed oral feeding is beneficial to reduce anastomotic leakage. However, there was no significant difference in anastomotic leakage between EOF and delayed oral feeding in patients with minimally invasive esophagectomy. This conclusion is contrary to the most meta-analyses. It may be that most of the included studies in the meta-analysis are retrospective studies with low quality, which may lead to low credibility of the conclusion. Willcutts et al. [13] also conducted a meta-analysis to compare the effect of EOF on clinical outcome after upper gastrointestinal surgery. They concluded that compared with conventional feeding, postoperative EOF was not associated with an increase in clinically relevant complications and was associated with a shorter length of hospital stay. There was inherent clinical heterogeneity in their meta-analysis because studies on multiple types of upper gastrointestinal surgery (gastrectomy, esophagectomy, hepatobiliary, and others) were pooled together.
Our meta-analysis evaluated the efficacy and safety of EOF in patients after upper gastrointestinal surgery. The results showed that compared with the traditional oral feeding group, EOF after upper gastrointestinal surgery significantly shorten the LOS and time of first exhaust. EOF also reduced the risk of pneumonia (RR: 0.74, 95% CI 0.55 to 0.99, I2 = 0.0%). And there is no significant difference in the risk of anastomotic leak, anastomotic bleeding, abdominal abscess, reoperation, readmission and mortality.
There is a large heterogeneity in the endpoint of the time of first exhaust (I2 = 62.1%). Through sensitivity analysis, we found that the heterogeneity mainly comes from the study of Hur et al. [20] We consider that this may be because the sample size of Hur’s study is small (n = 54). And the average age of the Hur’s study is unknown. We know that there are great differences in the tolerance of patients of different ages to surgery. Young people have significantly better tolerance than the elderly. Besides, these studies are conducted in different countries that the standard surgical practice may varies. Hur’s study [20] is from Korea and the other three studies are from China, which may lead to methodological heterogeneity.
The potential clinical implications of this meta-analysis are as follows: (1) this is an updated meta-analysis to evaluate the efficacy and safety of EOF in patients after upper gastrointestinal surgery. Compared to previous studies, we included 12 RCTs that contained a large sample size of 1771 participants; (2) Sensitivity analyses and subgroup analyses were conducted to decompose heterogeneity and explore the influence of sample size on the overall effect; (3) All the included studies were RCTs and the literature was of high quality; (4) Compared with previous studies, literature from different regions was included in this study, such as China, Japan, India, Norway, Iran, Netherlands, Sweden and USA, which was widely representative; (5) The heterogeneity of this meta-analysis is low and the conclusions are more reliable; (6) Only 2 of the studies [18, 28] had sample sizes less than 50; and (7) EOF not only did not increase the risk of pneumonia, but can significantly reduce the risk of pneumonia, which is different from the conclusion of previous studies. And it might be another potential benefit of EOF in upper gastrointestinal surgery, which needs to be further confirmed by higher quality RCTs.
The limitations of our study are as follows: (1) the types of surgery were mostly esophagectomy and gastrectomy. Other types of surgery accounted for a lower proportion; (2) Most of the EOF groups in the RCTs started the oral feeding within POD 1, but there was a large variation in when the control group started oral feeding. It leads to methodological heterogeneity; (3) Several baseline characteristics (diabetes, coronary heart disease, hypertension and neoplasm staging) were not considered which may lead to mixed bias; (4) Most of included RCTs didn’t describe the blinding method used, which may lead to confounding bias; and (5) The endpoint of LOS in half of the included studies had only the mean but no standard deviation, which made it impossible to use these data to calculate the effect size.
In summary, our meta-analysis has demonstrated that compared with the traditional oral feeding group, EOF could significantly shorten the LOS and time of first exhaust after upper gastrointestinal surgery. EOF also reduced the risk of pneumonia. There is no significant difference in the risk of other complications.
Future postoperative strategies for EOF in upper gastrointestinal surgery require safer and more effective multidisciplinary collaboration under better uniform standards and extraction of more large, high-quality samples for evidence-based analysis.
Availability of data and materials
Please contact author for data requests.
Abbreviations
- EOF:
-
Early oral feeding
- DOF:
-
Delay oral feeding
- ERAS:
-
Enhanced recovery after surgery
- LOS:
-
Length of stay
- RCTs:
-
Randomized controlled trials
- WMD:
-
Weighted mean difference
- RR:
-
Risk ratios
- CI:
-
Confidence intervals
References
Ishida Y, Inaba K, Suda K, et al. Upper gastrointestinal surgery on the esophagus and stomach. Nihon Geka Gakkai Zasshi. 2015;116:292–6.
Choi HJ, Lee BI, Kim JJ, et al. The temporary placement of covered self-expandable metal stents to seal various gastrointestinal leaks after surgery. Gut Liver. 2013;7(1):112–5.
Zevallos VF, Herencia LI, Chang F, et al. Gastrointestinal effects of eating quinoa (Chenopodium quinoa Willd.) in celiac patients. Am J Gastroenterol. 2014;109(2):270–8.
Dressman JB, Bass P, Ritschel WA, et al. Gastrointestinal parameters that influence oral medications. J Pharm Sci. 2010;82(9):857–72.
Ye RC, Yun JH, Choi SH, et al. Effect of early enteral nutrition on the incidence of acute acalculous cholecystitis among trauma patients. Asia Pac J Clin Nutr. 2020;29(1):35–40.
Alverdy J, Gilbert J, Defazio JR, et al. Proceedings of the 2013 A.S.P.E.N. research workshop: the interface between nutrition and the gut microbiome: implications and applications for human health. JPEN J Parenter Enteral Nutr. 2014;38(2):167–78.
Melloul E, Hübner M, Scott M, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40(10):2425–40.
Gianotti L, Nespoli L, Torselli L, et al. Safety, feasibility, and tolerance of early oral feeding after colorectal resection outside an enhanced recovery after surgery (ERAS) program. Int J Colorectal Dis. 2011;26(6):747–53.
Lassen K, Kjaeve J, Fetveit T, et al. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg. 2008;247(5):721–9.
Le Guen M, Fessler J, Fischler M. Early oral feeding after emergency abdominal operations. Curr Opin Clin Nutr Metab Care. 2014;17(5):477–82.
Liu X, Wang D, Zheng L, et al. Is early oral feeding after gastric cancer surgery feasible? A systematic review and meta-analysis of randomized controlled trials. PLoS ONE. 2014;9(11):e112062.
Zhang C, Zhang M, Gong L, et al. The effect of early oral feeding after esophagectomy on the incidence of anastomotic leakage: an updated review. Postgrad Med. 2020;132(5):419–25.
Willcutts KF, Chung MC, Erenberg CL, et al. Early oral feeding as compared with traditional timing of oral feeding after upper gastrointestinal surgery: a systematic review and meta-analysis. Ann Surg. 2016;264(1):54–63.
Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. J Am Med Assoc. 2015;313(16):1657–65.
Sterne J, Savovi J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ Clin Res. 2019;366:l4898.
Jiang M, Liu S, Deng H, et al. The efficacy and safety of fast track surgery (FTS) in patients after hip fracture surgery: a meta-analysis. J Orthop Surg Res. 2021. https://doi.org/10.1186/s13018-021-02277-w.
Jiang M, Deng H, Chen X, et al. The efficacy and safety of selective COX-2 inhibitors for postoperative pain management in patients after total knee/hip arthroplasty: a meta-analysis. J Orthop Surg Res. 2020. https://doi.org/10.1186/s13018-020-1569-z.
Suresh V. Feasibility and safety of early oral feeding after cervical esophagogastrostomy. Internet J Thorac Cardiovasc Surg. 2000;1:2.
Hirao M, Tsujinaka T, Takeno A, et al. Patient-controlled dietary schedule improves clinical outcome after gastrectomy for gastric cancer. World J Surg. 2005;29(7):853–7.
Hur H, Kim SG, Shim JH, et al. Effect of early oral feeding after gastric cancer surgery: a result of randomized clinical trial. Surgery. 2011;149(4):561–8.
Mi L, Zhong B, Zhang DL, et al. Effect of early oral enteral nutrition on clinical outcomes after gastric cancer surgery. Zhong hua Wei chang Wai ke Za zhi. 2012;15(5):464–7.
Peng S-S. Clinical effects of early postoperative oral feeding versus traditional oral feeding after bilioenteric anastomosis. World Chin J Digestol. 2014. https://doi.org/10.11569/wcjd.v22.i9.1312.
Mahmoodzadeh H, Shoar S, Sirati F, et al. Early initiation of oral feeding following upper gastrointestinal tumor surgery: a randomized controlled trial. Surg Today. 2015;45(2):203–8.
Sun HB, Li Y, Liu XB, et al. Early oral feeding following mckeown minimally invasive esophagectomy: an open-label, randomized, controlled noninferiority trial. Ann Surg. 2018;267(3):435–42.
Shimizu N, Oki E, Tanizawa Y, et al. Effect of early oral feeding on length of hospital stay following gastrectomy for gastric cancer: a Japanese multicenter, randomized controlled trial. Surg Today. 2018;48(9):865–74.
Gao L, Zhao ZG, Zhang LY, et al. Effect of early oral feeding on gastrointestinal function recovery in postoperative gastric cancer patients: a prospective study. J BUON. 2019;24(1):194–200.
Berkelmans GHK, Fransen LFC, Dolmans-Zwartjes ACP, et al. Direct oral feeding following minimally invasive esophagectomy (NUTRIENT II trial): an international, multicenter open-label randomized controlled trial. Ann Surg. 2020;271(1):41–7.
Masood A, Viqar S, Zia N, et al. Early oral feeding compared with traditional postoperative care in patients undergoing emergency abdominal surgery for perforated duodenal ulcer. Cureus. 2021;13(1):e12553.
Jiang X, Zhang XF. Anesthesia management on enhanced recovery after surgery (ERAS) during perioperative period in primary hospitals. World Latest Med Inform. 2019;19(67):40–1.
Pisarska M, Pędziwiatr M, Małczak P, et al. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int J Surg. 2016. https://doi.org/10.1016/j.clnesp.2016.02.060.
Pdziwiatr M, Mavrikis J, Witowski J, et al. Current status of enhanced recovery after surgery (ERAS) protocol in gastrointestinal surgery. Med Oncol. 2018;35(6):1–8.
Han-Geurts I, Hop W, Kok N, et al. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br J Surg. 2010;94(5):555–61.
Muller S, Zalunardo MP, Hubner M, et al. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Rev Gastroenterol Mex. 2009;136(3):842-847.e1.
Nimmo W. Pharmacology of “nil by mouth” after surgery. BMJ. 2015;323(1):773.
Li X, Yan S, Ma Y, et al. Impact of early oral feeding on anastomotic leakage rate after esophagectomy: a systematic review and meta-analysis. World J Surg. 2020;44(8):2709–18.
Acknowledgements
We thank the First Affiliated Hospital of Guangxi Medical University for their help in the statistical analysis.
Funding
Guangxi medical and health appropriate technology development and promotion application project (S201657).
Author information
Authors and Affiliations
Contributions
The author contributions are as follows: Huachu Deng and Baibei Li contributed equally to this manuscript. Yongjian Lin has made a significant contribution to the paper revision. HD designed the study; HD, BL developed the inclusion and exclusion criteria; BL and HD conducted the statistical analysis; HD wrote the article; XQ reviewed the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deng, H., Li, B. & Qin, X. Early versus delay oral feeding for patients after upper gastrointestinal surgery: a systematic review and meta-analysis of randomized controlled trials. Cancer Cell Int 22, 167 (2022). https://doi.org/10.1186/s12935-022-02586-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12935-022-02586-y